Exploring Alternative Radiolabeling Strategies for Sialic Acid-Binding Immunoglobulin-Like Lectin 9 Peptide: [68Ga]Ga- and [18F]AlF-NOTA-Siglec-9
Abstract
:1. Introduction
2. Results and Discussion
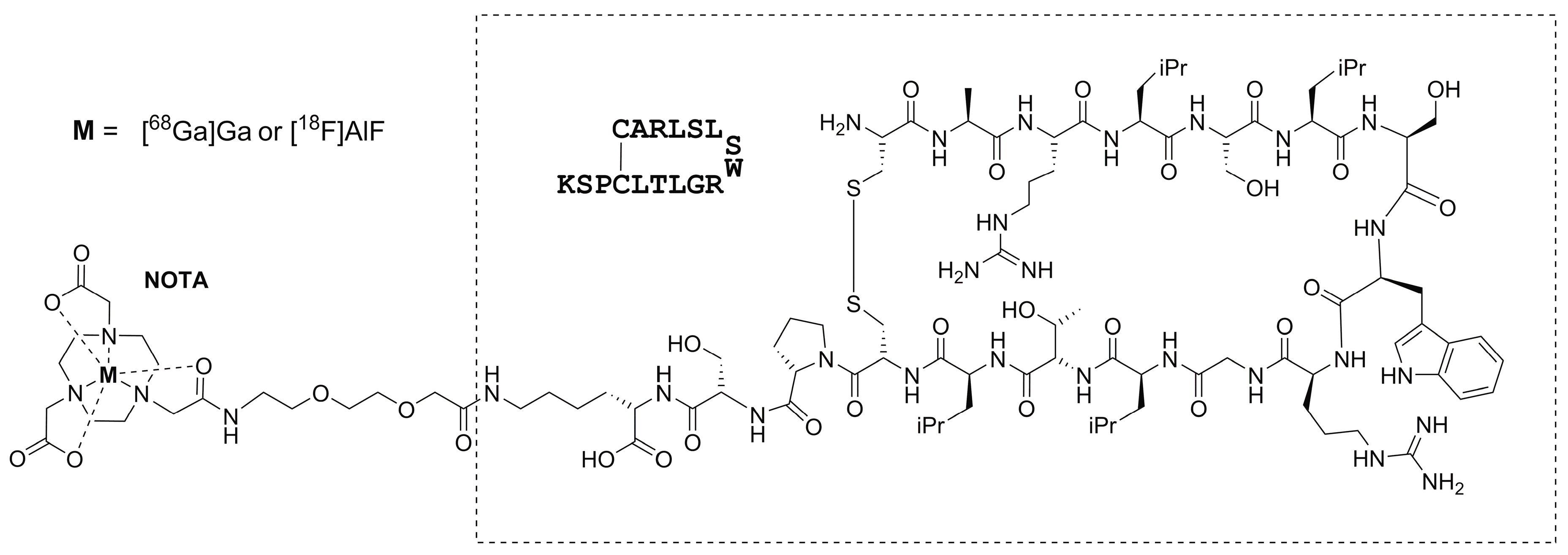
2.1. Radiosynthesis
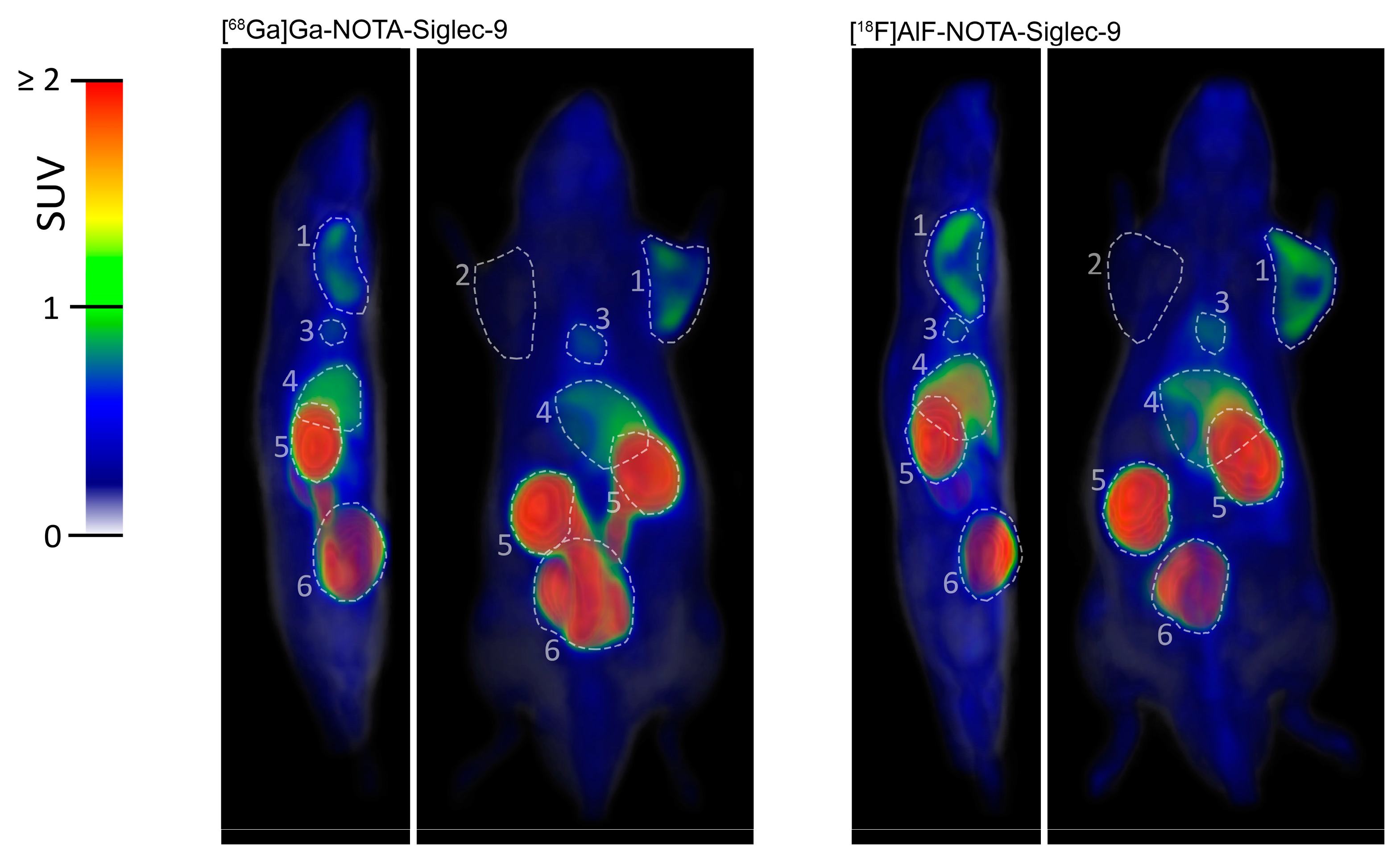
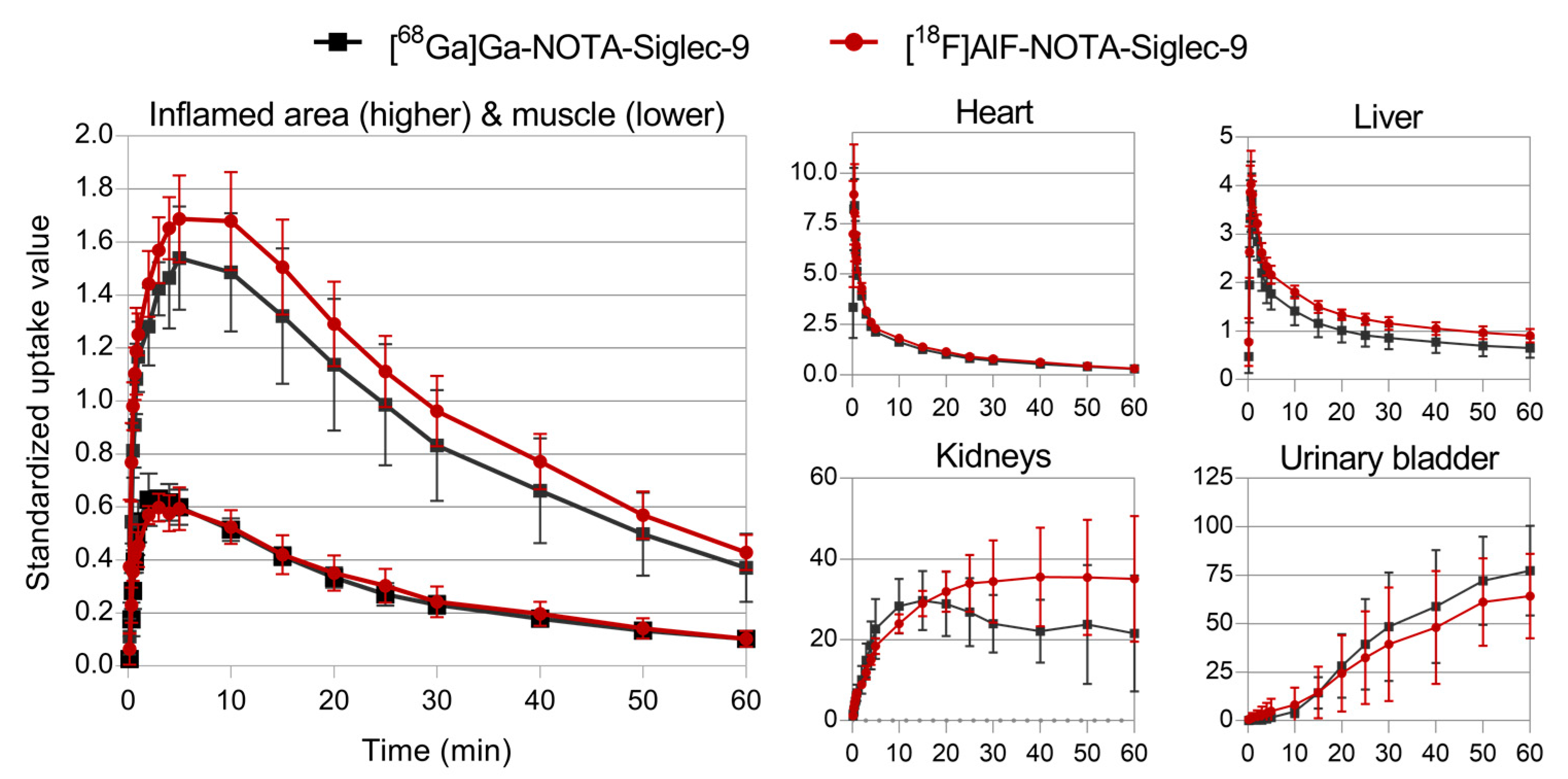
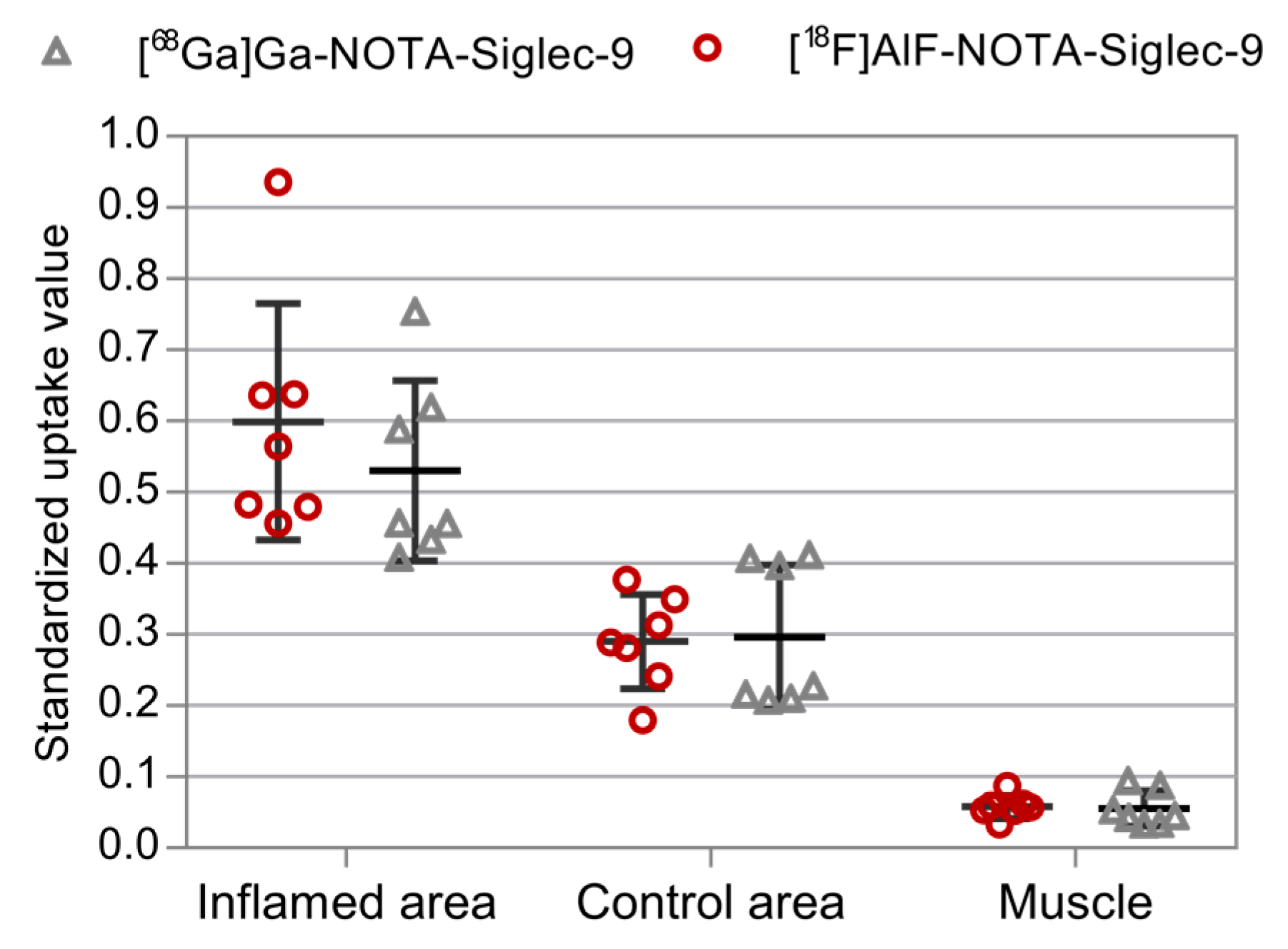
2.2. PET Imaging
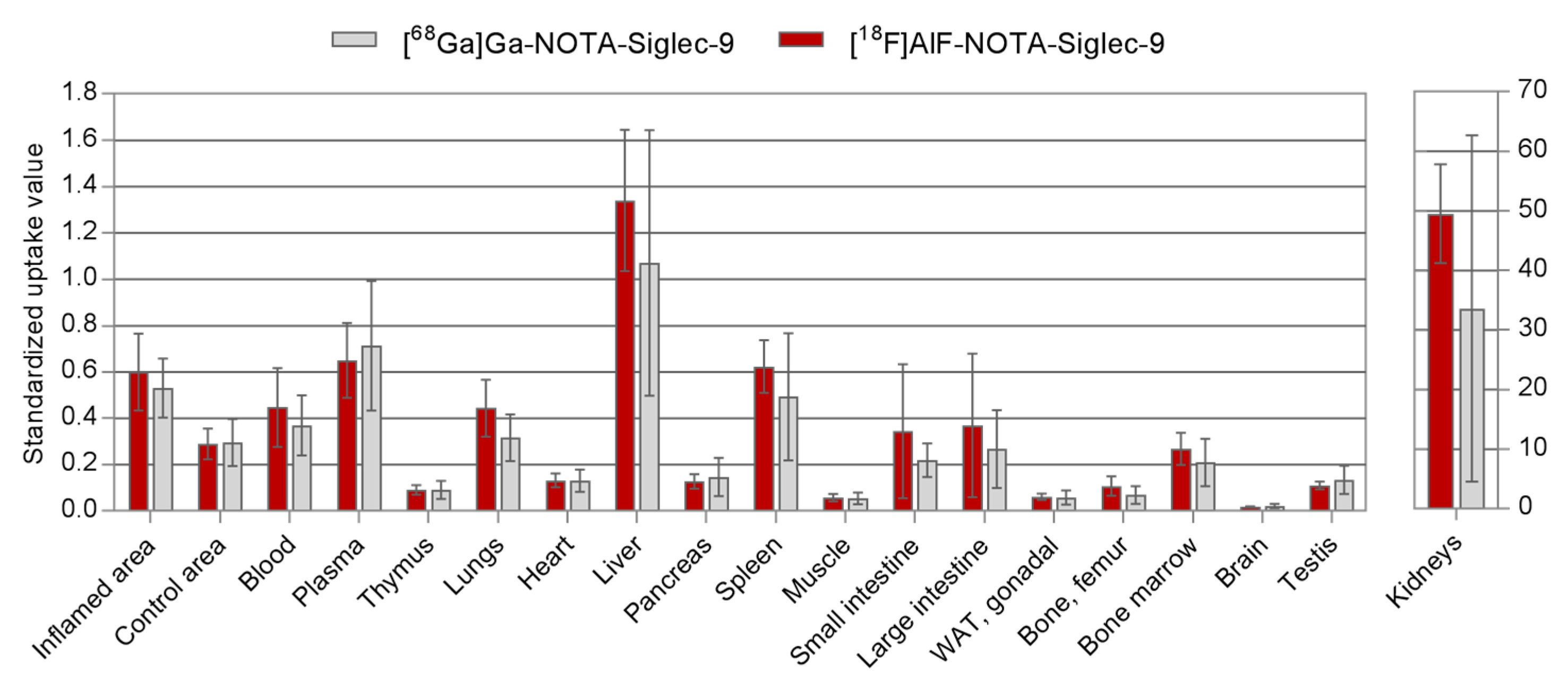
2.3. Ex Vivo Radioactivity Distribution
3. Materials and Methods
3.1. [68Ga]Ga-NOTA-Siglec-9 Radiosynthesis
3.2. [18F]AlF-NOTA-Siglec-9 Radiosynthesis
3.3. Distribution Coefficient (Log D)
3.4. PET Imaging of Sterile Skin/Muscle Inflammation
3.5. Plasma Protein Binding Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hammoud, D.A. Molecular imaging of inflammation: Current status. J. Nucl. Med. 2016, 57, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Roivainen, A.; Jalkanen, S.; Nanni, C. Gallium-labelled peptides for imaging of inflammation. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, S.; Patel, C.N.; Scarsbrook, A.F.; Chowdhury, F.U. FDG PET/CT in infection and inflammation—Current and emerging clinical applications. Clin. Radiol. 2015, 70, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.; Millon, A.; Fayad, Z.A. Molecular imaging in atherosclerosis: FDG PET. Curr. Atheroscler. Rep. 2012, 14, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Tarkin, J.M.; Joshi, F.R.; Evans, N.R.; Chowdhury, M.M.; Figg, N.L.; Shah, A.V.; Starks, L.T.; Martin-Garrido, A.; Manavaki, R.; Yu, E.; et al. Detection of atherosclerotic inflammation by 68Ga-DOTATATE PET compared to [18F]FDG PET imaging. J. Am. Coll. Cardiol. 2017, 69, 1774–1791. [Google Scholar] [CrossRef] [PubMed]
- Lapa, C.; Reiter, T.; Li, X.; Werner, R.A.; Samnick, S.; Jahns, R.; Buck, A.K.; Ertl, G.; Bauer, W.R. Imaging of myocardial inflammation with somatostatin receptor based PET/CT—A comparison to cardiac MRI. Int. J. Cardiol. 2015, 194, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Rinne, P.; Hellberg, S.; Kiugel, M.; Virta, J.; Li, X.-G.; Käkelä, M.; Helariutta, K.; Luoto, P.; Liljenbäck, H.; Hakovirta, H.; et al. Comparison of somatostatin receptor 2-targeting PET tracers in the detection of mouse atherosclerotic plaques. Mol. Imaging Biol. 2016, 18, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Crawshaw, A.A.; Robertson, N.P. The role of TSPO PET in assessing neuroinflammation. J. Neurol. 2017, 264, 1825–1827. [Google Scholar] [CrossRef] [PubMed]
- Malviya, G.; Anzola, K.L.; Podestà, E.; Laganà, B.; Del Mastro, C.; Dierckx, R.A.; Scopinaro, F.; Signore, A. 99mTc-labeled rituximab for imaging B lymphocyte infiltration in inflammatory autoimmune disease patients. Mol. Imaging Biol. 2012, 14, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Hyafil, F.; Pelisek, J.; Laitinen, I.; Schottelius, M.; Mohring, M.; Döring, Y.; van der Vorst, E.P.C.; Kallmayer, M.; Steiger, K.; Poschenrieder, A.; et al. Imaging the cytokine receptor CXCR4 in atherosclerotic plaques with the radiotracer 68Ga-pentixafor for PET. J. Nucl. Med. 2017, 58, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Bouter, C.; Meller, B.; Sahlmann, C.O.; Staab, W.; Wester, H.J.; Kropf, S.; Meller, J. Imaging chemokine receptor CXCR4 in chronic infection of the bone with 68Ga-Pentixafor-PET/CT—First insights. J. Nucl. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, J.T.; Derlin, T.; Haghikia, A.; Napp, L.C.; Wang, Y.; Ross, T.L.; Schäfer, A.; Tillmanns, J.; Wester, H.J.; Wollert, K.C.; et al. Molecular imaging of the chemokine receptor CXCR4 after acute myocardial infarction. J. Am. Coll. Cardiol. Imging 2015, 8, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Tohka, S.; Laukkanen, M.; Jalkanen, S.; Salmi, M. Vascular adhesion protein 1 (VAP-1) functions as a molecular brake during granulocyte rolling and mediates recruitment in vivo. FASEB J. 2001, 15, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Noonan, T.; Lukas, S.; Peet, G.W.; Pelletier, J.; Panzenbeck, M.; Hanidu, A.; Mazurek, S.; Wasti, R.; Rybina, I.; Roma, T.; et al. The oxidase activity of vascular adhesion protein-1 (VAP-1) is essential for function. Am. J. Clin. Exp. Immunol. 2013, 2, 172–185. [Google Scholar] [PubMed]
- Aalto, K.; Autio, A.; Kiss, E.A.; Elima, K.; Nymalm, Y.; Veres, T.Z.; Marttila-Ichihara, F.; Elovaara, H.; Saanijoki, T.; Crocker, P.R.; et al. Siglec-9 is a novel leukocyte ligand for vascular adhesion protein-1 and can be used in PET imaging of inflammation and cancer. Blood 2011, 118, 3725–3733. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Käkelä, M.; Jødal, L.; Moisio, O.; Alstrup, A.K.O.; Jalkanen, S.; Roivainen, A. Exploring the radiosynthesis and the in vitro characteristics of [68Ga]Ga-DOTA-Siglec-9. J. Label. Compd. Radiopharm. 2017, 60, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, H.; Autio, A.; Siitonen, R.; Liljenbäck, H.; Saanijoki, T.; Lankinen, P.; Mäkilä, J.; Käkelä, M.; Teuho, J.; Savisto, N.; et al. 68Ga-DOTA-Siglec-9—A new imaging tool to detect synovitis. Arthritis Res. Ther. 2015, 17, 308. [Google Scholar] [CrossRef] [PubMed]
- Silvola, J.M.U.; Virtanen, H.; Siitonen, R.; Hellberg, S.; Liljenbäck, H.; Metsälä, O.; Ståhle, M.; Saanijoki, T.; Käkelä, M.; Hakovirta, H.; et al. Leukocyte trafficking-associated vascular adhesion protein 1 is expressed and functionally active in atherosclerotic plaques. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ahtinen, H.; Kulkova, J.; Lindholm, L.; Eerola, E.; Hakanen, A.J.; Moritz, N.; Söderström, M.; Saanijoki, T.; Jalkanen, S.; Roivainen, A.; et al. 68Ga-DOTA-Siglec-9 PET/CT imaging of peri-implant tissue responses and staphylococcal infections. EJNMMI Res. 2014, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Retamal, J.; Sörensen, J.; Lubberink, M.; Suarez-Sipmann, F.; Borges, J.B.; Feinstein, R.; Jalkanen, S.; Antoni, G.; Hedenstierna, G.; Roivainen, A.; et al. Feasibility of 68Ga-labeled Siglec-9 peptide for the imaging of acute lung inflammation: A pilot study in a porcine model of acute respiratory distress syndrome. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 18–31. [Google Scholar] [PubMed]
- Sanchez-Crespo, A. Comparison of Gallium-68 and Fluorine-18 imaging characteristics in positron emission tomography. Appl. Radiat. Isot. 2013, 76, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-G.; Autio, A.; Ahtinen, H.; Helariutta, K.; Liljenbäck, H.; Jalkanen, S.; Roivainen, A.; Airaksinen, A.J. Translating the concept of peptide labeling with 5-deoxy-5-[18F]fluororibose into preclinical practice: 18F-labeling of Siglec-9 peptide for PET imaging of inflammation. Chem. Commun. 2013, 49, 3682–3684. [Google Scholar] [CrossRef] [PubMed]
- Siitonen, R.; Pietikäinen, A.; Liljenbäck, H.; Käkelä, M.; Söderström, M.; Jalkanen, S.; Hytönen, J.; Roivainen, A. Targeting of vascular adhesion protein-1 by positron emission tomography visualizes sites of inflammation in Borrelia burgdorferi-infected mice. Arthritis Res. Ther. 2017, 19, 254. [Google Scholar] [CrossRef] [PubMed]
- McBride, W.J.; Sharkey, R.M.; Goldenberg, D.M. Radiofluorination using aluminum-fluoride (Al18F). EJNMMI Res. 2013, 3, 36. [Google Scholar] [CrossRef] [PubMed]
| [68Ga]Ga-NOTA-Siglec-9 | [18F]AlF-NOTA-Siglec-9 | |
|---|---|---|
| Yield (%, n = 3) | 53 ± 2 AY 1; 64 ± 1 RCY 2 | 26 ± 3 AY 1; 39 ± 1 RCY 2 |
| Radiochemical purity (%, n = 3) | 98.5 ± 0.2 | 98.2 ± 1.4 |
| Total synthesis time (minutes) | 30 | 60 |
| Molar activity (MBq/nmol, n = 3) | 5.0 ± 0.2 | 8.8 ± 0.9 |
| Log D (n = 3) | −3.2 ± 0.3 | −2.3 ± 0.1 |
| Plasma protein binding (%, n = 5) 3 | 31.4 ± 3.4 | 37.4 ± 0.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moisio, O.; Siitonen, R.; Liljenbäck, H.; Suomela, E.; Jalkanen, S.; Li, X.-G.; Roivainen, A. Exploring Alternative Radiolabeling Strategies for Sialic Acid-Binding Immunoglobulin-Like Lectin 9 Peptide: [68Ga]Ga- and [18F]AlF-NOTA-Siglec-9. Molecules 2018, 23, 305. https://doi.org/10.3390/molecules23020305
Moisio O, Siitonen R, Liljenbäck H, Suomela E, Jalkanen S, Li X-G, Roivainen A. Exploring Alternative Radiolabeling Strategies for Sialic Acid-Binding Immunoglobulin-Like Lectin 9 Peptide: [68Ga]Ga- and [18F]AlF-NOTA-Siglec-9. Molecules. 2018; 23(2):305. https://doi.org/10.3390/molecules23020305
Chicago/Turabian StyleMoisio, Olli, Riikka Siitonen, Heidi Liljenbäck, Elli Suomela, Sirpa Jalkanen, Xiang-Guo Li, and Anne Roivainen. 2018. "Exploring Alternative Radiolabeling Strategies for Sialic Acid-Binding Immunoglobulin-Like Lectin 9 Peptide: [68Ga]Ga- and [18F]AlF-NOTA-Siglec-9" Molecules 23, no. 2: 305. https://doi.org/10.3390/molecules23020305






