Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review
Abstract
:1. Introduction
2. Key Factors for Effective Pretreatment
2.1. Structure of Lignocellulosic Biomass
2.2. Cellulose Crystallinity and Degree of Polymerization (DP)
2.3. Lignin
2.4. Hemicellulose
3. Physico-Chemical Pretreatment
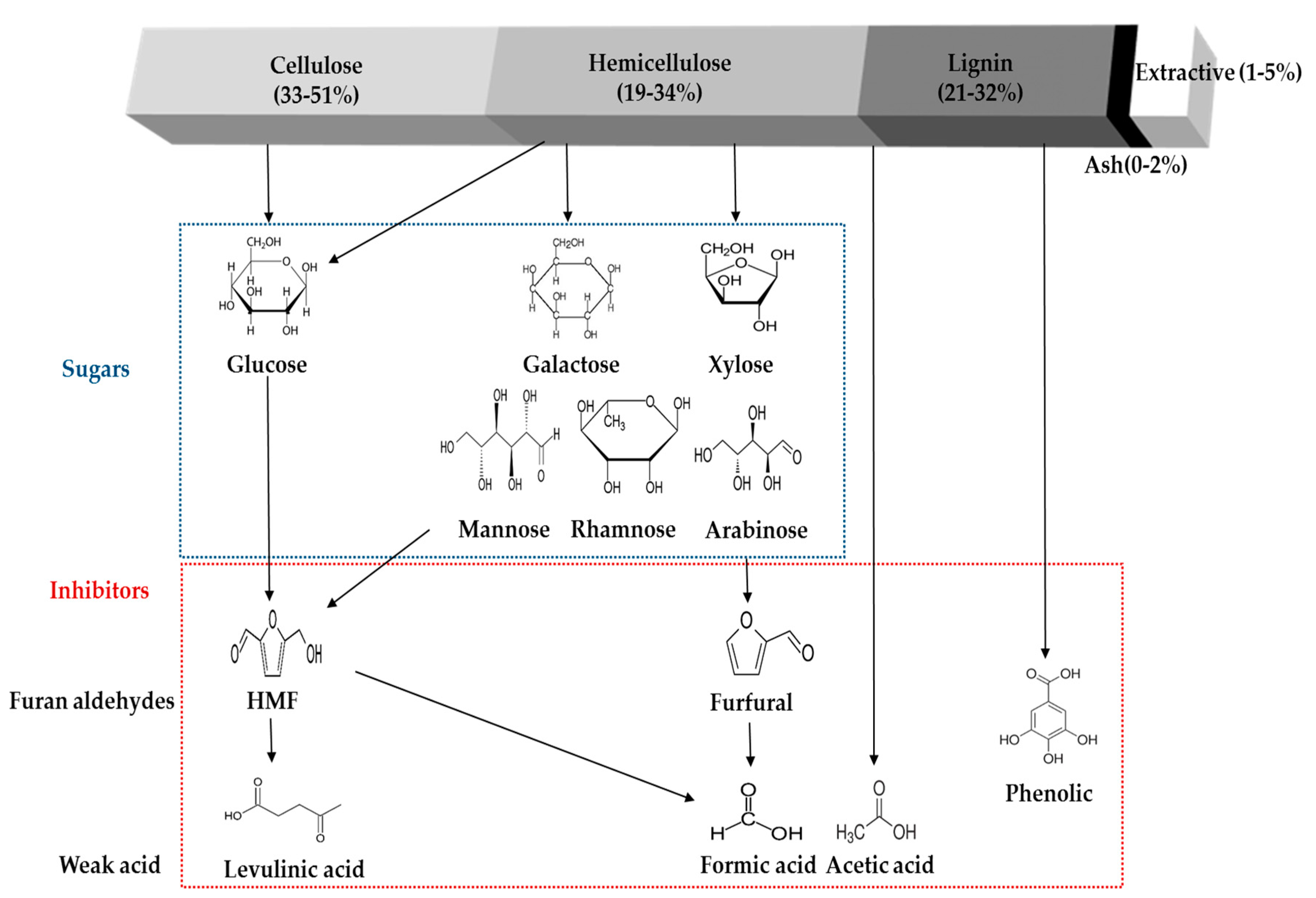
4. Formation of Inhibitory Compounds from Physico-Chemical Pretreatment
5. Pretreatment-Derived Inhibitors of Enzymatic Catalysts and Microbial Fermentations
5.1. Phenolic Compounds
5.2. Furan Derivatives
5.3. Small Organic Acids
5.4. Soluble Sugars
6. Strategies to Cope with Inhibition Issues
6.1. Selection and Modification of Feedstock
6.2. Removal of Inhibitory Compounds
6.3. Biological Detoxification
6.4. Adaptation of Microbial
6.5. Genetic/Metabolic Engineering
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Olofsson, K.; Bertilsson, M.; Lidén, G. A short review on SSF—An interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 2008, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Hägerdal, B.; Galbe, M.; Gorwa-Grauslund, M.F.; Lidén, G.; Zacchi, G. Bio-ethanol—The fuel of tomorrow from the residues of today. Trends Biotechnol. 2006, 24, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The Path Forward for Biofuels and Biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Binod, P.; Sindhu, R.; Singhania, R.R.; Vikram, S.; Devi, L.; Nagalakshmi, S.; Kurien, N.; Sukumaran, R.K.; Pandey, A. Bioethanol production from rice straw: An overview. Bioresour. Technol. 2010, 101, 4767–4774. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Chandrasekhar, G.; Radhika, K.; Ravinder, R.; Ravindra, P. Bioconversion of pentose sugars into ethanol: A review and future directions. Biotechnol. Mol. Biol. Rev. 2011, 6, 8–20. [Google Scholar]
- Rubin, E.M. Genomics of cellulosic biofuels. Nature 2008, 454, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Gírio, F.M.; Fonseca, C.; Carvalheiro, F.; Duarte, L.C.; Marques, S.; Bogel-Łukasik, R. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800. [Google Scholar] [CrossRef] [PubMed]
- Chiaramonti, D.; Prussi, M.; Ferrero, S.; Oriani, L.; Ottonello, P.; Torre, P.; Cherchi, F. Review of pretreatment processes for lignocellulosic ethanol production, and development of an innovative method. Biomass Bioenergy 2012, 46, 25–35. [Google Scholar] [CrossRef]
- Hu, G.; Heitmann, J.A.; Rojas, O.J. Feedstock pretreatment strategies for producing ethanol from wood, bark, and forest residues. BioResources 2008, 3, 270–294. [Google Scholar]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Templeton, D.W.; Scarlata, C.J.; Sluiter, J.B.; Wolfrum, E.J. Compositional analysis of lignocellulosic feedstocks. 2. Method uncertainties. J. Agric. Food Chem. 2010, 58, 9054–9062. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Day, D.F. Composition of sugar cane, energy cane, and sweet sorghum suitable for ethanol production at Louisiana sugar mills. J. Ind. Microbiol. Biotechnol. 2011, 38, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Taylor, F.; Hicks, K.B. Bioethanol production from barley hull using SAA (soaking in aqueous ammonia) pretreatment. Bioresour. Technol. 2008, 99, 5694–5702. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Orrego, D.; Ximenes, E.A.; Ladisch, M.R. Cellulose conversion of corn pericarp without pretreatment. Bioresour. Technol. 2017, 245, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ximenes, E.A.; Nichols, N.N.; Cao, G.; Frazer, S.E.; Ladisch, M.R. Maleic acid treatment of biologically detoxified corn stover liquor. Bioresour. Technol. 2016, 216, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, I.; Negro, M.J.; Oliva, J.M.; Cabanas, A.; Manzanare, P.; Ballesteros, M. Ethanol production from steam-explosion pretreated wheat straw. Appl. Biochem. Biotechnol. 2006, 129, 129–132. [Google Scholar]
- Ximenes, E.; Kim, Y.; Ladisch, M.R. Biological Conversion of Plants to Fuels and Chemicals and the Effects of Inhibitors. In Aqueous Pretreatment Plant Biomass Biological Chemical Conversion Fuels Chemicals; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 39–60. [Google Scholar]
- Chen, W.H.; Pen, B.L.; Yu, C.T.; Hwang, W.S. Pretreatment efficiency and structural characterization of rice straw by an integrated process of dilute-acid and steam explosion for bioethanol production. Bioresour. Technol. 2011, 102, 2916–2924. [Google Scholar] [CrossRef] [PubMed]
- García-Cubero, M.A.; González-Benito, G.; Indacoechea, I.; Coca, M.; Bolado, S. Effect of ozonolysis pretreatment on enzymatic digestibility of sugarcane bagasse. Agric. Eng. Int. CIGR J. 2009, 100, 1608–1613. [Google Scholar]
- Li, C.; Knierim, B.; Manisseri, C.; Arora, R.; Scheller, H.V.; Auer, M.; Vogel, K.P.; Simmons, B.A.; Singh, S. Comparison of dilute acid and ionic liquid pretreatment of switchgrass: Biomass recalcitrance, delignification and enzymatic saccharification. Bioresour. Technol. 2010, 101, 4900–4906. [Google Scholar] [CrossRef] [PubMed]
- Martín, C.; Klinke, H.B.; Thomsen, A.B. Wet oxidation as a pretreatment method for enhancing the enzymatic convertibility of sugarcane bagasse. Enzym. Microb. Technol. 2007, 40, 426–432. [Google Scholar] [CrossRef]
- Li, B.Z.; Balan, V.; Yuan, Y.J.; Dale, B.E. Process optimization to convert forage and sweet sorghum bagasse to ethanol based on ammonia fiber expansion (AFEX) pretreatment. Bioresour. Technol. 2010, 101, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Cara, C.; Ruiz, E.; Ballesteros, M.; Manzanares, P.; Negro, M.J.; Castro, E. Production of fuel ethanol from steam-explosion pretreated olive tree pruning. Fuel 2008, 87, 692–700. [Google Scholar] [CrossRef]
- Kumar, R.; Mago, G.; Balan, V.; Wyman, C.E. Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies. Bioresour. Technol. 2009, 100, 3948–3962. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Sharma, L.N.; Becker, C.; Chen, S.F.; Mowery, R.A.; van Walsum, G.P.; Chambliss, C.K. Effect of varying feedstock-pretreatment chemistry combinations on the formation and accumulation of potentially inhibitory degradation products in biomass hydrolysates. Biotechnol. Bioeng. 2010, 107, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, M.; Karimi, K.; Taherzadeh, M.J. Pretreatment of spruce and oak by N-methylmorpholine-N-oxide (NMMO) for efficient conversion of their cellulose to ethanol. Bioresour. Technol. 2010, 101, 4914–4918. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.P. Effect of crystallinity and degree of polymerization of cellulose on enzymatic saccharification. Biotechnol. Bioeng. 1984, 26, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.; Kim, Y.; Ximenes, E.; Ladisch, M.R. Effect of liquid hot water pretreatment severity on properties of hardwood lignin and enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 2015, 112, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.; Ximenes, E.; Kim, Y.; Ladisch, M.R. Adsorption of enzyme onto lignins of liquid hot water pretreated hardwoods. Biotechnol. Bioeng. 2015, 112, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Grabber, J.H.; Hatfield, R.D.; Lu, F.; Ralph, J. Coniferyl ferulate incorporation into lignin enhances the alkaline delignification and enzymatic degradation of cell walls. Biomacromolecules 2008, 9, 2510–2516. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Wyman, C.E. Access of cellulase to cellulose and lignin for poplar solids produced by leading pretreatment technologies. Biotechnol. Prog. 2009, 25, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Wyman, C.E. Effect of enzyme supplementation at moderate cellulase loadings on initial glucose and xylose release from corn stover solids pretreated by leading technologies. Biotechnol. Bioeng. 2009, 102, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Li, W.-C.; Liu, L.; Zhu, J.-Q.; Li, X.; Li, B.-Z.; Yuan, Y.-J. Inhibition of lignin-derived phenolic compounds to cellulase. Biotechnol. Biofuels 2016, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.; Kristensen, J.B.; Felby, C. Enzymatic conversion of lignocellulose into fermentable sugars: Challenges and opportunities. Biofuels Bioprod. Biorefin. 2007, 1, 119–134. [Google Scholar] [CrossRef]
- Kim, Y.; Kreke, T.; Ko, J.K.; Ladisch, M.R. Hydrolysis-determining substrate characteristics in liquid hot water pretreated hardwood. Biotechnol. Bioeng. 2015, 112, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, H.; Ahlgren, P.; Germgård, U. The degree of disorder in hardwood kraft pulps studied by means of LODP. Cellulose 2005, 12, 327–335. [Google Scholar] [CrossRef]
- Cao, S.; Pu, Y.; Studer, M.; Wyman, C.; Ragauskas, A.J. Chemical transformations of Populus trichocarpa during dilute acid pretreatment. RSC Adv. 2012, 2, 10925. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Foston, M.; Ragauskas, A.J. Changes in lignocellulosic supramolecular and ultrastructure during dilute acid pretreatment of Populus and switchgrass. Biomass Bioenergy 2010, 34, 1885–1895. [Google Scholar] [CrossRef]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Deactivation of cellulases by phenols. Enzym. Microb. Technol. 2011, 48, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Inhibition of cellulases by phenols. Enzym. Microb. Technol. 2010, 46, 170–176. [Google Scholar] [CrossRef]
- Chang, V.S.; Holtzapple, M.T. Fundamental Factors Affecting Biomass Enzymatic Reactivity. Appl. Biochem. Biotechnol. 2000, 84, 5–38. [Google Scholar] [CrossRef]
- Nakagame, S.; Chandra, R.P.; Kadla, J.F.; Saddler, J.N. Enhancing the enzymatic hydrolysis of lignocellulosic biomass by increasing the carboxylic acid content of the associated lignin. Biotechnol. Bioeng. 2011, 108, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Nakagame, S.; Chandra, R.P.; Kadla, J.F.; Saddler, J.N. The isolation, characterization and effect of lignin isolated from steam pretreated Douglas-fir on the enzymatic hydrolysis of cellulose. Bioresour. Technol. 2011, 102, 4507–4517. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.G.S.; Wayman, M. Characterization of autohydrolysis aspen (P. tremuloides) lignins. Part 1. Composition and molecular weight distribution of extracted autohydrolysis lignin. Can. J. Chem. 1979, 57, 1141–1149. [Google Scholar] [CrossRef]
- Li, J.; Henriksson, G.; Gellerstedt, G. Lignin depolymerization/repolymerization and its critical role for delignification of aspen wood by steam explosion. Bioresour. Technol. 2007, 98, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.; Um, Y.; Park, Y.C.; Seo, J.H.; Kim, K.H. Compounds inhibiting the bioconversion of hydrothermally pretreated lignocellulose. Appl. Microbiol. Biotechnol. 2015, 99, 4201–4212. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, C.I.; Davis, M.F.; Schell, D.F.; Johnson, D.K. Porosity and its effect on the digestibility of dilute sulfuric acid pretreated corn stover. J. Agric. Food Chem. 2007, 55, 2575–2581. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Gilkes, N.; Saddler, J.N. Effect of acetyl groups on enzymatic hydrolysis of cellulosic substrates. Holzforschung 2006, 60, 398–401. [Google Scholar] [CrossRef]
- Chen, X.; Shekiro, J.; Franden, M.A.; Wang, W.; Zhang, M.; Kuhn, E.; Johnson, D.K.; Tucker, M.P. The impacts of deacetylation prior to dilute acid pretreatment on the bioethanol process. Biotechnol. Biofuels 2012, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Selig, M.J.; Adney, W.S.; Himmel, M.E.; Decker, S.R. The impact of cell wall acetylation on corn stover hydrolysis by cellulolytic and xylanolytic enzymes. Cellulose 2009, 16, 711–722. [Google Scholar] [CrossRef]
- Pu, Y.; Hu, F.; Huang, F.; Davison, B.H.; Ragauskas, A.J. Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol. Biofuels 2013, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Avellar, B.K.; Glasser, W.G. Steam-assisted biomass fractionation. I. Process considerations and economic evaluation. Biomass Bioenergy 1998, 14, 205–218. [Google Scholar] [CrossRef]
- Pan, X.; Xie, D.; Gilkes, N.; Gregg, D.J.; Saddler, J.N. Strategies to Enhance the Enzymatic Hydrolysis of Pretreated Softwood with High Residual Lignin Content. Appl. Biochem. Biotechnol. 2005, 124, 1069–1080. [Google Scholar] [CrossRef]
- Sassner, P.; Mårtensson, C.G.; Galbe, M.; Zacchi, G. Steam pretreatment of H2SO4-impregnated Salix for the production of bioethanol. Bioresour. Technol. 2008, 99, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Monavari, S.; Galbe, M.; Zacchi, G. Impact of impregnation time and chip size on sugar yield in pretreatment of softwood for ethanol production. Bioresour. Technol. 2009, 100, 6312–6316. [Google Scholar] [CrossRef] [PubMed]
- Linde, M.; Jakobsson, E.L.; Galbe, M.; Zacchi, G. Steam pretreatment of dilute H2SO4-impregnated wheat straw and SSF with low yeast and enzyme loadings for bioethanol production. Biomass Bioenergy 2008, 32, 326–332. [Google Scholar] [CrossRef]
- Rabemanolontsoa, H.; Saka, S. Various pretreatments of lignocellulosics. Bioresour. Technol. 2016, 199, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Jedvert, K.; Saltberg, A.; Lindström, M.E.; Theliander, H. Mild steam explosion and chemical pre-treatment of Norway spruce. BioResources 2012, 7, 2051–2074. [Google Scholar] [CrossRef]
- Alfani, F.; Gallifuoco, A.; Saporosi, A.; Spera, A.; Cantarella, M. Comparison of SHF and SSF processes for the bioconversion of steam-exploded wheat straw. J. Ind. Microbiol. Biotechnol. 2000, 25, 184–192. [Google Scholar] [CrossRef]
- Sunna, A.; Antranikian, G. Xylanolytic Enzymes from Fungi and Bacteria. Crit. Rev. Biotechnol. 1997, 17, 39–67. [Google Scholar] [CrossRef] [PubMed]
- Bondesson, P.-M.; Galbe, M.; Zacchi, G. Ethanol and biogas production after steam pretreatment of corn stover with or without the addition of sulphuric acid. Biotechnol. Biofuels 2013, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Hongzhang, C.; Liying, L. Unpolluted fractionation of wheat straw by steam explosion and ethanol extraction. Bioresour. Technol. 2007, 98, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, Ó.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.S.; Hendrickson, R.; Brewer, M.; Ho, N.; Sedlak, M.; Dreshel, R.; Welch, G.; Dien, B.S.; Aden, A.; Ladisch, M.R. Industrial scale-up of pH-controlled liquid hot water pretreatment of corn fiber for fuel ethanol production. Appl. Biochem. Biotechnol. 2005, 125, 77–97. [Google Scholar] [CrossRef]
- Kim, Y.; Mosier, N.S.; Ladisch, M.R. Enzymatic digestion of liquid hot water pretreated hybrid poplar. Biotechnol. Prog. 2009, 25, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.; Hendrickson, R.; Ho, N.; Sedlak, M.; Ladisch, M.R. Optimization of pH controlled liquid hot water pretreatment of corn stover. Bioresour. Technol. 2005, 96, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.P.; Sun, Z.J.; Shi, Z.J.; Xu, F.; Sun, R.C. Impact of hot compressed water pretreatment on the structural changes of woody biomass for bioethanol production. BioResources 2011, 6, 1576–1598. [Google Scholar]
- Laureano-Perez, L.; Teymouri, F.; Alizadeh, H.; Dale, B.E. Understanding factors that limit enzymatic hydrolysis of biomass. Appl. Biochem. Biotechnol. 2005, 124, 1081–1099. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, Z.; Zhang, R. Overview of biomass pretreatment for cellulosic ethanol production. Int. J. Agric. Biol. Eng. 2009, 2, 51–68. [Google Scholar]
- Teymouri, F.; Laureano-Perez, L.; Alizadeh, H.; Dale, B.E. Optimization of the ammonia fiber explosion (AFEX) treatment parameters for enzymatic hydrolysis of corn stover. Bioresour. Technol. 2005, 96, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Sendich, E.N.; Laser, M.; Kim, S.; Alizadeh, H.; Laureano-Perez, L.; Dale, B.; Lynd, L. Recent process improvements for the ammonia fiber expansion (AFEX) process and resulting reductions in minimum ethanol selling price. Bioresour. Technol. 2008, 99, 8429–8435. [Google Scholar] [CrossRef] [PubMed]
- Sendich, E.D.; Dale, B.E.; Kim, S. Comparison of crop and animal simulation options for integration with the biorefinery. Biomass Bioenergy 2008, 32, 1162–1174. [Google Scholar] [CrossRef]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.Y. Coordinated development of leading biomass pretreatment technologies. Bioresour. Technol. 2005, 96, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Chundawat, S.P.; Krishnan, C.; Bals, B.; Sousa, L.; Thelen, K.D.; Dale, B.E.; Balan, V. Enzymatic digestibility and ethanol fermentability of AFEX-treated starch-rich lignocellulosics such as corn silage and whole corn plant. Biotechnol. Biofuels 2010, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.W.; Dale, B.E. Effect of primary degradation-reaction products from Ammonia Fiber Expansion (AFEX)-treated corn stover on the growth and fermentation of Escherichia coli KO11. Bioresour. Technol. 2010, 101, 7849–7855. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Lau, M.W.; Balan, V.; Dale, B.E. Two-step SSCF to convert AFEX-treated switchgrass to ethanol using commercial enzymes and Saccharomyces cerevisiae 424A(LNH-ST). Bioresour. Technol. 2010, 101, 8171–8178. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Ximenes, E.; Nichols, N.N.; Frazer, S.E.; Kim, D.; Cotta, M.A.; Ladisch, M. Bioabatement with hemicellulase supplementation to reduce enzymatic hydrolysis inhibitors. Bioresour. Technol. 2015, 190, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Ximenes, E.; Nichols, N.N.; Zhang, L.; Ladisch, M. Biological abatement of cellulase inhibitors. Bioresour. Technol. 2013, 146, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ximenes, E.; Mosier, N.S.; Ladisch, M.R. Soluble inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass. Enzym. Microb. Technol. 2011, 48, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kreke, T.; Hendrickson, R.; Parenti, J.; Ladisch, M.R. Fractionation of cellulase and fermentation inhibitors from steam pretreated mixed hardwood. Bioresour. Technol. 2013, 135, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Mansfield, S.D.; Mooney, C.; Saddler, J.N. Substrate and enzymatic characteristics that limit cellulose hydrolysis. Biotechnol. Prog. 1999, 15, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.P.; Lynd, L.R. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol. Bioeng. 2004, 88, 797–824. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, G.; Toussaint, B.; Vignon, M.R. Saccharification of steam-exploded poplar wood. Biotechnol. Bioeng. 1991, 38, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Gurram, R.N.; Datta, S.; Lin, Y.J.; Snyder, S.W.; Menkhaus, T.J. Removal of enzymatic and fermentation inhibitory compounds from biomass slurries for enhanced biorefinery process efficiencies. Bioresour. Technol. 2011, 102, 7850–7859. [Google Scholar] [CrossRef] [PubMed]
- Klinke, H.B.; Thomsen, A.B.; Ahring, B.K. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol. 2004, 66, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Michelin, M.; Ximenes, E.; de Lourdes Teixeira de Moraes Polizeli, M.; Ladisch, M.R. Effect of phenolic compounds from pretreated sugarcane bagasse on cellulolytic and hemicellulolytic activities. Bioresour. Technol. 2016, 199, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam—Aqueous pretreatments. Philos. Trans. R. Soc. Lond. A 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Chundawat, S.P.S.; Beckham, G.T.; Himmel, M.E.; Dale, B.E. Deconstruction of Lignocellulosic Biomass to Fuels and Chemicals. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.; Verma, P. An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. 3 Biotech 2013, 3, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Balan, V.; Da Costa Sousa, L.; Chundawat, S.P.S.; Marshall, D.; Sharma, L.N.; Chambliss, C.K.; Dale, B.E. Enzymatic digestibility and pretreatment degradation products of AFEX-treated hardwoods (Populus nigra). Biotechnol. Prog. 2009, 25, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Ezeji, T.; Qureshi, N.; Blaschek, H.P. Butanol production from agricultural residues: Impact of degradation products on Clostridium beijerinckii growth and butanol fermentation. Biotechnol. Bioeng. 2007, 97, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Adeboye, P.T.; Bettiga, M.; Aldaeus, F.; Larsson, P.T.; Olsson, L. Catabolism of coniferyl aldehyde, ferulic acid and p-coumaric acid by Saccharomyces cerevisiae yields less toxic products. Microb. Cell Fact. 2015, 14, 149. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, D.J.; Stratford, M.; Gasson, M.J.; Ueckert, J.; Bos, A.; Narbad, A. Mode of antimicrobial of vanillin against Escherichia coli, Lactobacillus plantarum and Listeria innocua. J. Appl. Microbiol. 2004, 97, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, M.J.; Gustafsson, L.; Niklasson, C.; Lidén, G. Conversion of furfural in aerobic and anaerobic batch fermentation of glucose by Saccharomyces cerevisiae. J. Biosci. Bioeng. 1999, 87, 169–174. [Google Scholar] [CrossRef]
- Roberto, I.C.; Lacis, L.S.; Barbosa, M.F.S.; de Mancilha, I.M. Utilization of sugar cane bagasse hemicellulosic hydrolysate by pichia stipitis for the production of ethanol. Process Biochem. 1991, 26, 15–21. [Google Scholar] [CrossRef]
- Nigam, J.N. Ethanol production from wheat straw hemicellulose hydrolysate by Pichia stipitis. J. Biotechnol. 2001, 87, 17–27. [Google Scholar] [CrossRef]
- Liu, Z.L. Molecular mechanisms of yeast tolerance and in situ detoxification of lignocellulose hydrolysates. Appl. Microbiol. Biotechnol. 2011, 90, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Zaldivar, J.; Martinez, A.; Ingram, L.O. Effect of Selected Aldehydes on the Growth and Fermentation of Ethanologeic Escherichia coli. Biotechnol. Bioeng. 1999, 65, 24–33. [Google Scholar] [CrossRef]
- Hadi, S.M.; Rehman, A. Specificity of the interaction of furfural with DNA. Mutat. Res. Lett. 1989, 225, 101–106. [Google Scholar] [CrossRef]
- Guo, G.L.; Chen, W.H.; Chen, W.H.; Men, L.C.; Hwang, W.S. Characterization of dilute acid pretreatment of silvergrass for ethanol production. Bioresour. Technol. 2008, 99, 6046–6053. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.A.; Clark, W.; McCaffery, J.M.; Cai, Z.; Lanctot, A.; Slininger, P.J.; Liu, Z.L.; Gorsich, S.W. Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnol. Biofuels 2010, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Blaschek, H.P. Biomass Conversion Inhibitors and In Situ Detoxification. In Biomass to Biofuels: Strategies for Global Industries; John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 233–259. [Google Scholar]
- Taherzadeh, M.J.; Karimi, K. Acid-Based Hydrolysis Processes For Ethanol From Lignocellulosic Materials: A Review. Bioresources 2015, 2, 472–499. [Google Scholar] [CrossRef]
- Pampulha, M.E.; Loureiro-Dias, M.C. Combined effect of acetic acid, pH and ethanol on intracellular pH of fermenting yeast. Appl. Microbiol. Biotechnol. 1989, 31, 547–550. [Google Scholar] [CrossRef]
- Verduyn, C.; Postma, E.; Scheffers, W.A.; van Duken, J.P. Energetics of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. Biotechnol. Bioeng. 1990, 136, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.A.; Tucker, M.P.; Keller, F.A.; Beaty, D.A.; Connors, K.M.; Eddy, F.P. Dilute acid hydrolysis of softwoods. Appl. Biochem. Biotechnol. 1999, 77, 133–142. [Google Scholar] [CrossRef]
- Axe, D.D.; Bailey, J.E. Transport of lactate and acetate through the energized cytoplasmic membrane of Escherichia coli. Biotechnol. Bioeng. 1995, 47, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.S.; Ladisch, M.R.; Tsao, G.T. Cellobiase from Trichoderma viride: Purification, Properties, Kinetics, and Mechanism. Biotechnol. Bioleng. 1977, 19, 959–981. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Ladisch, M.R.; Gong, C.-S.; Wankat, P.C.; Tsao, G.T. Combined product and substrate inhibition equation for cellobiase. Biotechnol. Bioeng. 1981, 23, 2779–2788. [Google Scholar] [CrossRef]
- Gusakov, A.V.; Sinitsyn, A.P. A Theoretical Analysis of Cellulase Product Inhibition: Effect of Cellulase Binding Constant, Enzyme/Substrate Ratio, Inhibition Pattern T1. Biotechnology 1992, 40, 663–671. [Google Scholar]
- Holtzapple, M.; Cognata, M.; Shu, Y.; Hendrickson, C. Inhibition of Trichoderma reesei cellulase by sugars and solvents. Biotechnol. Bioeng. 1990, 36, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Philippidis, G.P.; Smith, T.K.; Wyman, C.E. Study of the enzymatic hydrolysis of cellulose for production of fuel ethanol by the simultaneous saccharification and fermentation process. Biotechnol. Bioeng. 1993, 41, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Ladisch, M.R.; Gong, C.S.; Tsao, G.T. Corn Crop Residues as a Potential Source of Single Cell Protein Kinetics of Trichoderma Viride Cellobiase Action; Developments in Industrial Microbilogy; National Agricultural Library: Beltsville, MD, USA, 1997; 18, pp. 157–158.
- Qing, Q.; Yang, B.; Wyman, C.E. Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresour. Technol. 2010, 101, 9624–9630. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in Dietary Polyphenols as α-Glucosidases Inhibitors: A Review on Structure-Activity Relationship Aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef] [PubMed]
- Qing, Q.; Wyman, C.E. Supplementation with xylanase and β-xylosidase to reduce xylo-oligomer and xylan inhibition of enzymatic hydrolysis of cellulose and pretreated corn stover. Biotechnol. Biofuels 2011, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Jung, S.; Ragauskas, A. Pseudo-lignin formation and its impact on enzymatic hydrolysis. Bioresour. Technol. 2012, 117, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Studer, M.H.; DeMartini, J.D.; Davis, M.F.; Sykes, R.W.; Davison, B.; Keller, M.; Tuskan, G.A.; Wyman, C.E. Lignin content in natural Populus variants affects sugar release. Proc. Natl. Acad. Sci. USA 2011, 108, 6300–6305. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.; Østergaard Haven, M.; Thirup, L. Inbicon makes lignocellulosic ethanol a commercial reality. Biomass Bioenergy 2012, 46, 36–45. [Google Scholar] [CrossRef]
- Li, X.; Ximenes, E.; Kim, Y.; Slininger, M.; Meilan, R.; Ladisch, M.; Chapple, C. Lignin monomer composition affects Arabidopsis cell-wall degradability after liquid hot water pretreatment. Biotechnol. Biofuels 2010, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, P.N.; Resch, M.G.; Hewetson, B.; Killgore, J.P.; Curtin, A.; Anderson, N.; Chiaramonti, A.N.; Hurley, D.C.; Sanders, A.; Himmel, M.E.; et al. Engineering plant cell walls: Tuning lignin monomer composition for deconstructable biofuel feedstocks or resilient biomaterials. Green Chem. 2014, 16, 2627. [Google Scholar] [CrossRef]
- Alriksson, B.; Cavka, A.; Jönsson, L.J. Improving the fermentability of enzymatic hydrolysates of lignocellulose through chemical in-situ detoxification with reducing agents. Bioresour. Technol. 2011, 102, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Alriksson, B.; Sjöde, A.; Nilvebrant, N.-O.; Jönsson, L.J. Optimal Conditions for Alkaline Detoxification of Dilute-Acid Lignocellulose Hydrolysates. Appl. Biochem. Biotechnol. 2006, 130, 599–611. [Google Scholar] [CrossRef]
- Cannella, D.; Sveding, P.V.; Jørgensen, H. PEI detoxification of pretreated spruce for high solids ethanol fermentation. Appl. Energy 2014, 132, 394–403. [Google Scholar] [CrossRef]
- Nichols, N.N.; Dien, B.S.; Cotta, M.A. Fermentation of bioenergy crops into ethanol using biological abatement for removal of inhibitors. Bioresour. Technol. 2010, 101, 7545–7550. [Google Scholar] [CrossRef] [PubMed]
- Nichols, N.N.; Dien, B.S.; Guisado, G.M.; López, M.J. Bioabatement to remove inhibitors from biomass-derived sugar hydrolysates. Appl. Biochem. Biotechnol. 2005, 121–124, 379–390. [Google Scholar] [CrossRef]
- Nichols, N.N.; Sharma, L.N.; Mowery, R.A.; Chambliss, C.K.; van Walsum, G.P.; Dien, B.S.; Iten, L.B. Fungal metabolism of fermentation inhibitors present in corn stover dilute acid hydrolysate. Enzym. Microb. Technol. 2008, 42, 624–630. [Google Scholar] [CrossRef]
- Koppram, R.; Albers, E.; Olsson, L. Evolutionary engineering strategies to enhance tolerance of xylose utilizing recombinant yeast to inhibitors derived from spruce biomass. Biotechnol. Biofuels 2012, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Almario, M.P.; Reyes, L.H.; Kao, K.C. Evolutionary engineering of Saccharomyces cerevisiae for enhanced tolerance to hydrolysates of lignocellulosic biomass. Biotechnol. Bioeng. 2013, 110, 2616–2623. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Cassland, P.; Jönsson, L.J. Development of a Saccharomyces cerevisiae Strain with Enhanced Resistance to Phenolic Fermentation Inhibitors in Lignocellulose Hydrolysates by Heterologous Expression of Laccase. Appl. Environ. Microbiol. 2001, 67, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yomano, L.P.; Lee, J.Y.; York, S.W.; Zheng, H.; Mullinnix, M.T.; Shanmugam, K.T.; Ingram, L.O. Engineering furfural tolerance in Escherichia coli improves the fermentation of lignocellulosic sugars into renewable chemicals. Proc. Natl. Acad. Sci. USA 2013, 110, 4021–4026. [Google Scholar] [CrossRef] [PubMed]
- Sanda, T.; Hasunuma, T.; Matsuda, F.; Kondo, A. Repeated-batch fermentation of lignocellulosic hydrolysate to ethanol using a hybrid Saccharomyces cerevisiae strain metabolically engineered for tolerance to acetic and formic acids. Bioresour. Technol. 2011, 102, 7917–7924. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Mielenz, J.R.; Xiao, X.; Ge, Y.; Hamilton, C.Y.; Rodriguez, M.; Chen, F.; Foston, M.; Ragauskas, A.; Bouton, J.; et al. Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proc. Natl. Acad. Sci. USA 2011, 108, 3803–3808. [Google Scholar] [CrossRef] [PubMed]
- Pienkos, P.T.; Zhang, M. Role of pretreatment and conditioning processes on toxicity of lignocellulosic biomass hydrolysates. Cellulose 2009, 16, 743–762. [Google Scholar] [CrossRef]
- Larsson, S.; Reimann, A.; Nilvebrant, N.-O.; Jönsson, L.J. Comparison of Different Methods for the Detoxification of Lignocellulose Hydrolyzates of Spruce. Appl. Biochem. Biotechnol. 1999, 77, 91–104. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Ladisch, M.R.; Engelberth, A.S. Acetic acid removal from corn stover hydrolysate using ethyl acetate and the impact on Saccharomyces cerevisiae bioethanol fermentation. Biotechnol. Prog. 2016, 32, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, T.; Börjesson, J.; Tjerneld, F. Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzym. Microb. Technol. 2002, 31, 353–364. [Google Scholar] [CrossRef]
- Kumar, L.; Arantes, V.; Chandra, R.; Saddler, J. The lignin present in steam pretreated softwood binds enzymes and limits cellulose accessibility. Bioresour. Technol. 2012, 103, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Sipos, B.; Dienes, D.; Schleicher, Á.; Perazzini, R.; Crestini, C.; Siika-aho, M.; Réczey, K. Hydrolysis efficiency and enzyme adsorption on steam-pretreated spruce in the presence of poly(ethylene glycol). Enzym. Microb. Technol. 2010, 47, 84–90. [Google Scholar] [CrossRef]
- Silva, A.; Sampaio-Marques, B.; Fernandes, Â.; Carreto, L.; Rodrigues, F.; Holcik, M.; Santos, M.A.S.; Ludovico, P. Involvement of Yeast HSP90 Isoforms in Response to Stress and Cell Death Induced by Acetic Acid. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duque, S.H.; Cardona, C.A.; Moncada, J. Techno-economic and environmental analysis of ethanol production from 10 agroindustrial residues in Colombia. Energy Fuels 2015, 29, 775–783. [Google Scholar] [CrossRef]
- Nichols, N.N.; Hector, R.E.; Saha, B.C.; Frazer, S.E.; Kennedy, G.J. Biological abatement of inhibitors in rice hull hydrolyzate and fermentation to ethanol using conventional and engineered microbes. Biomass Bioenergy 2014, 67, 79–88. [Google Scholar] [CrossRef]
- López, M.J.; Moreno, J.; Nichols, N.N.; Dien, B.S.; Bothast, R.J. Isolation of microorganisms for biological detoxification of lignocellulosic hydrolysates. Appl. Microbiol. Biotechnol. 2004, 64, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, D.J.; Hector, R.E.; Riley, R.; Lipzen, A.; Kuo, R.C.; Amirebrahimi, M.; Barry, K.W.; Grigoriev, I.V.; Van Elsas, J.D.; Nichols, N. Draft Genome Sequence of Coniochaeta ligniaria NRRL 30616, a Lignocellulolytic Fungus for Bioabatement of Inhibitors in Plant Biomass Hydrolysates. Genome Announc. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Dien, B.S.; Cotta, M.A. Functional expression of bacterial Zymobacter palmae pyruvate decarboxylase gene in Lactococcus lactis. Curr. Microbiol. 2005, 50, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.A.; Keller, F.A.; Tucker, M.P.; Lombard, C.K.; Jenkins, B.M.; Yomogida, D.E.; Tiangco, V.M. Bioconversion of mixed solids waste to ethanol. Appl. Biochem. Biotechnol. 1999, 77–79, 455–471. [Google Scholar] [CrossRef]
- Favaro, L.; Basaglia, M.; Trento, A.; Van Rensburg, E.; García-Aparicio, M.; Van Zyl, W.H.; Casella, S. Exploring grape marc as trove for new thermotolerant and inhibitor-tolerant Saccharomyces cerevisiae strains for second-generation bioethanol production. Biotechnol. Biofuels 2013, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hasunuma, T.; Ismail, K.S.K.; Nambu, Y.; Kondo, A. Co-expression of TAL1 and ADH1 in recombinant xylose-fermenting Saccharomyces cerevisiae improves ethanol production from lignocellulosic hydrolysates in the presence of furfural. J. Biosci. Bioeng. 2014, 117, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Nevoigt, E. Progress in Metabolic Engineering of Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2008, 72, 379–412. [Google Scholar] [CrossRef] [PubMed]
- Wuddineh, W.A.; Mazarei, M.; Zhang, J.-Y.; Turner, G.B.; Sykes, R.W.; Decker, S.R.; Davis, M.F.; Udvardi, M.K.; Stewart, C.N. Identification and Overexpression of a Knotted1-Like Transcription Factor in Switchgrass (Panicum virgatum L.) for Lignocellulosic Feedstock Improvement. Front. Plant Sci. 2016, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Voorend, W.; Nelissen, H.; Vanholme, R.; De Vliegher, A.; Van Breusegem, F.; Boerjan, W.; Roldán-Ruiz, I.; Muylle, H.; Inzé, D. Overexpression of GA20-OXIDASE1 impacts plant height, biomass allocation and saccharification efficiency in maize. Plant Biotechnol. J. 2016, 14, 997–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Wyman, E.C. Lignin Blockers and Uses Thereof. U.S. Patant US8580541 B2, 13 November 2013. [Google Scholar]
- Han, Y.J.; Chen, H.Z. Synergism between hydrophobic proteins of corn stover and cellulase in lignocellulose hydrolysis. Biochem. Eng. J. 2010, 48, 218–224. [Google Scholar] [CrossRef]
| Biomass | Cellulose | Hemicellulose | Lignin | Reference |
|---|---|---|---|---|
| Bagasse | 39.0 | 24.4 | 24.8 | [14,15] |
| Barley hull | 33.6 | 37.2 | 19.3 | [16] |
| Corn fiber | 14.3 | 16.8 | 8.4 | [17] |
| Corn pericarp | 22.5 | 23.7 | 4.7 | [18] |
| Corn stover | 37.0 | 22.7 | 18.6 | [19] |
| Wheat straw | 30.2 | 21.0 | 17 | [20] |
| Red maple | 41.0 | 15.0 | 29.1 | [21] |
| Rice straw | 31.1 | 22.3 | 13.3 | [22] |
| Rye straw | 30.9 | 21.5 | 22.1 | [23] |
| Switchgrass | 39.5 | 20.3 | 17.8 | [24] |
| Sugarcane bagasse | 43.1 | 31.1 | 11.4 | [25] |
| Sweet sorghum bagasse | 27.3 | 13.1 | 14.3 | [26] |
| Olive tree pruning | 25.0 | 11.1 | 16.2 | [27] |
| Poplar | 43.8 | 14.8 | 29.1 | [28] |
| Pinewood | 40.0 | 28.5 | 27.7 | [29] |
| Spruce | 43.8 | 6.3 | 28.3 | [30] |
| Biomass Property | Effects on Pretreatment and Enzymatic Hydrolysis | Reference |
|---|---|---|
| Cellulose crystallinity | The intramolecular and intermolecular chemical linkages such as hydrogen bonding in the linear cellulose chains increase the feedstock recalcitrance, enzyme loading, and pretreatment severe condition. The high cellulose crystallinity contributes to the feedstock recalcitrance, and subsequently decreases the cellulose conversion. | [37,38,39,40] |
| Degree of polymerization (DP) | Cellulose DP is normally in the range of 800–10,000 (up to 17,000). Since the high DP structure has less reducing sugar ends that could affect feedstock disobedience and enzyme catalyst, the reduction of DP is required for effective cellulose conversion | [41,42,43,44,45] |
| Lignin | Lignin plays a key role in the lignocellulosic materials as a biological glue and secondary cell wall. Both lignin and its roles have negative effects on pretreatment, enzyme usage, cellulose conversion, and total costs. Delignification and/or reduction of lignin content using pretreatments, genetic/system engineering, and feedstock selection/modification are required to improve the final conversion yield and productivity. | [33,34,36,46,47,48,49,50,51,52,53] |
| Hemicellulose | Xyan, the most plentiful hemicellulose in plants, forms a coating layer with cellulose by hydrogen bonding and covalently links with lignin to protect the plant cells. Primary role of the pretreatment is to solubilize the hemicellulose components, and it could improve the cellulose digestibility and hydrolysis. | [54,55,56,57] |
| Method | Feedstock (Solid Concen.) | Pretreatment Conditions | Soluble Inhibitors in Pre-Hydrolysate (g/L) | Ref. | |||
|---|---|---|---|---|---|---|---|
| Phenols | Furans | Acetic Acid | Others | ||||
| Steam explosion | Olive tree pruning (20%) | Temp. 190–240 °C, residence time 5 min, sulfuric acid 0–2% | nm 1 | 0–3.2 | 0.4–4.2 | Formic acid, 0.8–1.8 | [27] |
| Steam explosion | Wheat straw (30%) | Temp. 190–210 °C, residence time 2–10 min, sulfuric acid 0.2% | nm | 0.16–2.14 | 0.04–1.01 | nm | [63] |
| Steam explosion | Wood chip(38–41%) | Temp. 180–210 °C, residence time 4–12 min, sulfuric acid 0.25–0.5% | nm | 0.5–3.2 | up to 7.5 | nm | [61] |
| LHW | Maple (23%) | Temp. 180–200 °C, residence time 24 min | 1.3 | 4.1 | 13.1 | Sugar oligomer 12.7, xylo-oligomers 11.2 | [86] |
| LHW | Hardwood (15%) | Temp. 195 °C, residence time 10 min | 5.9 | 0.7 | 2.5 | Gluco-oligomers 3.4, xylo-oligomers 56, formic acid 1.9, bound acetyl 12.9 | [87] |
| LHW | Sugarcane bagasse (10%) | Temp. 180–200 °C, residence time 30 min | 1.4–2.4 | 0.5–5.1 | 1.1–3.4 | Gluco-oligomers 0.8, xylo-oligomers 6.5–12.5 | [95] |
| LHW | Corn stover(10–20%) | Temp. 190 °C, residence time 45 min | 181–246 AU 2 | 0.74–8.37 | 2.0–2.8 | Xylo-oligomers 9.71–21.7 | [19,84] |
| AFEX | Poplar | Temp. 180 °C, 233% moisture ammonia 1:1, 2:1, and 3:1 w/w biomass | 2.1 mg/g solids | 8.6 µg/g solids | nm | Aliphatic acid 1.8 µg/g solids | [97] |
| Strategy | Main Effect | Considerations | Reference |
|---|---|---|---|
| Biomass selection and modification | Screen adequate feedstock and/or engineering which produce less undesirable compounds | A range of suitable agricultural residues, requiring time for selection and engineering | [127,128,129,130] |
| Detoxification/conditioning | Chemical supplementation, i.e., alkaline, BSA, polymers | Chemical needs, additional process may be required | [33,34,131,132,133] |
| Biological detoxification | Use microbes | Time consuming, loss of sugars | [19,84,85,134,135,136] |
| Adaptation of microbes | Adaptive evolution of specific microbe in the inhibitory environment | May not be applied to other feedstock, pretreatment conditions | [137,138] |
| Genetic/metabolic engineering | Use genetically modified microbes to lignocellulosic hydrolysates | Require following the genetically modified micro-organisms (GMM) process | [139,140,141] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309. https://doi.org/10.3390/molecules23020309
Kim D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules. 2018; 23(2):309. https://doi.org/10.3390/molecules23020309
Chicago/Turabian StyleKim, Daehwan. 2018. "Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review" Molecules 23, no. 2: 309. https://doi.org/10.3390/molecules23020309





