Recent Theoretical and Experimental Progress in Circularly Polarized Luminescence of Small Organic Molecules
Abstract
:1. Introduction
2. Progress on Theoretical Calculations
3. Progress on Experimental Researches
3.1. Improvement on the CPL Measurement Instrument
3.2. Improvement on the CPL Experimental Results
3.2.1. Central Chirality
3.2.2. Axial Chirality
3.2.3. Planar Chirality
3.2.4. Helical Chirality
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Bradshaw, D.S.; Leeder, J.M.; Coles, M.M.; Andrews, D.L. Signatures of material and optical chirality: Origins and measures. Chem. Phys. Lett. 2015, 626, 106–110. [Google Scholar] [CrossRef]
- Yan, B.; Gao, F.; Ma, H.F.; Zhong, K.S.; Lv, B.; Chen, N.B.; Cai, P.G.; Ye, Z.R.; Li, Y.; Sui, C.H.; et al. Chirality-dependent electromagnetically induced transparency based on a double semi-periodic helix metastructure. Optics Lett. 2018, 43, 3722–3725. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.P.; Cao, C.H.; Zheng, Y.G. Enzymatic asymmetric synthesis of chiral amino acids. Chem. Soc. Rev. 2018, 47, 1516–1561. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Xu, L.G.; de Moura, A.F.; Wu, X.L.; Kuang, H.; Xu, C.L.; Kotov, N.A. Chiral inorganic nanostructures. Chem. Rev. 2017, 117, 8041–8093. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, M.; Schäferling, M.; Duan, X.Y.; Giessen, H.; Liu, N. Chiral plasmonics. Sci. Adv. 2017, 3, e1602735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanganyado, E.; Lu, Z.J.; Fu, Q.G.; Schlenk, D.; Gan, J. Chiral pharmaceuticals: A review on their environmental occurrence and fate processes. Water Res. 2017, 124, 527–542. [Google Scholar] [CrossRef]
- Schadt, M. Liquid crystal materials and liquid crystal displays. Annu. Rev. Mater. Sci. 1997, 27, 305–379. [Google Scholar] [CrossRef]
- Sherson, J.F.; Krauter, H.; Olsson, R.K.; Julsgaard, B.; Hammerer, K.; Cirac, I.; Polzik, E.S. Quantum teleportation between light and matter. Nature 2006, 443, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Wagenknecht, C.; Li, C.M.; Reingruber, A.; Bao, X.H.; Goebel, A.; Chen, Y.A.; Zhang, Q.; Chen, K.; Pan, J.W. Experimental demonstration of a heralded entanglement source. Nat. Photon. 2010, 4, 549–552. [Google Scholar] [CrossRef] [Green Version]
- Feringa, B.L. In control of motion: From molecular switches to molecular motors. Acc. Chem. Res. 2001, 34, 504–513. [Google Scholar] [CrossRef]
- Farshchi, R.; Ramsteiner, M.; Herfort, J.; Tahraoui, A.; Grahn, H.T. Optical communication of spin information between light emitting diodes. Appl. Phys. Lett. 2011, 98, 162508. [Google Scholar] [CrossRef]
- Yuasa, J.; Ohno, T.; Tsumatori, H.; Shiba, R.; Kamikubo, H.; Kataoka, M.; Hasegawa, Y.; Kawai, T. Fingerprint signatures of lanthanide circularly polarized luminescence from proteins covalently labeled with a β-diketonate europium (III) chelate. Chem. Commun. 2013, 49, 4604–4606. [Google Scholar] [CrossRef]
- Furumi, S. Recent progress in chiral photonic band-gap liquid crystals for laser applications. Chem. Rec. 2010, 10, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Wei, G.; Jiang, X.; Li, F.; Zhu, C.; Cheng, Y. Chiral sensing for induced circularly polarized luminescence using an Eu (III)-containing polymer and d- or l-proline. Chem. Commun. 2013, 49, 5772–5774. [Google Scholar] [CrossRef] [PubMed]
- Sato, I.; Yamashima, R.; Kadowaki, K.; Yamamoto, J.; Shibata, T.; Soai, K. Asymmetric induction by helical hydrocarbons: [6]- and [5]Helicenes. Angew. Chem. Int. Ed. 2001, 40, 1096–1098. [Google Scholar] [CrossRef]
- Cave, R.J. Inducing chirality with circularly polarized light. Science 2009, 323, 1435–1436. [Google Scholar] [CrossRef] [PubMed]
- Gussakovsky, E. Circularly polarised luminescence (CPL) of proteins and protein complexes. In Reviews in Fluorescence 2008; Geddes, C.D., Ed.; Springer: New York, NY, USA, 2008; Volume 3, pp. 425–459. [Google Scholar]
- Chen, S.H.; Katsis, D.; Schmid, A.W.; Mastrangelo, J.C.; Tsutsui, T.; Blanton, T.N. Circularly polarized light generated by photoexcitation of luminophores in glassy liquid-crystal films. Nature 1999, 397, 506–508. [Google Scholar] [CrossRef]
- Montali, A.; Bastiaansen, C.; Smith, P.; Weder, C. Polarizing energy transfer in photoluminescent materials for display applications. Nature 1998, 392, 261–264. [Google Scholar] [CrossRef]
- Emeis, C.A.; Oosterhoff, L.J. The n-π* absorption and emission of optically active trans-β-hydrindanone and trans-β-thiohydrindanone. J. Chem. Phys. 1971, 54, 4809–4819. [Google Scholar] [CrossRef]
- Berova, N.; Nakanishi, K.; Woody, R.W. Circular Dichroism: Principles and Applications, 2nd ed.; Wiley-VCH: New York, NY, USA, 2000; pp. 196–197. [Google Scholar]
- Zinna, F.; Bari, L.D. Lanthanide circularly polarized luminescence: Bases and applications. Chirality 2015, 27, 1–13. [Google Scholar] [CrossRef]
- Lunkley, J.L.; Shirotani, D.; Yamanari, K.; Kaizaki, S.; Muller, G. Extraordinary circularly polarized luminescence activity exhibited by cesium tetrakis (3-heptafluoro-butylryl-(+)-camphorato) Eu(III) complexes in EtOH and CHCl3 Solutions. J. Am. Chem. Soc. 2008, 130, 13814–13815. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Carnerero, E.M.; Agarrabeitia, A.R.; Moreno, F.; Maroto, B.L.; Muller, G.; Ortiz, M.J.; de la Moya, S. Circularly polarized luminescence from simple organic molecules. Chem. Eur. J. 2015, 21, 13488–13500. [Google Scholar] [CrossRef] [PubMed]
- Feuillastre, S.; Pauton, M.; Gao, L.; Desmarchelier, A.; Riives, A.J.; Prim, D.; Tondelier, D.; Geffroy, B.; Muller, G.; Clavier, G.; et al. Design and synthesis of new circularly polarized thermally activated delayed fluorescence emitters. J. Am. Chem. Soc. 2016, 138, 3990–3993. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Inoue, Y.; Mori, T. Circularly polarized luminescence and circular dichroisms in small organic molecules: Correlation between excitation and emission dissymmetry factors. Chem. Photo. Chem. 2018, 2, 1–18. [Google Scholar] [CrossRef]
- Pritchard, B.; Autschbach, J. Calculation of vibrationally resolved circularly polarized luminescence of d-camphorquinone and (S,S)-trans-β-hydrindanone. Chem. Phys. Chem. 2010, 11, 2409–2415. [Google Scholar] [CrossRef]
- Pecul, M.; Ruud, K. The optical activity of beta, gamma-enones in ground and excited states using circular dichroism and circularly polarized luminescence. Phys. Chem. Chem. Phys. 2011, 13, 643–650. [Google Scholar] [CrossRef]
- Longhi, G.; Castiglioni, E.; Abbate, S.; Lebon, F.; Lightner, D.A. Experimental and calculated CPL spectra and related spectroscopic data of camphor and other simple chiral bicyclic ketones. Chirality 2013, 25, 589–599. [Google Scholar] [CrossRef]
- Autschbach, J. Computing chiroptical properties with first-principles theoretical methods: Background and illustrative examples. Chirality 2009, 21, E116–E152. [Google Scholar] [CrossRef] [PubMed]
- Richardson, F.S.; Riehl, J.P. Circularly polarized luminescence spectroscopy. Chem. Rev. 1977, 77, 773–792. [Google Scholar] [CrossRef]
- Abbate, S.; Longhi, G.; Lebon, F.; Castiglioni, E.; Superchi, S.; Pisani, L.; Fontana, F.; Torricelli, F.; Caronna, T.; Villani, C.; et al. Helical sense-responsive and substituent-sensitive features in vibrational and electronic circular dichroism, in circularly polarized luminescence, and in Raman spectra of some simple optically active hexahelicenes. J. Phys. Chem. C 2014, 118, 1682–1695. [Google Scholar] [CrossRef]
- McAlexander, H.R.; Crawforda, T.D. Simulation of circularly polarized luminescence spectra using coupled cluster theory. J. Chem. Phys. 2015, 142, 154101. [Google Scholar] [CrossRef] [PubMed]
- Longhi, G.; Castiglioni, E.; Villani, C.; Sabia, R.; Menichetti, S.; Viglianisi, C.; Devlin, F.; Abbate, S. Chiroptical properties of the ground and excited states of two thia-bridged triarylamine heterohelicenes. J. Photochem. Photobiol. A 2016, 331, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Zinna, F.; Bruhn, T.; Guido, C.A.; Ahrens, J.; Broering, M.; Di Bari, L.; Pescitelli, G. Circularly polarized luminescence from axially chiral BODIPY DYEmers: An experimental and computational study. Chem. Eur. J. 2016, 22, 16089–16098. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cerezo, J.; Mazzeo, G.; Lin, N.; Zhao, X.; Longhi, G.; Abbate, S.; Santoro, F. Vibronic coupling explains the different shape of electronic circular dichroism and of circularly polarized luminescence spectra of hexahelicenes. J. Chem. Theory Comput. 2016, 12, 2799–2819. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Ikenosako, M.; Kato, Y.; Fujiki, M.; Inoue, Y.; Mori, T. Symmetry-based rational design for boosting chiroptical responses. Commun. Chem. 2018, 1, 38. [Google Scholar] [CrossRef]
- Steinberg, I.Z.; Gafni, A. Sensitive instrument for the study of circular polarization of luminescence. Rev. Sci. Instrum. 1972, 43, 409–413. [Google Scholar] [CrossRef]
- Castiglioni, E.; Abbate, S.; Longhi, G. Revisiting with updated hardware an old spectroscopic technique: Circularly polarized luminescence. Appl. Spectrosc. 2010, 64, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, E.; Abbate, S.; Lebon, F.; Longhi, G. Chiroptical spectroscopic techniques based on fluorescence. Methods Appl. Fluoresc. 2014, 2, 024006. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; Su, H.M.; Meng, L.M.; Zhao, Y.H.; Deng, C.M.; Ng, J.C.Y.; Lu, P.; Faisal, M.; Lam, J.W.Y.; Huang, X.H.; et al. What makes efficient circularly polarised luminescence in the condensed phase: Aggregation-induced circular dichroism and light emission. Chem. Sci. 2012, 3, 2737–2747. [Google Scholar] [CrossRef]
- Harada, T.; Hayakawa, H.; Watanabe, M.; Takamoto, M. A solid-state dedicated circularly polarized luminescence spectrophotometer: Development and application. Rev. Sci. Instrum. 2016, 87, 075102. [Google Scholar] [CrossRef] [PubMed]
- Cahn, R.S.; Ingold, S.C.; Prelog, V. Specification of Molecular Chirality. Angew. Chem. Internat. Edit. 1966, 5, 385–415. [Google Scholar] [CrossRef]
- Amako, T.; Nakabayashi, K.; Mori, T.; Inoue, Y.; Fujiki, M.; Imai, Y. Sign inversion of circularly polarized luminescence by geometry manipulation of four naphthalene units introduced into a tartaric acid scaffold. Chem. Commun. 2014, 50, 12836–12839. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Ma, J.; Liu, S.; Wang, Y.; Zhu, C.; Cheng, Y. Strong and reversible circularly polarized luminescence emission of a chiral 1,8-naphthalimide fluorophore induced by excimer emission and orderly aggregation. Chem. Eur. J. 2016, 22, 9519–9522. [Google Scholar] [CrossRef]
- Gobo, Y.; Yamamura, M.; Nakamura, T.; Nabeshima, T. Synthesis and chiroptical properties of a ring-fused BODIPY with a skewed chiral π skeleton. Org. Lett. 2016, 18, 2719–2721. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Ikeda, K.; Nakanishi, S.; Imai, Y.; Asami, M. Concentration-dependent circularly polarized luminescence (CPL) of chiral N,N′-dipyrenyldiamines: Sign-inverted CPL switching between monomer and excimer regions under retention of the monomer emission for photoluminescence. Chem. Commun. 2017, 53, 6323–6326. [Google Scholar] [CrossRef]
- Hassan, K.; Yamashita, K.; Hirabayashi, K.; Shimizu, T.; Nakabayashi, K.; Imai, Y.; Matsumoto, T.; Yamano, A.; Sugiura, K.I. π-expanded axially chiral biaryls and their emissions: Molecular design, syntheses, optical resolution, absolute configuration, and circularly polarized luminescence of 1,1′-bipyrene-2,2′-diols. Chem. Lett. 2015, 44, 1607–1609. [Google Scholar] [CrossRef]
- Hara, N.; Kaji, D.; Okuda, K.; Shizuma, M.; Tajima, N.; Imai, Y. Substituent-induced Preservation/Inversion of the Sign of Circularly Polarized Luminescence in Binaphthyl Organic Fluorophores. Chem. Lett. 2018, 47, 894–896. [Google Scholar] [CrossRef]
- Nakanishi, S.; Hara, N.; Kuroda, N.; Tajima, N.; Fujiki, M.; Imai, Y. Solvent-sensitive signs and magnitudes of circularly polarised luminescence and circular dichroism spectra: Probing two phenanthrenes as emitters endowed with BINOL derivatives. Org. Biomol. Chem. 2018, 16, 1093–1100. [Google Scholar] [CrossRef]
- Kitayama, Y.; Amako, T.; Suzuki, N.; Fujiki, M.; Imai, Y. Enhancing circularly polarised luminescence by extending the π-conjugation of axially chiral compounds. Org. Biomol. Chem. 2014, 12, 4342–4346. [Google Scholar] [CrossRef]
- Kitayama, Y.; Nakabayashi, K.; Wakabayashi, T.; Tajima, N.; Fujiki, M.; Imai, Y. Circularly polarized luminescence of biaryl atropisomers: Subtle but significant structural dependency. RSC Adv. 2015, 5, 410–415. [Google Scholar] [CrossRef]
- Benincori, T.; Appoloni, G.; Mussini, P.R.; Arnaboldi, S.; Cirilli, R.; Procopio, E.Q.; Panigati, M.; Abbate, S.; Mazzeo, G.; Longhi, G. Searching for models exhibiting high circularly polarized luminescence: Electroactive inherently chiral oligothiophenes. Chem. Eur. J. 2018, 24, 12660–12668. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Nakabayashi, K.; Kitamura, S.; Kuroda, R.; Fujiki, M.; Imai, Y. A comparison of circularly polarised luminescent BINAP and BINAPO as chiral binaphthyl luminophores. Tetrahedron 2015, 71, 3985–3989. [Google Scholar] [CrossRef]
- Sato, T.; Tajima, N.; Ueno, H.; Harada, T.; Fujiki, M.; Imai, Y. Binaphthyl luminophores with triphenylsilyl groups: Sign inversion of circularly polarized luminescence and circular dichroism. Tetrahedron 2016, 72, 7032–7038. [Google Scholar] [CrossRef]
- Okazakia, M.; Mizusawa, T.; Nakabayashi, K.; Yamashita, M.; Tajima, N.; Harada, T.; Fujikid, M.; Imai, Y. Solvent-controlled sign inversion of circularly polarized luminescent binaphthylacetic acid derivative. J. Photochem. Photobiol. A 2016, 331, 115–119. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Amako, T.; Tajima, N.; Fujiki, M.; Imai, Y. Nonclassical dual control of circularly polarized luminescence modes of binaphthyl–pyrene organic fluorophores in fluidic and glassy media. Chem. Commun. 2014, 50, 13228–13230. [Google Scholar] [CrossRef]
- Sato, T.; Hara, N.; Tajima, N.; Sudo, A.; Fujiki, M.; Imai, Y. Turn-on circularly polarized luminescent (CPL) molecular system realized by thermo-driven Newman-Kwart rearrangement reaction from CPL-silent O- to CPL-active S-thiocarbamate groups at peripheral position of 1,1’-binapthyl rings. Tetrahedron Lett. 2018, 59, 1619–1622. [Google Scholar] [CrossRef]
- Kitatobe, T.; Mimura, Y.; Tsujimoto, S.; Tajima, N.; Fujiki, M.; Imai, Y. Circularly polarized luminescence from open- and closed-style axially chiral amphipathic binaphthyl fluorophores in water. Tetrahedron 2017, 73, 6856–6862. [Google Scholar] [CrossRef]
- Taniguchi, N.; Nakabayashi, K.; Harada, T.; Tajima, N.; Shizuma, M.; Fujiki, M.; Imai, Y. Circularly polarized luminescence of chiral binaphthyl with achiral terthiophene fluorophores. Chem. Lett. 2015, 44, 598–600. [Google Scholar] [CrossRef]
- Sanchez-Carnerero, E.M.; Moreno, F.; Maroto, B.L.; Agarrabeitia, A.R.; Ortiz, M.J.; Vo, B.G.; Muller, G.; de la Moya, S. Circularly polarized luminescence by visible-light absorption in a chiral O-BODIPY dye: Unprecedented design of CPL organic molecules from achiral chromophores. J. Am. Chem. Soc. 2014, 136, 3346–3349. [Google Scholar] [CrossRef]
- Jimenez, J.; Cerdan, L.; Moreno, F.; Maroto, B.L.; Garcia-Moreno, I.; Lunkley, J.L.; Muller, G.; de la Moya, S. Chiral organic dyes endowed with circularly polarized laser emission. J. Phys. Chem. C 2017, 121, 5287–5292. [Google Scholar] [CrossRef]
- Takase, K.; Noguchi, K.; Nakano, K. Circularly polarized luminescence from chiral spiro molecules: Synthesis and optical properties of 10,10′-spirobi(indeno[1,2-b][1]benzothiophene) derivatives. Org. Lett. 2017, 19, 5082–5085. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, K.; Yamamoto, T.; Hinoide, S.; Ema, T. Helical oligonaphthodioxepins showing intense circularly polarized luminescence (CPL) in solution and in the solid state. Chem. Eur. J. 2017, 23, 9249–9252. [Google Scholar] [CrossRef]
- Sato, T.; Hara, N.; Yoshida, K.; Tajima, N.; Tsubaki, K.; Imai, Y. Extensive effect of p-conjugation in rotatable oligonaphthyl derivatives on circularly polarised luminescence in solution and solid films. Tetrahedron 2018, 74, 4471–4475. [Google Scholar] [CrossRef]
- Morisaki, Y.; Gon, M.; Sasamori, T.; Tokitoh, N.; Chujo, Y. Planar chiral tetrasubstituted [2.2]paracyclophane: Optical resolution and functionalization. J. Am. Chem. Soc. 2014, 136, 3350–3353. [Google Scholar] [CrossRef]
- Morisaki, Y.; Inoshita, K.; Chujo, Y. Planar-chiral through-space conjugated oligomers: Synthesis and characterization of chiroptical properties. Chem. Eur. J. 2014, 20, 8386–8390. [Google Scholar] [CrossRef] [PubMed]
- Gon, M.; Morisaki, Y.; Chujo, Y. Highly emissive optically active conjugated dimers consisting of a planar chiral [2.2]paracyclophane showing circularly polarized luminescence. Eur. J. Org. Chem. 2015, 7756–7762. [Google Scholar] [CrossRef]
- Gon, M.; Morisaki, Y.; Sawada, R.; Chujo, Y. Synthesis of optically active, X-shaped, conjugated compounds and dendrimers based on planar chiral [2.2]paracyclophane, leading to highly emissive circularly polarized luminescence. Chem. Eur. J. 2016, 22, 2291–2298. [Google Scholar] [CrossRef]
- Sasai, Y.; Tsuchida, H.; Kakuta, T.; Ogoshi, T.; Morisaki, Y. Synthesis of optically active π-stacked compounds based on planar chiral tetrasubstituted [2.2]paracyclophane. Mater. Chem. Front. 2018, 2, 791–795. [Google Scholar] [CrossRef]
- Gon, M.; Morisaki, Y.; Chujo, Y. Optically active cyclic compounds based on planar chiral [2.2]paracyclophane: Extension of the conjugated systems and chiroptical properties. J. Mater. Chem. C 2015, 3, 521–529. [Google Scholar] [CrossRef]
- Morisaki, Y.; Sawada, R.; Gon, M.; Chujo, Y. New types of planar chiral [2.2]paracyclophanes and construction of one-handed double helices. Chem. Asian J. 2016, 11, 2524–2527. [Google Scholar] [CrossRef]
- Gon, M.; Morisaki, Y.; Chujo, Y. Optically active phenylethene dimers based on planar chiral tetrasubstituted [2.2]paracyclophane. Chem. Eur. J. 2017, 23, 6323–6329. [Google Scholar] [CrossRef] [PubMed]
- Gon, M.; Morisaki, Y.; Chujo, Y. A silver (I)-induced higher-ordered structure based on planar chiral tetrasubstituted [2.2]paracyclophane. Chem. Commun. 2017, 53, 8304–8307. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Yamanoi, Y.; Ohto, T.; Pham, S.T.; Yamada, R.; Tada, H.; Omoto, K.; Tashiro, S.; Shionoya, M.; Hattori, M.; et al. Multifunctional octamethyltetrasila[2.2]cyclophanes: Conformational variations, circularly polarized luminescence, and organic electroluminescence. J. Am. Chem. Soc. 2017, 139, 11214–11221. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Yoshii, A.; Takahashi, S.; Furumi, S.; Takeuchi, M.; Isobe, H. Chiral intertwined spirals and magnetic transition dipole moments dictated by cylinder helicity. Proc. Natl. Acad. Sci. USA 2017, 114, 13097–13101. [Google Scholar] [CrossRef]
- Sakai, H.; Shinto, S.; Kumar, J.; Araki, Y.; Sakanoue, T.; Takenobu, T.; Wada, T.; Kawai, T.; Hasobe, T. Highly fluorescent [7]carbohelicene fused by asymmetric 1,2-dialkyl-substituted quinoxaline for circularly polarized luminescence and electroluminescence. J. Phys. Chem. C 2015, 119, 13937–13947. [Google Scholar] [CrossRef]
- Sakai, H.; Kubota, T.; Yuasa, J.; Araki, Y.; Sakanoue, T.; Takenobu, T.; Wada, T.; Kawai, T.; Hasobe, T. Synthetic control of photophysical process and circularly polarized luminescence of [5]carbohelicene derivatives substituted by maleimide units. J. Phys. Chem. C 2016, 120, 7860–7869. [Google Scholar] [CrossRef]
- Sakai, H.; Kubota, T.; Yuasa, J.; Araki, Y.; Sakanoue, T.; Takenobu, T.; Wada, T.; Kawai, T.; Hasobe, T. Protonation-induced red-coloured circularly polarized luminescence of [5]carbohelicene fused by benzimidazole. Org. Biomol. Chem. 2016, 14, 6738–6743. [Google Scholar] [CrossRef]
- Dhbaibi, K.; Favereau, L.; Hooper, M.S.; Jean, M.; Vanthuyne, N.; Zinna, F.; Jamoussi, B.; Bari, L.D.; Autschbach, J.; Crassous, J. Exciton coupling in diketopyrrolopyrrole–helicene derivatives leads to red and near-infrared circularly polarized luminescence. Chem. Sci. 2018, 9, 735–742. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Furumi, S.; Takeuchi, M.; Shibuya, T.; Tanaka, K. Enantioselective synthesis and enhanced circularly polarized luminescence of S-shaped double azahelicenes. J. Am. Chem. Soc. 2014, 136, 5555–5558. [Google Scholar] [CrossRef]
- Saleh, N.; Moore, B., II; Srebro, M.; Vanthuyne, N.; Toupet, L.; Williams, J.A.G.; Roussel, C.; Deol, K.K.; Muller, G.; Autschbach, J.; et al. Acid/base-triggered switching of circularly polarized luminescence and electronic circular dichroism in organic and organometallic helicences. Chem. Eur. J. 2015, 21, 1673–1681. [Google Scholar] [CrossRef]
- Saleh, N.; Srebro, M.; Reynaldo, T.; Vanthuyne, N.; Toupet, L.; Chang, V.Y.; Muller, G.; Williams, J.A.G.; Roussel, C.; Autschbach, J.; et al. Enantio-enriched CPL-active helicene-bipyridine–rhenium complexes. Chem. Commun. 2015, 51, 3754–3757. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, T.; Koyama, Y.; Hiroto, S.; Kumar, J.; Kawai, T.; Shinokubo, H. Isolation of a 1,4-diketone intermediate in oxidative dimerization of 2-hydroxyanthracene and its conversion to oxahelicence. Chem. Commun. 2015, 51, 4607–4610. [Google Scholar] [CrossRef]
- Murayama, K.; Oike, Y.; Furumi, S.; Takeuchi, M.; Noguchi, K.; Tanaka, K. Enantioselective synthesis, crystal structure, and photophysical properties of a 1,1’-bitriphenylene-based sila [7] helicene. Eur. J. Org. Chem. 2015, 2015, 1409–1414. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sakai, H.; Yuasa, J.; Araki, Y.; Wada, T.; Sakanoue, T.; Takenobu, T.; Kawai, T.; Hasobe, T. Controlled excited-state dynamics and enhanced fluorescence property of tetrasulfone[9]helicene by a simple synthetic process. J. Phys. Chem. C 2016, 120, 7421–7427. [Google Scholar] [CrossRef]
- Bosson, J.; Labrador, G.M.; Pascal, S.; Miannay, F.A.; Yushchenko, O.; Li, H.D.; Bouffier, L.; Sojic, N.; Tovar, R.C.; Muller, G.; et al. Physicochemical and electronic properties of cationic[6]helicenes: From chemical and electrochemical stabilities to far-red (polarized) luminescence. Chem. Eur. J. 2016, 22, 18394–18403. [Google Scholar] [CrossRef]
- Pascal, S.; Besnard, C.; Zinna, F.; Bari, L.D.; Guennic, B.L.; Jacquemin, D.; Lacour, J. Zwitterionic [4]helicene: A water-soluble and reversible pH-triggered ECD/CPL chiroptical switch in the UV and red spectral regions. Org. Biomol. Chem. 2016, 14, 4590–4594. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Nakatsuka, S.; Hirai, H.; Yasuda, N.; Kumar, J.; Kawai, T.; Hatakeyama, T. Two-step synthesis of boron-fused double helicenes. J. Am. Chem. Soc. 2016, 138, 5210–5213. [Google Scholar] [CrossRef] [PubMed]
- Isla, H.; Srebro-Hooper, M.; Jean, M.; Vanthuyne, N.; Roisnel, T.; Lunkley, J.L.; Muller, G.; Williams, J.A.G.; Autschbach, J.; Crassous, J. Conformational changes and chiroptical switching of enantiopure bis-helicenic terpyridine upon Zn2+ binding. Chem. Commun. 2016, 52, 5932–5935. [Google Scholar] [CrossRef]
- Shen, C.; Srebro-Hooper, M.; Jean, M.; Vanthuyne, N.; Toupet, L.; Williams, J.A.G.; Torres, A.R.; Riives, A.J.; Muller, G.; Autschbach, J.; et al. Synthesis and chiroptical properties of hexa-, octa-, and deca-azaborahelicenes: Influence of helicene size and of the number of boron atoms. Chem. Eur. J. 2017, 23, 407–418. [Google Scholar] [CrossRef]
- Otani, T.; Tsuyuki, A.; Iwachi, T.; Someya, S.; Tateno, K.; Kawai, H.; Saito, T.; Kanyiva, K.S.; Shibata, T. Facile two-step synthesis of 1,10-phenanthroline-derived polyaza-[7]helicenes with high fluorescence and CPL efficiency. Angew. Chem. Int. Ed. 2017, 56, 3906–3910. [Google Scholar] [CrossRef]
- Ushiyama, A.; Hiroto, S.; Yuasa, J.; Kawai, T.; Shinokubo, H. Synthesis of a figure-eight azahelicene dimer with high emission and CPL properties. Org. Chem. Front. 2017, 4, 664–667. [Google Scholar] [CrossRef]
- Shen, C.; Anger, E.; Srebro, M.; Vanthuyne, N.; Deol, K.K.; Jefferson, T.D.; Muller, G.; Williams, J.A.G.; Toupet, L.; Roussel, C.; et al. Straightforward access to mono- and biscycloplatinated helicenes displaying circularly polarized phosphorescence by using crystallization resolution methods. Chem. Sci. 2014, 5, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Biet, T.; Cauchy, T.; Sun, Q.; Ding, J.; Hauser, A.; Oulevey, P.; Burgi, T.; Jacquemin, D.; Vanthuyne, N.; Crassous, J.; et al. Triplet state CPL active helicene–dithiolene platinum bipyridine complexes. Chem. Commun. 2017, 53, 9210–9213. [Google Scholar] [CrossRef] [PubMed]
- Hellou, N.; Srebro-Hooper, M.; Favereau, L.; Zinna, F.; Caytan, E.; Toupet, L.; Dorcet, V.; Jean, M.; Vanthuyne, N.; Williams, J.A.G.; et al. Enantiopure cycloiridiated complexes bearing a pentahelicenic n-heterocyclic carbene and displaying long-lived circularly polarized phosphorescence. Angew. Chem. Int. Ed. 2017, 56, 8236–8239. [Google Scholar] [CrossRef] [PubMed]
- Sundar, M.S.; Talele, H.R.; Mande, H.M.; Bedekar, A.V.; Tovar, R.C.; Muller, G. Synthesis of enantiomerically pure helicene like bis-oxazines from atropisomeric 7,7’-dihydroxy BINOL: Preliminary measurements of the circularly polarized luminescence. Tetrahedron Lett. 2014, 55, 1760–1764. [Google Scholar] [CrossRef] [PubMed]
- Oyama, H.; Akiyama, M.; Nakano, K.; Naito, M.; Nobusawa, K.; Nozaki, K. Synthesis and properties of [7]helicene-like compounds fused with a fluorene unit. Org. Lett. 2016, 18, 3654–3657. [Google Scholar] [CrossRef]
- Yamano, R.; Hara, J.; Murayama, K.; Sugiyama, H.; Teraoka, K.; Uekusa, H.; Kawauchi, S.; Shibata, Y.; Tanaka, K. Rh-mediated enantioselective synthesis, crystal structures, and photophysical/chiroptical properties of phenanthrenol-based [9]helicene-like molecules. Org. Lett. 2017, 19, 42–45. [Google Scholar] [CrossRef]
- Ray, C.; Sanchez-Carnerero, E.M.; Moreno, F.; Maroto, B.L.; Agarrabeitia, A.R.; Ortiz, M.J.; Lopez-Arbeloa, I.; Banuelos, J.; Cohovi, K.D.; Lunkley, J.L.; et al. Bis(haloBODIPYs) with labile helicity: Valuable simple organic molecules that enable circularly polarized luminescence. Chem. Eur. J. 2016, 22, 8805–8808. [Google Scholar] [CrossRef]
- Alnoman, R.B.; Rihn, S.; O’Connor, D.C.; Black, F.A.; Costello, B.; Waddell, P.G.; Clegg, W.; Peacock, R.D.; Herrebout, W.; Knight, J.G.; et al. Circularly polarized luminescence from helically chiral N,N,O,O-boron-chelated dipyrromethenes. Chem. Eur. J. 2016, 22, 93–96. [Google Scholar] [CrossRef]
- Saikawa, M.; Nakamura, T.; Uchida, J.; Yamamura, M.; Nabeshima, T. Synthesis of figure-of-eight helical bisBODIPY macrocycles and their chiroptical properties. Chem. Commun. 2016, 52, 10727–10730. [Google Scholar] [CrossRef]
- Clarke, R.; Ho, K.L.; Alsimaree, A.A.; Woodford, O.J.; Waddell, P.G.; Bogaerts, J.; Herrebout, W.; Knight, J.G.; Pal, R.; Penfold, T.J.; et al. Circularly polarised luminescence from helically chiral “confused” N,N,O,C-boron-chelated dipyrromethenes (BODIPYs). Chem. Photo. Chem. 2017, 1, 513–517. [Google Scholar] [CrossRef]
- Li, M.; Lu, H.Y.; Zhang, C.; Shi, L.; Tang, Z.; Chen, C.F. Helical aromatic imide based enantiomers with full-color circularly polarized luminescence. Chem. Commun. 2016, 52, 9921–9924. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, C.; Fang, L.; Shi, L.; Tang, Z.Y.; Lu, H.Y.; Chen, C.F. Chiral nanoparticles with full-color and white CPL properties based on optically stable helical aromatic imide enantiomers. ACS Appl. Mater. Interfaces 2018, 10, 8225–8230. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Li, M.; Lin, W.B.; Shen, Y.; Chen, C.F. Synthesis, structures, and photophysical properties of optically stable 1,16-diphenyl-3,14-diaryl-substituted tetrahydrobenzo [5]helicenediol derivatives:enantioselective recognition toward tryptophan methyl esters. J. Org. Chem. 2017, 82, 7402–7409. [Google Scholar] [CrossRef] [PubMed]
- He, D.Q.; Lu, H.Y.; Li, M.; Chen, C.F. Intense blue circularly polarized luminescence from helical aromatic esters. Chem. Commun. 2017, 53, 6093–6096. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| No. | Structure | Solvent | λexc (nm) | λlum (nm) | ϕF (%) | |glum| (10−3) | Ref. | Year |
|---|---|---|---|---|---|---|---|---|
| C1 | 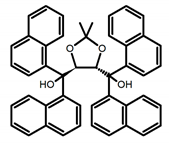 | CHCl3 (1 × 10−3 M) | - | 410 | 2.0 | 9.40 | [44] | 2014 |
| C2 | 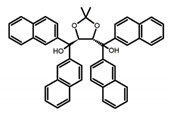 | CHCl3 (1 × 10−3 M) | - | 375 | 2.0 | 3.90 | [44] | 2014 |
| C3 | 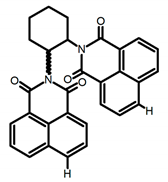 | (CH2)4O (1 × 10−5 M) | 330 | 450 | - | 14.00 | [45] | 2016 |
| C4 |  | CHCl3 (1 × 10−5 M) | - | 641 | 73.0 | 0.60 | [46] | 2016 |
| C5 | 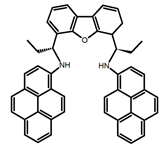 | C7H8 (1 × 10−5 M) | - | 424 | 60.0 | 0.69 | [47] | 2017 |
| 500 | 60.0 | 3.90 |
| No. | Structure | Solvent | λexc (nm) | λlum (nm) | ϕF (%) | |glum| (10−3) | Ref. | Year | |
|---|---|---|---|---|---|---|---|---|---|
| A1 | 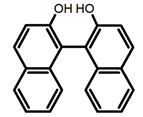 | CHCl3 (1 × 10−4 M) | 320 | 364 | 4.0 | 0.47 | [48] | 2015 | |
| A2 | 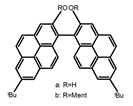 | CHCl3 (1 × 10−4 M) | a | 340 | 438 | 57.0 | 0.36 | [48] | 2015 |
| b | 300 | 401 | 80.0 | 1.20 | |||||
| A3 | 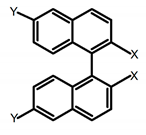  | CHCl3 (1 × 10−4 M) | a | 280 | 370 | - | 0.79 | [49] | 2018 |
| b | 340 | 418 | - | 0.73 | |||||
| c | 280 | 358 | - | 0.67 | |||||
| d | 340 | 412 | - | 0.85 | |||||
| e | 280 | 365 | - | 0.59 | |||||
| A4 |  | CHCl3 (1 × 10−4 M) | a | 300 | 374 | 2.0 | 0.61 | [50] | 2018 |
| b | 300 | 364 | 4.0 | 0.80 | |||||
| DMF (1 × 10−4 M) | a | 320 | 391 | 38.0 | 0.55 | ||||
| b | 320 | 363 | 6.0 | 0.87 | |||||
| A5 | 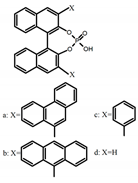 | CHCl3 (1 × 10−4 M) | a | 300 | 375 | 10.0 | 0.79 | [50] | 2018 |
| b | 300 | 422 | - | 1.00 | |||||
| c | 300 | 350 | 22.0 | 1.20 | |||||
| d | 300 | 350 | 29.0 | 1.50 | |||||
| DMF (1 × 10−4 M) | a | 300 | 373 | 21.0 | 0.32 | ||||
| b | 300 | 415 | - | 0.43 | |||||
| c | 300 | 370 | 22.0 | 1.90 | |||||
| d | 300 | 361 | 28.0 | 1.90 | |||||
| A6 |  | CHCl3 (1 × 10−5 M) | 282 | 376 | 20.0 | 1.30 | [51] | 2014 | |
| CHCl3 (1 × 10−4 M) | 289 | 378 | 16.0 | - | |||||
| CHCl3 (1 × 10−3 M) | 320 | 391 | 13.0 | 1.10 | |||||
| A7 |  | CHCl3 (1 × 10−4 M) | 307 | 372 | 12.0 | 3.50 | [52] | 2015 | |
| PMMA | 320 | 372 | 16.0 | 2.00 | |||||
| A8 |  | CHCl3 (1 × 10−4 M) | 335 | 373 | 9.0 | 1.30 | [52] | 2015 | |
| PMMA | 330 | 376 | 19.0 | 0.79 | |||||
| A9 |  | CHCl3 (2 × 10−4 M) | - | ~520 | 21.0 | 9.30 | [53] | 2018 | |
| A10 | 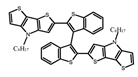 | CHCl3 (2 × 10−4 M) | - | ~500 | 12.0 | 8.00 | [53] | 2018 | |
| A11 |  | CHCl3 (1 × 10−4 M) | 300 | ~355 | 2.0 | 1.20 | [54] | 2015 | |
| PMMA | 300 | 354 | 7.0 | 1.00 | |||||
| KBr pellet | 330 | 372 | 7.0 | 0.67 | |||||
| A12 | 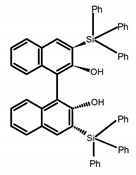 | CHCl3 (1 × 10−4 M) | 280 | 375 | 18.0 | 1.00 | [55] | 2016 | |
| PMMA | - | 368 | 5.0 | 0.61 | |||||
| KBr pellet | - | 372 | 8.0 | 0.25 | |||||
| A13 |  | CHCl3 (1 × 10−4M) | 280 | 363 | 35.0 | 1.30 | [55] | 2016 | |
| PMMA | - | 362 | 15.0 | 1.30 | |||||
| KBr pellet | - | 367 | 29.0 | 0.36 | |||||
| A14 |  | CHCl3 (1 × 10−4 M) | 300 | ~360 | 25.0 | 0.18 | [56] | 2016 | |
| DMF (1 × 10−4 M) | 300 | ~375 | 39.0 | 0.19 | |||||
| CH3CN (1 × 10−4 M) | 290 | ~370 | 29.0 | 0.03 | |||||
| MeOH (1 × 10−4 M) | 290 | ~360 | 32.0 | 0.14 | |||||
| PMMA | 300 | ~360 | 11.0 | 0.42 | |||||
| A15 |  | CHCl3 (1 × 10−3 M) | 340 | 480 | 20.0 | 0.78 | [57] | 2014 | |
| PMMA | 340 | ~400 | 46.0 | 0.36 | |||||
| A16 | 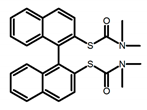 | CHCl3 (1 × 10−4 M) | 300 | 400 | 2.0 | 1.20 | [58] | 2018 | |
| PMMA | 300 | no | 4.0 | no | |||||
| ARTON | 300 | no | 2.0 | no | |||||
| A17 | 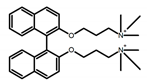 | Water (1 × 10−4 M) | 300 | 374 | 18.0 | 0.30 | [59] | 2017 | |
| A18 | 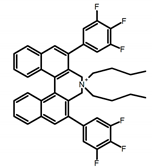 | Water (1 × 10−4 M) | 310 | 371 | 16.0 | 0.20 | [59] | 2017 | |
| A19 | 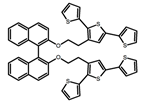 | KBr pellet | 297 | ~410 | 3.0 | 0.50 | [60] | 2015 | |
| A20 |  | CHCl3 (1 × 10−3 M) | 529 | 570 | 45.0 | 0.78 | [61] | 2014 | |
| A21 | 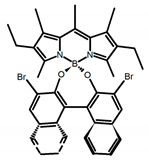 | CHCl3 (1 × 10−3 M) | 445 | 575 | 69.0 | 0.70 | [62] | 2017 | |
| A22 | 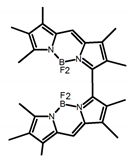 | CH2Cl2 (5 × 10−5 M) | - | 655 | - | 3.80 | [35] | 2016 | |
| A23 | 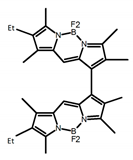 | CH2Cl2 (5 × 10−5 M) | - | 603 | - | 0.40 | [35] | 2016 | |
| A24 |  | CH2Cl2 (1 × 10−5 M) | a | 300 | 368 | 6.0 | 1.50 | [63] | 2017 |
| CH2Cl2 (6 × 10−6 M) | b | 330 | 459 | 1.0 | 3.00 | ||||
| CH2Cl2 (1 × 10−5 M) | c | 350 | 444 | 76.0 | 1.50 | ||||
| A25 |  | C4H8O2 (1 × 10−5 M) | 310 | ~360 | 44.0 | 0.68 | [64] | 2017 | |
| Solid state | 310 | ~360 | 13.0 | 1.10 | |||||
| A26 | 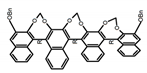 | C4H8O2 (5 × 10−6 M) | 330 | ~400 | 78.0 | 1.60 | [64] | 2017 | |
| Solid state | 330 | ~400 | 29.0 | 1.40 | |||||
| A27 | 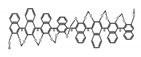 | C4H8O2(2.5 × 10−6 M) | 330 | ~410 | 90.0 | 2.20 | [64] | 2017 | |
| Solid state | 330 | ~410 | 22.0 | 7.00 | |||||
| A28 | 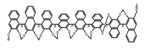 | C4H8O2 (2.5 × 10−6 M) | 330 | ~410 | 79.0 | 0.57 | [64] | 2017 | |
| Solid state | 330 | ~410 | 24.0 | - | |||||
| A29 | 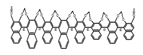 | C4H8O2 (2.5 × 10−6 M) | 330 | ~410 | 84.0 | 0.38 | [64] | 2017 | |
| Solid state | 330 | ~410 | 17.0 | - | |||||
| A30 | 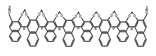 | C4H8O2 (2.5 × 10−6 M) | 330 | ~410 | 64.0 | 0.31 | [64] | 2017 | |
| Solid state | 330 | ~410 | 13.0 | - | |||||
| A31 | 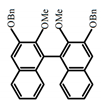 | CHCl3 (1 × 10−4 M) | 290 | 343 | 7.0 | 0.58 | [65] | 2018 | |
| PMMA | 290 | 348 | 5.0 | 0.65 | |||||
| A32 | 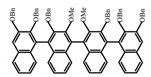 | CHCl3 (1 × 10−4 M) | 300 | 354 | 40.0 | 0.61 | [65] | 2018 | |
| PMMA | 300 | 354 | 16.0 | 0.51 | |||||
| A33 | 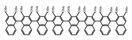 | CHCl3 (1 × 10−4 M) | 290 | 363 | 52.0 | 0.33 | [65] | 2018 | |
| PMMA | 305 | 358 | 29.0 | 0.49 | |||||
| No. | Structure | Solvent | λexc (nm) | λlum (nm) | ϕF (%) | |glum| (10−3) | Ref. | Year |
|---|---|---|---|---|---|---|---|---|
| P1 | 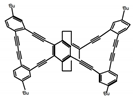 | CHCl3 (1 × 10−5 M) | 314 | 460 | 45.0 | 11.00 | [66] | 2014 |
| P2 | 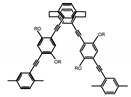 | CHCl3 (1 × 10−5 M) | 320 | 407 | 50.0 | 1.80 | [67] | 2014 |
| P3 | 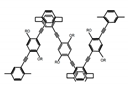 | CHCl3 (1 × 10−5 M) | 320 | 414 | 47.0 | 2.20 | [67] | 2014 |
| P4 |  | CHCl3 (1 × 10−5 M) | 320 | 413 | 64.0 | 2.20 | [67] | 2014 |
| P5 |  | CHCl3 (1 × 10−5 M) | 320 | 415 | 60.0 | 2.60 | [67] | 2014 |
| P6 | 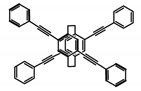 | CHCl3 (1 × 10−5 M) | 300 | 412 | 60.0 | 1.20 | [68] | 2015 |
| P7 | 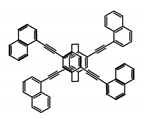 | CHCl3 (1 × 10−5 M) | 300 | 421 | 78.0 | 1.70 | [68] | 2015 |
| P8 | 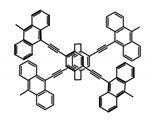 | CHCl3 (1 × 10−5 M) | 350 | 503 | 42.0 | 0.50 | [68] | 2015 |
| P9 | 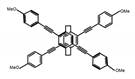 | CHCl3 (1 × 10−5 M) | - | 416 | 66.0 | 1.50 | [69] | 2016 |
| P10 | 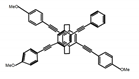 | CHCl3 (1 × 10−5 M) | 280 | 425 | >70.0 | 1.70 | [70] | 2018 |
| P11 | 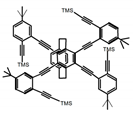 | CHCl3 (1 × 10−5 M) | 300 | 418 | 46.0 | 1.40 | [71] | 2015 |
| P12 | 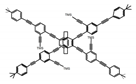 | CHCl3 (1 × 10−5 M) | 350 | 438 | 80.0 | 1.20 | [71] | 2015 |
| P13 | 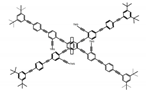 | CHCl3 (1 × 10−5 M) | 350 | 443 | 88.0 | 1.00 | [71] | 2015 |
| P14 | 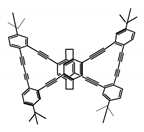 | CHCl3 (1 × 10−5 M) | 314 | 453 | 41.0 | 13.00 | [71] | 2015 |
| P15 | 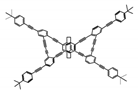 | CHCl3 (1 × 10−5 M) | 350 | 471 | 60.0 | 10.00 | [71] | 2015 |
| P16 | 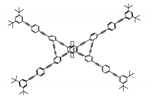 | CHCl3 (1 × 10−5 M) | 355 | 474 | 70.0 | 7.50 | [71] | 2015 |
| P17 |  | CHCl3 (1 × 10−5 M) | 379 | 419 | 62.0 | 1.60 | [72] | 2016 |
| P18 | 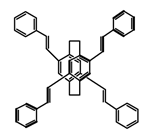 | C4H8O2 (1 × 10−5 M) | 350 | 455 | 78.0 | 3.70 | [73] | 2017 |
| P19 | 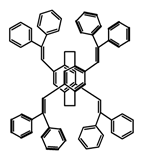 | C4H8O2 (1 × 10−5 M) | 350 | 494 | 58.0 | 0.73 | [73] | 2017 |
| P20 | 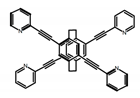 | CH2Cl2 (1 × 10−5 M) | 300 | 421 | 59.0 | 2.80 | [74] | 2017 |
| P21 |  | CH2Cl2 (1 × 10−5 M) | 300 | 417 | 56.0 | 1.20 | [74] | 2017 |
| P22 | 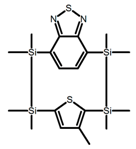 | Cyclohexane (1.5 × 10−5 M) | 370–380 | 500 | 5.0 | 1.70 | [75] | 2017 |
| P23 | 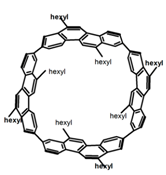 | C7H8 (4.90–6.77 × 10−6 M) | - | 443 | 80.0 | 15.00 | [76] | 2017 |
| P24 | 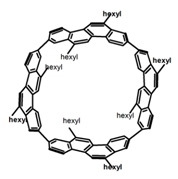 | C7H8 (4.90–6.77 × 10−6 M) | - | ~450 | 74.0 | 10.00 | [76] | 2017 |
| No. | Structure | Solvent | λexc (nm) | λlum (nm) | ϕF (%) | |glum| (10−3) | Ref. | Year | |
|---|---|---|---|---|---|---|---|---|---|
| H1 | 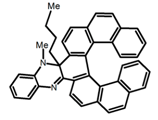 | (CH2)4O | 330 | ~550 | 25.0 | 4.00 | [77] | 2015 | |
| H2 |  | (CH2)4O | - | ~480 | 37.0 | 2.40 | [78] | 2016 | |
| H3 | 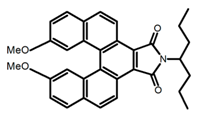 | (CH2)4O | - | ~480 | 22.0 | 2.30 | [78] | 2016 | |
| H4 | 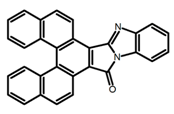 | CH2Cl2 | - | 550 | ~6.0 | 9.45 | [79] | 2016 | |
| H5 |  | CH2Cl2 | - | 650 | ~6.0 | 5.92 | [79] | 2016 | |
| H6 | 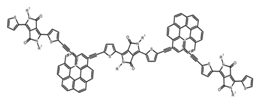 | CH2Cl2 | - | 610 | 41.0 | 0.30 | [80] | 2018 | |
| H7 |  | CHCl3 (1 × 10−6 M) | 375 | 492 | 19.0 | 28.00 | [81] | 2014 | |
| H8 | 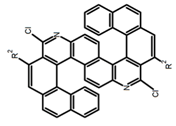 | CHCl3 (1 × 10−6 M) | 375 | 454 | 9.0 | 11.00 | [81] | 2014 | |
| H9 | 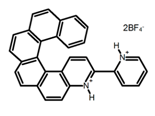 | CH2Cl2 | - | 590 | 8.0 | 2.90 | [82] | 2015 | |
| H10 | 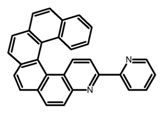 | CH2Cl2 | - | 421 | 8.0 | 3.20 | [83] | 2015 | |
| H11 |  | CH2Cl2 (1 × 10−5 M) | - | ~550 | 66.0 | 1.20 | [84] | 2015 | |
| H12 | 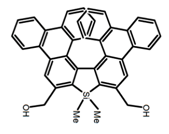 | CHCl3 | - | 482 | 15.0 | 16.00 | [85] | 2015 | |
| H13 | 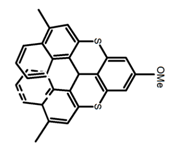 | CHCl3 (2 × 10−4 M) | 390 | ~500 | 1.6 | 9.00 | [34] | 2016 | |
| H14 | 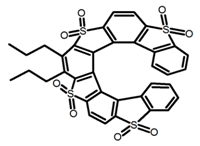 | C7H8 | - | ~430 | 27.0 | 0.83 | [86] | 2016 | |
| H15 | 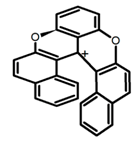 | CH2Cl2 (2 × 10−3 M) | 435 | 595 | 12.0 | 0.40 | [87] | 2016 | |
| H16 | 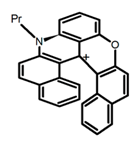 | CH2Cl2 (2 × 10−3 M) | 442 | 614 | 22.0 | 2.10 | [87] | 2016 | |
| H17 | 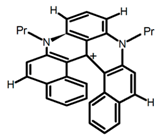 | CH2Cl2 (2 × 10−3 M) | 473 | 658 | 31.0 | 1.10 | [87] | 2016 | |
| H18 |  | CH3CN (1 × 10−5 M) | - | 654 | 29.0 | 0.50 | [88] | 2016 | |
| H19 | 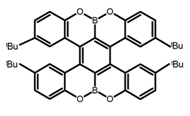 | CH2Cl2 (2 × 10-3 M) | - | 436 | 65.0 | 1.70 | [89] | 2016 | |
| H20 | 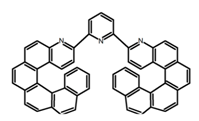 | CH2Cl2 (2 × 10−3 M) | - | 421 | 8.4 | 8.50 | [90] | 2016 | |
| H21 |  | CH2Cl2 (2 × 10−3 M) | - | 480 | 19.0 | 1.30 | [90] | 2016 | |
| H22 | 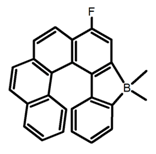 | CH2Cl2 | 395 | 430 | 21.0 | 0.90 | [91] | 2017 | |
| H23 | 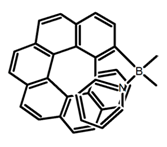 | CH2Cl2 | 405 | 430 | 49.0 | 2.30 | [91] | 2017 | |
| H24 |  | CH2Cl2 | 405 | 442 | 7.0 | 0.70 | [91] | 2017 | |
| H25 | 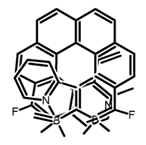 | CH2Cl2 | 420 | 473 | 7.0 | 1.00 | [91] | 2017 | |
| H26 | 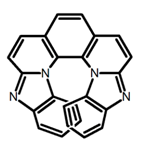 | CH2Cl2 (1 × 10−5 M) | 380 | 473 | 39.0 | 9.00 | [92] | 2017 | |
| H27 | 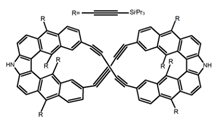 | CH2Cl2 | - | 588 | 55.0 | 8.50 | [93] | 2017 | |
| H28 | 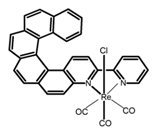 | CH2Cl2 (1 × 10−3 M) | - | 673 | 0.2 | 3.00 | [83] | 2015 | |
| H29 | 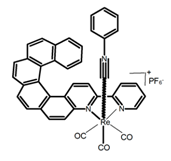 | CH2Cl2 (1 × 10−3 M) | - | 598 | 6.0 | 1.40 | [83] | 2015 | |
| H30 | 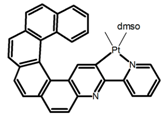 | CH2Cl2 | - | 547 | 0.4 | 1.10 | [82] | 2015 | |
| H31 | 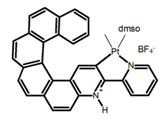 | CH2Cl2 | - | 555 | 3.0 | 2.00 | [82] | 2015 | |
| H32 | 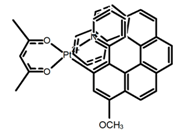 | CH2Cl2 (1 × 10−3 M) | 452 | 648 | 5.6 | 4.50 | [94] | 2014 | |
| H33 | 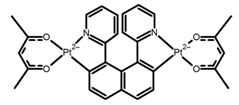 | CH2Cl2(1 × 10−3 M) | 459–469 | 633 | 13.0 | 0.50 | [94] | 2014 | |
| H34 | 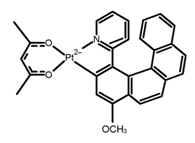 | CH2Cl2 (1 × 10−3 M) | 452 | 644 | 10.0 | 12.00 | [94] | 2014 | |
| H35 | 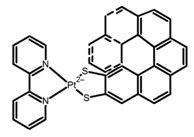 | CH3CN (3 × 10−4 M) | - | 715 | 15.0 | 0.30 | [95] | 2017 | |
| H36 | 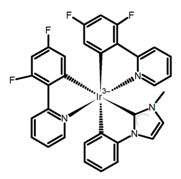 | CH2Cl2 (5 × 10−5 M) | - | 493 | - | 0.90 | [96] | 2017 | |
| H37 | 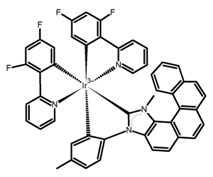 | CH2Cl2 (5 × 10−5 M) | - | 530 | 9.0~ 13.0 | 1.50 | [96] | 2017 | |
| H38 | 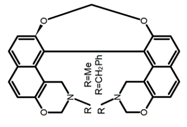 | CH3CN (1 × 10−3 M) | 357 | - | - | ~1.30 | [97] | 2014 | |
| H39 | 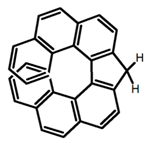 | CH2Cl2 (1 × 10−5 M) | - | 417 | 39.0 | 3.00 | [98] | 2016 | |
| H40 | 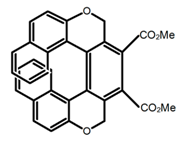 | CHCl3 (2 × 10−5 M) | 350 | 473 | 23.0 | 0.95 | [99] | 2017 | |
| H41 | 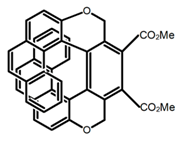 | CHCl3 (2 × 10−5 M) | 350 | 547 | 18.0 | 1.10 | [99] | 2017 | |
| H42 |  | CHCl3 (1 × 10−3 M) | - | ~570 | 14.0 | 1.00 | [100] | 2016 | |
| H43 | 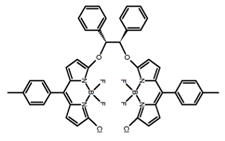 | CHCl3 (1 × 10−3 M) | - | ~550 | 16.0 | 1.00 | [100] | 2016 | |
| H44 | 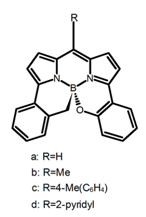 | MeCN | a | 540 | 623 | 65.0 | 4.70 | [101] | 2016 |
| b | 635 | 73.0 | 3.30 | ||||||
| c | 637 | 52.0 | 4.30 | ||||||
| d | 675 | 28.0 | 4.20 | ||||||
| H45 | 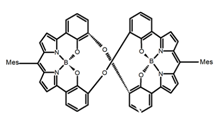 | CHCl3 (1 × 10−5 M) | 400 | 663 | 58.0 | 9.00 | [102] | 2016 | |
| H46 |  | C6H14 | - | 622 | 49.0 | 3.70 | [103] | 2017 | |
| H47 | 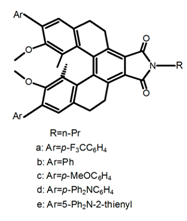 | (CH2)4O (1 × 10−5 M) | a | 366 | 445 | 13.0 | ~1.00 | [104] | 2016 |
| b | 366 | 457 | 19.0 | 1.45 | |||||
| c | 371 | 482 | 65.0 | ~1.25 | |||||
| d | 387 | 556 | 40.0 | ~0.80 | |||||
| e | 415 | 617 | 7.0 | 0.30 | |||||
| Water (2.5 × 10−5 M) | a | - | 463 | 12.0 | 1.70 | [105] | 2018 | ||
| b | - | 478 | 13.0 | 1.10 | |||||
| c | - | 492 | 21.0 | 1.30 | |||||
| d | - | 545 | 16.0 | 0.70 | |||||
| e | - | 603 | 3.0 | 0.10 | |||||
| H48 | 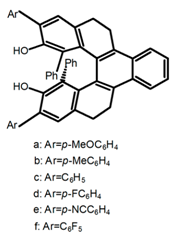 | CH2Cl2 (1 × 10−5 M) | a | - | 408 | 27.0 | 0.89 | [106] | 2017 |
| b | - | 407 | 33.0 | 0.90 | |||||
| c | - | 409 | 23.0 | 1.00 | |||||
| d | - | 406 | 35.0 | 0.61 | |||||
| e | - | 416 | 22.0 | 0.77 | |||||
| f | - | 408 | 27.0 | 0.76 | |||||
| H49 | 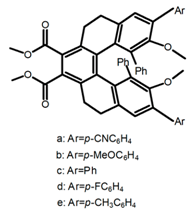 | (CH2)4O (5 × 10−5 M) | a | - | 459 | 48.0 | 5.33 | [107] | 2017 |
| b | - | 453 | 59.0 | 3.53 | |||||
| c | - | 453 | 40.0 | 6.08 | |||||
| d | - | 459 | 41.0 | 6.50 | |||||
| e | - | 453 | 42.0 | 4.63 | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, N.; Yan, B. Recent Theoretical and Experimental Progress in Circularly Polarized Luminescence of Small Organic Molecules. Molecules 2018, 23, 3376. https://doi.org/10.3390/molecules23123376
Chen N, Yan B. Recent Theoretical and Experimental Progress in Circularly Polarized Luminescence of Small Organic Molecules. Molecules. 2018; 23(12):3376. https://doi.org/10.3390/molecules23123376
Chicago/Turabian StyleChen, Naibo, and Bo Yan. 2018. "Recent Theoretical and Experimental Progress in Circularly Polarized Luminescence of Small Organic Molecules" Molecules 23, no. 12: 3376. https://doi.org/10.3390/molecules23123376





