Antioxidant Activity of Zein Hydrolysates from Zea Species and Their Cytotoxic Effects in a Hepatic Cell Culture
Abstract
:1. Introduction
2. Results
2.1. Protein Content, Determination, and Zein Quantification
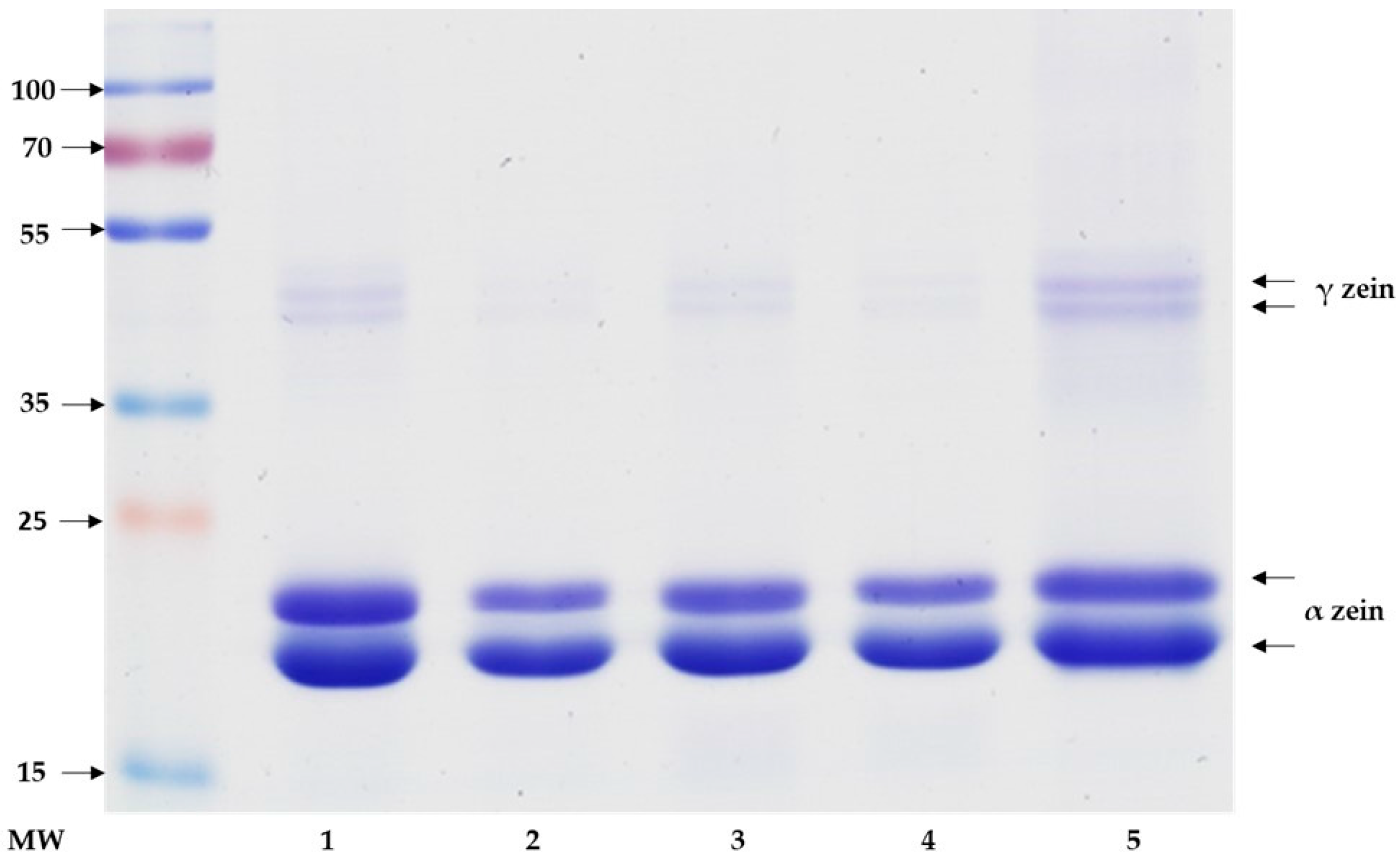
2.2. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
2.3. Antioxidant Capacity Determination
2.4. Cytotoxicity Assay
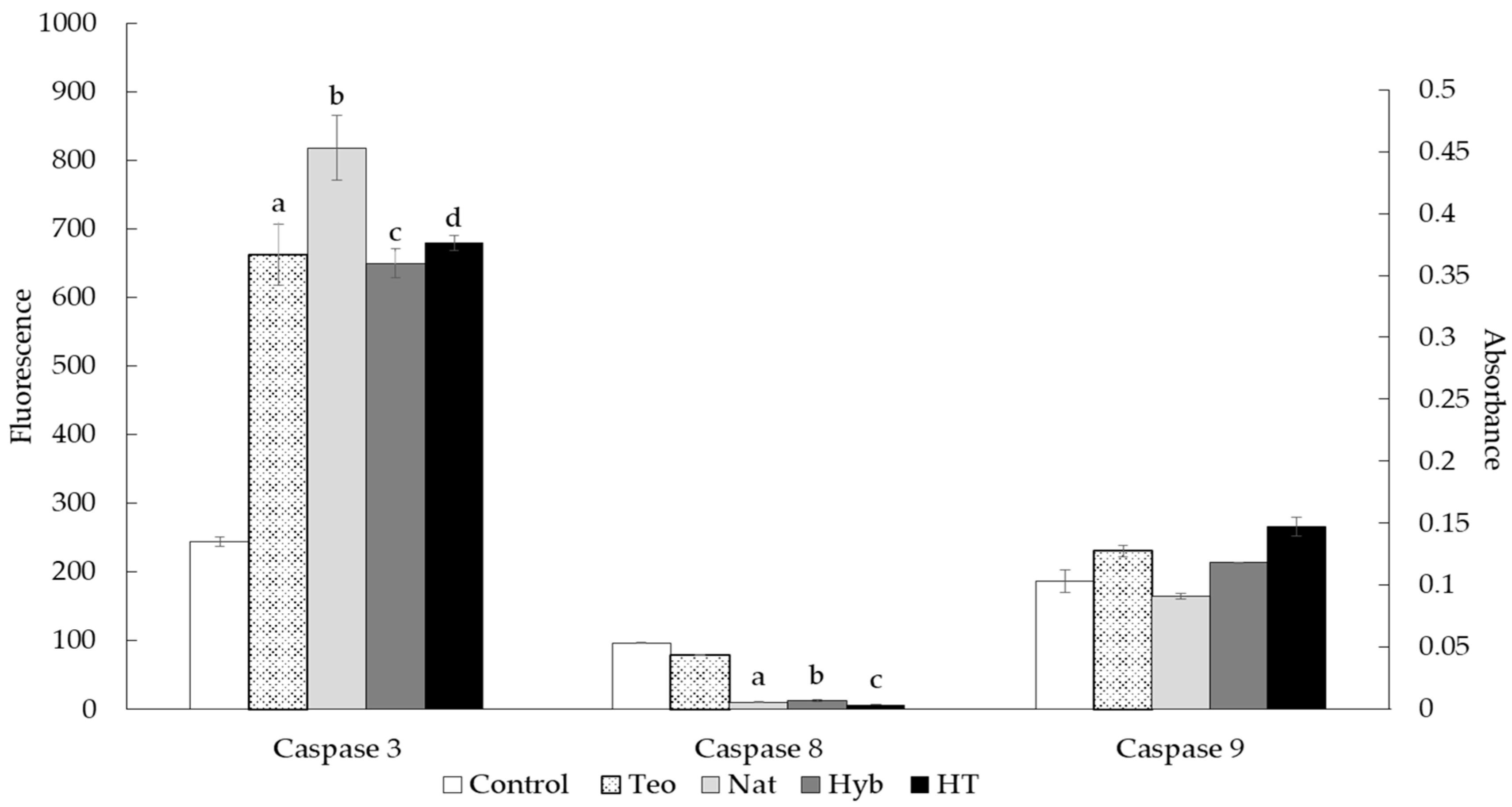
2.5. Caspase Activity Assay
3. Discussion
3.1. Total Protein and Zein Content in Maize Kernels
3.2. Antioxidant and Cytotoxic Activity of Zein and Its Peptides
3.3. Proapoptotic Activity of Zein Peptides
4. Materials and Methods
4.1. Germplasm Description
4.2. Flour Sample Preparation
4.3. Zein Extraction and Quantification
4.4. Zein Hydrolysis
4.5. Electrophoresis of Native Zein
4.6. Antioxidant Activity
4.7. Cytotoxicity Assay
4.8. Caspase Activity Evaluation
4.8.1. Caspase 3 Activity Evaluation
4.8.2. Caspase 8 Activity Evaluation
4.8.3. Caspase 9 Activity Evaluation
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WHO Projections of Mortality and Causes of Death, 2015 and 2030. Available online: www.who.int/entity/healthinfo/global_burden_disease/GHE_DthGlobal_Proj_2015_2030.xls?ua=1 (accessed on 1 August 2017).
- Li, J.-T.; Zhang, J.-L.; He, H.; Ma, Z.-L.; Nie, Z.-K.; Wang, Z.-Z.; Xu, X.-G. Apoptosis in human hepatoma HepG2 cells induced by corn peptides and its anti-tumor efficacy in H22 tumor bearing mice. Food Chem. Toxicol. 2013, 51, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.Y. Hepatocellular carcinoma--cause, treatment and metastasis. World J. Gastroenterol. 2001, 7, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Delhaye, M.; Louis, H.; Degraef, C.; Le Moine, O.; Devière, J.; Gulbis, B.; Jacobovitz, D.; Adler, M.; Galand, P. Relationship between hepatocyte proliferative activity and liver functional reserve in human cirrhosis. Hepatology 1996, 23, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.; Ye, Y.; Xie, L.; Li, W. Oxidative stress and liver cancer: Etiology and therapeutic targets. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. Mol. Mech. Mutagen. 2011, 711, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Ralph, S.J.; Rodríguez-Enríquez, S.; Neuzil, J.; Saavedra, E.; Moreno-Sánchez, R. The causes of cancer revisited: “Mitochondrial malignancy” and ROS-induced oncogenic transformation—Why mitochondria are targets for cancer therapy. Mol. Aspects Med. 2010, 31, 145–170. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Tsai, W.-L.; Shao, R.-X.; Wu, G.; Peng, L.F.; Barlow, L.L.; Chung, W.J.; Zhang, L.; Zhao, H.; Jang, J.-Y.; et al. Hepatitis C Virus Regulates Transforming Growth Factor β1 Production through the Generation of Reactive Oxygen Species in a Nuclear Factor κB–Dependent Manner. Gastroenterology 2010, 138, 2509–2518. [Google Scholar] [CrossRef] [PubMed]
- Osawa, T. Nephroprotective and hepatoprotective effects of curcuminoids. Adv. Exp. Med. Biol. 2007, 595, 407–423. [Google Scholar] [PubMed]
- Esrefoglu, M. Oxidative Stress and Benefits of Antioxidant Agents in Acute and Chronic Hepatitis. Hepat. Mon. 2012, 12, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Takaki, A.; Tsuzaki, R.; Yasunaka, T.; Koike, K.; Shimomura, Y.; Seki, H.; Matsushita, H.; Miyake, Y.; Ikeda, F.; et al. L-Carnitine Prevents Progression of Non-Alcoholic Steatohepatitis in a Mouse Model with Upregulation of Mitochondrial Pathway. PLoS ONE 2014, 9, e100627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoofnagle, J.H.; Van Natta, M.L.; Kleiner, D.E.; Clark, J.M.; Kowdley, K.V.; Loomba, R.; Neuschwander-Tetri, B.A.; Sanyal, A.J.; Tonascia, J. Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2013, 38, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Hu, X.; Cai, B.; Wang, Q.; Li, Y.; Tan, X.; Hu, H.; Chen, X.; Huang, J.; Cheng, J.; et al. Metformin suppresses hepatocellular carcinoma cell growth through induction of cell cycle G1/G0 phase arrest and p21CIP and p27KIP expression and downregulation of cyclin D1 in vitro and in vivo. Oncol. Rep. 2013, 30, 2449–2457. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-M.; Hu, X.; Guo, P.; Fu, P.; Xu, L.; Zhang, X.-Z. Antioxidant Properties and Possible Mode of Action of Corn Protein Peptides and Zein Peptides. J. Food Biochem. 2010, 34, 44–60. [Google Scholar] [CrossRef]
- FAO Crops. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 13 February 2017).
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Soto, F.E.; Serna-Saldívar, S.O.; García-Lara, S.; Pérez-Carrillo, E. Hydroxycinnamic acids, sugar composition and antioxidant capacity of arabinoxylans extracted from different maize fiber sources. Food Hydrocoll. 2014, 35, 471–475. [Google Scholar] [CrossRef]
- Del Pozo-Insfran, D.; Brenes, C.H.; Serna Saldivar, S.O.; Talcott, S.T. Polyphenolic and antioxidant content of white and blue corn (Zea mays L.) products. Food Res. Int. 2006, 39, 696–703. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Antioxidant, α-Glucosidase and Xanthine Oxidase Inhibitory Activity of Bioactive Compounds From Maize (Zea mays L.). Chem. Biol. Drug Des. 2014, 83, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Urias-Peraldí, M.; Gutiérrez-Uribe, J.A.; Preciado-Ortiz, R.E.; Cruz-Morales, A.S.; Serna-Saldívar, S.O.; García-Lara, S. Nutraceutical profiles of improved blue maize (Zea mays) hybrids for subtropical regions. F. Crop. Res. 2013, 141, 69–76. [Google Scholar] [CrossRef]
- Zilić, S.; Serpen, A.; Akıllıoğlu, G.; Gökmen, V.; Vančetović, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Díaz, S.; Ortiz-Valerio, M.D.C.; Castaño-Tostado, E.; Figueroa-Cárdenas, J.D.D.; Reynoso-Camacho, R.; Ramos-Gómez, M.; Campos-Vega, R.; Loarca-Piña, G. Antioxidant capacity and antimutagenic activity of anthocyanin and carotenoid extracts from nixtamalized pigmented Creole maize races (Zea mays L.). Plant Foods Hum. Nutr. 2012, 67, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Martinez, M.; Winkler, R.; García-Lara, S. Preventive and therapeutic potential of peptides from cereals against cancer. J. Proteomics 2014, 111, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Martinez, M.; Gonzalez de Mejia, E.; García-Lara, S.; Aguilar, O.; Lopez-Castillo, L.M.; Otero-Pappatheodorou, J.T. Antiproliferative effect of peptide fractions isolated from a quality protein maize, a white hybrid maize, and their derived peptides on hepatocarcinoma human HepG2 cells. J. Funct. Foods 2017, 34, 36–48. [Google Scholar] [CrossRef]
- Shukla, R.; Cheryan, M. Zein: The industrial protein from corn. Ind. Crops Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Anderson, T.J.; Lamsal, B.P. REVIEW: Zein Extraction from Corn, Corn Products, and Coproducts and Modifications for Various Applications: A Review. Cereal Chem. 2011, 88, 159–173. [Google Scholar] [CrossRef]
- Argos, P.; Pedersen, K.; Marks, M.D.; Larkins, B.A. A structural model for maize zein proteins. J. Biol. Chem. 1982, 257, 9984–9990. [Google Scholar] [PubMed]
- Wang, L.; Xu, C.; Qu, M.; Zhang, J. Kernel amino acid composition and protein content of introgression lines from Zea mays ssp. mexicana into cultivated maize. J. Cereal Sci. 2008, 48, 387–393. [Google Scholar] [CrossRef]
- Berardo, N.; Mazzinelli, G.; Valoti, P.; Laganà, P.; Redaelli, R. Characterization of maize germplasm for the chemical composition of the grain. J. Agric. Food Chem. 2009, 57, 2378–2384. [Google Scholar] [CrossRef] [PubMed]
- Ignjatovic-Micic, D.; Vancetovic, J.; Trbovic, D.; Dumanovic, Z.; Kostadinovic, M.; Bozinovic, S. Grain nutrient composition of maize (Zea mays L.) drought-tolerant populations. J. Agric. Food Chem. 2015, 63, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; He, X.; Luo, Y.; Ma, L.; Tang, X.; Huang, K. Nutritional assessment of transgenic lysine-rich maize compared with conventional quality protein maize. J. Sci. Food Agric. 2013, 93, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Giuberti, G.; Gallo, A.; Masoero, F. A comparison of methods to quantify prolamin contents in cereals. Ital. J. Anim. Sci. 2011, 10, 7–13. [Google Scholar] [CrossRef]
- Giuberti, G.; Gallo, A.; Masoero, F. Technical note: quantification of zeins from corn, high-moisture corn, and corn silage using a turbidimetric method: comparative efficiencies of isopropyl and tert-butyl alcohols. J. Dairy Sci. 2012, 95, 3384–3389. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yuan, L.; Chen, H.; Sato, S.J.; Clemente, T.E.; Holding, D.R. Nonredundant Function of Zeins and Their Correct Stoichiometric Ratio Drive Protein Body Formation in Maize Endosperm. Plant Physiol. 2013, 162, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Sofi, P.A.; Wani, S.A.; Rather, A.G.; Wani, S.H. Quality protein maize (QPM): Genetic manipulation for the nutritional fortification of maize. J. Plant Breed. Crop Sci. 2009, 1, 244–253. [Google Scholar]
- Momany, F.A.; Sessa, D.J.; Lawton, J.W.; Selling, G.W.; Hamaker, S.A.H.; Willett, J.L. Structural Characterization of α-Zein. J. Agric. Food Chem. 2006, 54, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Luo, Y.; Wang, Q. Effect of acid and base treatments on structural, rheological, and antioxidant properties of α-zein. Food Chem. 2011, 124, 210–220. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Li, L.T.; Liu, X.L.; Wang, X.J.; Lin, J.; Li, D. Production of hydrolysate with antioxidative activity by enzymatic hydrolysis of extruded corn gluten. Appl. Microbiol. Biotechnol. 2006, 73, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, L.; Chen, L. In vitro antioxidant activity of protein hydrolysates prepared from corn gluten meal. J. Sci. Food Agric. 2008, 88, 1660–1666. [Google Scholar] [CrossRef]
- Tang, N.; Zhuang, H. Evaluation of Antioxidant Activities of Zein Protein Fractions. J. Food Sci. 2014, 79, C2174–C2184. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; He, Z.; Dai, Y.; Xiong, Y.L.; Xie, M.; Chen, J. Peptide fractionation and free radical scavenging activity of zein hydrolysate. J. Agric. Food Chem. 2010, 58, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Tang, N.; Yuan, Y. Purification and identification of antioxidant peptides from corn gluten meal. J. Funct. Foods 2013, 5, 1810–1821. [Google Scholar] [CrossRef]
- Zhou, K.; Sun, S.; Canning, C. Production and functional characterisation of antioxidative hydrolysates from corn protein via enzymatic hydrolysis and ultrafiltration. Food Chem. 2012, 135, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, L.; Wang, Y.; Zhang, Y.; Liu, J. Isolation and characterisation of in vitro and cellular free radical scavenging peptides from corn peptide fractions. Molecules 2015, 20, 3221–3237. [Google Scholar] [CrossRef] [PubMed]
- Kannan, A.; Hettiarachchy, N.S.; Lay, J.O.; Liyanage, R. Human cancer cell proliferation inhibition by a pentapeptide isolated and characterized from rice bran. Peptides 2010, 31, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Barrio, D.A.; Añón, M.C. Potential antitumor properties of a protein isolate obtained from the seeds of Amaranthus mantegazzianus. Eur. J. Nutr. 2010, 49, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Jeong, J.B.; Hsieh, C.C.; Hernández-Ledesma, B.; de Lumen, B.O. Lunasin is prevalent in barley and is bioavailable and bioactive in in vivo and in vitro studies. Nutr. Cancer 2010, 62, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Hernández-Ledesma, B.; De Lumen, B.O. Soybean Peptide Lunasin Suppresses In Vitro and In Vivo 7,12-Dimethylbenz[a]anthracene-Induced Tumorigenesis. J. Food Sci. 2010, 75. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Yu, W.; Liu, Z.; Wu, M.; Wang, J. Induction of apoptosis in cervix neoplasms hela cells by a rapeseed peptide hydrolysate fraction. J. Food Biochem. 2011, 35, 1283–1297. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Dia, V.P.; Mejia, E.G.D. Lunasin promotes apoptosis in human colon cancer cells by mitochondrial pathway activation and induction of nuclear clusterin expression. Cancer Lett. 2010, 295, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez de Mejia, E.; Wang, W.; Dia, V.P. Lunasin, with an arginine-glycine-aspartic acid motif, causes apoptosis to L1210 leukemia cells by activation of caspase-3. Mol. Nutr. Food Res. 2010, 54, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Dia, V.P.; Gonzalez de Mejia, E. Lunasin induces apoptosis and modifies the expression of genes associated with extracellular matrix and cell adhesion in human metastatic colon cancer cells. Mol. Nutr. Food Res. 2011, 55, 623–634. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.J.; Devapatla, B.; Yaddanapudi, K.; Davis, K.R. The soybean-derived peptide lunasin inhibits non-small cell lung cancer cell proliferation by suppressing phosphorylation of the retinoblastoma protein. Oncotarget 2015, 6, 4649–4662. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.-T.; Zhou, T.-T.; Liu, B.; Bao, J.-K. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Burz, C.; Berindan-Neagoe, I.; Balacescu, O.; Irimie, A. Apoptosis in cancer: key molecular signaling pathways and therapy targets. Acta Oncol. 2009, 48, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Llaca, V.; Linton, E.; Messing, J. Sequence, regulation, and evolution of the maize 22-kD α zein gene family. Genome Res. 2001, 11, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Park, W.D.; Lewis, E.D.; Rubenstein, I. Heterogeneity of Zein mRNA and Protein in Maize. PLANT Physiol. 1980, 65, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Gómez, J.L.; Castorena-Torres, F.; Preciado-Ortiz, R.E.; García-Lara, S. Anti-Cancer Activity of Maize Bioactive Peptides. Front. Chem. 2017, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mestres, C.; Matencio, F. Biochemical Basis of Kernel Milling Characteristics and Endosperm Vitreousness of Maize. J. Cereal Sci. 1996, 24, 283–290. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists (AACC). Approved Methods of the American Association of Cereal Chemists, 10th ed.; AACC: St. Paul, MN, USA, 2000; ISBN 9781891127120. [Google Scholar]
- García-Lara, S.; Arnason, J.T.; Díaz-Pontones, D.; Gonzalez, E.; Bergvinson, D.J. Soluble peroxidase activity in maize endosperm associated with maize weevil resistance. Crop Sci. 2007, 47, 1125–1130. [Google Scholar] [CrossRef]
- Malumba, P.; Vanderghem, C.; Deroanne, C.; Béra, F. Influence of drying temperature on the solubility, the purity of isolates and the electrophoretic patterns of corn proteins. Food Chem. 2008, 111, 564–572. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Wu, C.; Yu, C.; Liao, H. Prolamin, a rice protein, augments anti-leukaemia immune response. J. Cereal Sci. 2010, 51, 189–197. [Google Scholar] [CrossRef]
- González-González, M.; Mayolo-Deloisa, K.; Rito-Palomares, M.; Winkler, R. Colorimetric protein quantification in aqueous two-phase systems. Process Biochem. 2011, 46, 413–417. [Google Scholar] [CrossRef]
- Starcher, B. A ninhydrin-based assay to quantitate the total protein content of tissue samples. Anal. Biochem. 2001, 292, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Bamdad, F.; Wu, J.; Chen, L. Effects of enzymatic hydrolysis on molecular structure and antioxidant activity of barley hordein. J. Cereal Sci. 2011, 54, 20–28. [Google Scholar] [CrossRef]
- LaemmLi, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Uribe, J.A.; Rojas-García, C.; García-Lara, S.; Serna-Saldivar, S.O. Phytochemical analysis of wastewater (nejayote) obtained after lime-cooking of different types of maize kernels processed into masa for tortillas. J. Cereal Sci. 2010, 52, 410–416. [Google Scholar] [CrossRef]
- American Type Culture Collection (ATCC). Animal Cell Culture Guide. Available online: https://www.atcc.org/~/media/PDFs/Culture%20Guides/AnimCellCulture_Guide.ashx (accessed on 28 January 2018).
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of maize, zein hydrolysates and peptides are available from the authors. |
| Germplasm | Protein | Native Zein | |
|---|---|---|---|
| (%) | mg/g Dry Flour | % Zein/Total Protein | |
| Teo | 18.86 ± 0.94 a | 46.00 ± 5.39 a | 24.39 ± 2.85 a |
| Nat | 9.27 ± 0.11 c | 21.39 ± 1.66 b | 23.07 ± 2.07 ab |
| Hyb | 13.13 ± 0.21 b | 28.55 ± 1.33 b | 21.74 ± 1.17 ab |
| HT | 8.51 ± 0.22 d | 14.83 ± 2.55 b | 17.43 ± 3.46 b |
| Germplasm | Native Protein | Hydrolyzed Zein |
|---|---|---|
| (µM TE/g of Zein) | (µM TE/g of Peptide) | |
| Teo | 673.40 ± 82.93 a | 987.53 ± 2.88 b |
| Nat | 87.86 ± 3.60 b | 814.15 ± 5.92 c |
| Hyb | 98.92 ± 1.27 b | 1055.45 ± 14.69 a |
| HT | 90.84 ± 1.33 b | 724.32 ± 3.26 d |
| Germplasm | IC50 (ng/mL) | |
|---|---|---|
| 12 h | 24 h | |
| Teo | 1198.69 ± 14.82 dc | 1781.63 ± 100.10 a |
| Nat | 2233.74 ± 100.28 a | 1546.23 ± 183.77 a |
| Hyb | 1526.44 ± 29.25 b | 1252.25 ± 4.8 b |
| HT | 1377.36 ± 21.09 c | 1155.56 ± 33.07 b |
| Germplasm | Acronym | Classification * | Ecology | Test Weight kg/hL | 1000 Kernel Weight (g) | Endosperm Proportion (%) | Endosperm Texture ** |
|---|---|---|---|---|---|---|---|
| Teosinte (sub mexicana) | Teo | Ancestral maize | Highlands | 75.35 ± 0.25 b | 80.67 ± 1.20 c | 41.88 ± 5.35 b | 2.09 ± 0.2 b |
| Azul de Chiapas | Nat | Local land race | Tropical | 71.63 ± 0.34 c | 375.90 ± 5.70 a | 83.81 ± 1.45 a | 4.20 ± 0.1 a |
| Hybrid Pioneer 30T83 | Hyb | Conventional hybrid | Tropical | 76.38 ± 0.17 a | 373.51 ± 3.34 a | 86.80 ± 0.65 a | 4.34 ± 0.1 a |
| Hybrid HT Hercules Plus | HT | Transgenic hybrid | Temperate | 75.65 ± 0.37 b | 286.62 ± 3.98 b | 86.39 ± 0.86 a | 4.32 ± 0.1 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Gómez, J.L.; Ortíz-Martínez, M.; Aguilar, O.; García-Lara, S.; Castorena-Torres, F. Antioxidant Activity of Zein Hydrolysates from Zea Species and Their Cytotoxic Effects in a Hepatic Cell Culture. Molecules 2018, 23, 312. https://doi.org/10.3390/molecules23020312
Díaz-Gómez JL, Ortíz-Martínez M, Aguilar O, García-Lara S, Castorena-Torres F. Antioxidant Activity of Zein Hydrolysates from Zea Species and Their Cytotoxic Effects in a Hepatic Cell Culture. Molecules. 2018; 23(2):312. https://doi.org/10.3390/molecules23020312
Chicago/Turabian StyleDíaz-Gómez, Jorge L., Margarita Ortíz-Martínez, Oscar Aguilar, Silverio García-Lara, and Fabiola Castorena-Torres. 2018. "Antioxidant Activity of Zein Hydrolysates from Zea Species and Their Cytotoxic Effects in a Hepatic Cell Culture" Molecules 23, no. 2: 312. https://doi.org/10.3390/molecules23020312





