Multielemental Analysis Associated with Chemometric Techniques for Geographical Origin Discrimination of Tea Leaves (Camelia sinensis) in Guizhou Province, SW China
Abstract
:1. Introduction
2. Results and Discussion
2.1. Differences in the Elemental Concentrations
2.2. Principal Component Analysis (PCA)
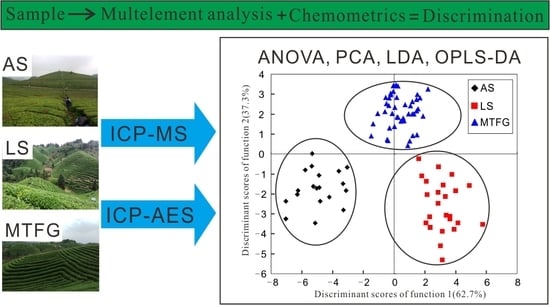
2.3. Linear Discriminant Analysis (LDA)
- Function 1 = − 0.492 As + 0.888 K + 0.368 La + 0.563 Na + 0.100 Nb − 0.880 Pb − 0.875 S + 0.591 Sb − 0.036 U
- Function 2 = 0.246 As + 0.366 K − 0.011 La − 0.365 Na + 0.593 Nb − 0.924 Pb + 0.148 S − 0.648 Sb + 0.625 U
- Group 1 (Anshun) = 175.669 As − 0.001 K − 23.971 La − 0.152 Na − 984.548 Nb + 69.683 Pb + 0.071 S − 291.927 Sb − 3695.292 U − 115.915
- Group 2 (Leishan) = 10.517 As + 0.003 K + 27.5 La + 0.162 Na − 813.812 Nb + 6.788 Pb + 0.041 S + 264.153 Sb − 4417.603 U − 94.021
- Group 3 (Meitan and Fenggang) = 110.136 As + 0.002 K + 9.008 La − 0.05 Na + 718.577 Nb − 12.620 Pb + 0.054 S − 225.517 Sb − 1800.63 U − 110.986
2.4. Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
3. Materials and Methods
3.1. Sample Collection
3.2. Sample preparation
3.3. Chemical Analysis
3.4. Quality Control
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Y.X.; Yu, M.G.; Xu, J.; Chen, X.C.; Shi, J.Y. Differentiation of eight tea (Camellia sinensis) cultivars in China by elemental fingerprint of their leaves. J. Sci. Food Agric. 2009, 89, 2350–2355. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, R.D.; Chen, R.; Peng, Y.S.; Wen, X.F.; Gao, L. Accumulation of heavy metals in tea leaves and potential health risk assessment: a case study from Puan county, Guizhou Province, China. Int. J. Environ. Res. Public Health 2018, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.T.; You, C.F.; Aggarwal, S.K.; Chung, C.H.; Chao, H.C.; Liu, H.C. Boron and strontium isotope ratios and major/trace elements concentrations in tea leaves at four major tea growing gardens in Taiwan. Environ. Geochem. Health 2016, 38, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.C.; Zhang, Y.B.; Zhang, J.Y.; Wang, G.Q.; Chen, L.Y.; Zhang, M.L.; Liu, T.; Liu, X.; Lu, C.Y. Determining the geographical origin of Chinese green tea by linear discriminant analysis of trace metals and rare earth elements: taking Dongting Biluochun as an example. Food Control 2016, 59, 714–720. [Google Scholar] [CrossRef]
- Ariyama, K.; Horita, H.; Yasui, A. Application of inorganic element ratios to chemometrics for determination of the geographic origin of Welsh onions. J. Agric. Food Chem. 2004, 52, 5803–5809. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.J.; Fechner, D.C.; Marchevsky, E.J.; Pelleranoa, R.G. Determining the geographical origin of Sechium edule fruits by multielement analysis and advanced chemometric techniques. Food Chem. 2016, 210, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, J.; Xiong, Y.B.; Qin, W.; Tang, C. Identification of the geographical origins of pomelos using multielement fingerprinting. J. Food Sci. 2015, 80, C228–C233. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Kim, J.K.; Lee, J.K.; Kim, S.H. Discrimination of geographical origin of rice (Oryza sativa L.) by multielement analysis using inductively coupled plasma atomic emission spectroscopy and multivariate analysis. J. Cereal Sci. 2015, 65, 252–259. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, J.K.; Prabakaran, M.; Yang, J.H.; Kim, S.H. Authenticity of rice (Oryza sativa L.) geographical origin based on analysis of C, N, O and S stable isotope ratios: a preliminary case report in Korea, China and Philippine. J. Sci. Food Agric. 2015, 96, 2433–2439. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, D.; Shree, P.; Singh, D.K. Stable isotope ratio analysis in determining the geographical traceability of Indian wheat. Food Control 2017, 79, 169–176. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, J.K.; Jin, Y.I.; Oh, Y.T.; Prabakaran, M.; Youn, K.J.; Kim, S.H. Discriminative study of a potato (Solanum tuberosum L.) cultivation region by measuring the stable isotope ratios of bio-elements. Food Chem. 2016, 212, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Wei, Y.M.; Wei, S.; Liu, H.Y.; Guo, B.L. Authentication of Zhongning wolfberry with geographical indication by mineral profile. Int. J. Food Sci. Tech. 2017, 52, 457–463. [Google Scholar] [CrossRef]
- Choi, Y.H.; Hong, C.K.; Kim, M.; Jung, S.O.; Park, J.; Oh, Y.H.; Kwon, J.H. Multivariate analysis to discriminate the origin of sesame seeds by multi-element analysis inductively coupled plasma-mass spectrometry. Food Sci. Biotech. 2017, 26, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Potortì, A.G.; Di Bella, G.; Mottese, A.F.; Bua, G.D.; Fede, M.R.; Sabatino, G.; Salvo, A.; Somma, R.; Dugo, G.; Turco, V.L. Traceability of protected geographical indication (PGI) interdonato lemon pulps by chemometric analysis of the mineral composition. J. Food Compos. Anal. 2018, 69, 122–128. [Google Scholar] [CrossRef]
- Albergamo, A.; Rotondo, A.; Salvo, A.; Pellizzeri, V.; Bua, D.G.; Maggio, A.; Cicero, N.; Dugo, G. Metabolite and mineral profiling of “Violetto di Niscemi” and “Spinoso di Menfi” globe artichokes by 1H-NMR and ICP-MS. Nat. Prod. Res. 2017, 31, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, A.; Salvo, A.; Giuffrida, D.; Dugo, G.; Rotondo, E. NMR analysis of aldehydes in Sicilian extra-virgin olive oils by DPFGSE techniques. AAPP Sci. Fis. Mat. Nat. 2011, 89, C1A8901002. [Google Scholar]
- Fragni, R.; Trifirò, A.; Nucci, A.; Seno, A.; Allodi, A.; Di Rocco, M. Italian tomato-based products authentication by multi-element approach: a mineral elements database to distinguish the domestic provenance. Food Control 2018, 93, 211–218. [Google Scholar] [CrossRef]
- Fan, L.X.; Chen, L.; Ding, R.Y.; Wang, L.; Zhang, B.C. Geographical discrimination of honeysuckle (Lonicera japonica Thunb.) from China by characterization of the stable isotope ratio and multielemental analysis. Anal. Lett. 2018, 51, 2507–2516. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Ma, X.F.; Fan, L.L.; Mao, F.Y.; Tian, H.L.; Xu, R.; Cao, Z.; Zhang, X.H.; Hu, X.Y.; Sui, H. Discrimination of geographical origin of cultivated Polygala tenuifolia based on multi-element fingerprinting by inductively coupled plasma mass spectrometry. Sci. Rep. 2017, 7, 12577. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Dong, Q.H.; Sun, W.J.; Huang, Y.; Wang, Q.Q.; Zhou, W.L. Discrimination of Chinese teas with different fermentation degrees by stepwise linear discriminant analysis (S-LDA) of the chemical compounds. J. Agric. Food Chem. 2014, 62, 9336–9344. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lee, B.J.; Chung, J.O.; Hwang, J.A.; Lee, S.J.; Lee, C.H.; Hong, Y.S. Geographical and climatic dependencies of green tea (Camellia sinensis) metabolites: a 1H NMR-based metabolomics study. J. Agri. Food Chem. 2010, 58, 10582–10589. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.X.; Wang, S.P.; Ning, J.M.; Xu, R.R.; Wang, Y.X.; Xing, Z.Q.; Wan, X.C.; Zhang, Z.Z. Quantitative analysis and geographical traceability of black tea using Fourier transform near-infrared spectroscopy (FT-NIRS). Food Res. Int. 2013, 53, 822–826. [Google Scholar] [CrossRef]
- Ye, N.S.; Zhang, L.Q.; Gu, X.X. Discrimination of green teas from different geographical origins by using HS-SPME/GC-MS and pattern recognition methods. Food Anal. Method 2012, 5, 856–860. [Google Scholar] [CrossRef]
- Chen, Q.S.; Zhao, J.W.; Guo, Z.M.; Wang, X.Y. Determination of caffeine content and main catechins contents in green tea (Camellia sinensis L.) using taste sensor technique and multivariate calibration. J. Food Compos. Anal. 2010, 23, 353–358. [Google Scholar] [CrossRef]
- Ni, K.; Wang, J.; Zhang, Q.F.; Yi, X.Y.; Ma, L.F.; Shi, Y.Z.; Ruan, J.Y. Multi-element composition and isotopic signatures for the geographical origin discrimination of green tea in China: A case study of Xihu Longjing. J. Food Compos. Anal. 2018, 67, 104–109. [Google Scholar] [CrossRef]
- Kelly, S.; Heaton, K.; Hoogewerff, J. Tracing the geographical origin of food: The application of multi-element and multi-isotope analysis. Trends Food Sci. Tech. 2005, 16, 555–567. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Zhang, S.L.; Zhang, Z.W. Relationship between multi-element composition in tea leaves and in provenance soils for geographical traceability. Food Control 2017, 76, 82–87. [Google Scholar] [CrossRef]
- Marcos, A.; Fisher, A.; Rea, G.; Hill, S.J. Preliminary study using trace element concentrations and a chemometrics approach to determine the geographical origin of tea. J. Anal. Atom. Spectrom. 1998, 13, 521–525. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, A.; Fisher, A.; Hill, S.J. The classification of tea according to region of origin using pattern recognition techniques and trace metal data. J. Food Compos. Anal. 2003, 16, 195–211. [Google Scholar] [CrossRef]
- Ye, X.H.; Jin, S.; Wang, D.H.; Zhao, F.; Yu, Y.; Zheng, D.Y.; Ye, N.X. Identification of the origin of white tea based on mineral element content. Food Anal. Methods 2017, 10, 191–199. [Google Scholar] [CrossRef]
- Brzezicha-Cirocka, J.; Grembecka, M.; Szefer, P. Monitoring of essential and heavy metals in green tea from different geographical origins. Environ. Monit. Assess. 2016, 188, 183. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture of the People’s Republic of China (MAPRC). The Limit of Chromium, Cadmium, Mercury, Arsenic and Fluoride in Tea; NY 659-2003; MAPRC: Beijing, China, 2003. (In Chinese) [Google Scholar]
- National Health and Family Planning Commission of the People’s Republic of China (NHFPCPRC) and China Food and Drug Administration (CFDA). National Standard for Food Safety: Limit of Contaminants in Food; GB2762-2017; NHFPCPRC and CFDA: Beijing, China, 2017. (In Chinese) [Google Scholar]
- Ministry of Agriculture of the People’s Republic of China (MAPRC). Green Food: Tea; NY/T 288-2012; MAPRC: Beijing, China, 2012. (In Chinese) [Google Scholar]
- Commission Regulation (EC), No. 629/2008 amending Regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official J. Eur. Union 2008, L173, 6–9. [Google Scholar]
- Gasser, U.; Klier, B.; Kuhn, A.V.; Steinhoff, B. Current findings on the heavy metal content in herbal drugs. Pharmeuropa 2009, 1, 37–49. [Google Scholar]
- Chen, Q.; Li, M.H.; Lin, L.R.; Xu, X.N. Research on determination and correlation of rare earth content in tea soil, root, leaf of tea area in Eastern Fujian. Qual Tech Supervision Res. 2013, 4, 19–23. (In Chinese) [Google Scholar]
- Shi, J.C.; Wang, G.; He, Y.; Wu, J.J.; Xu, J.M. Lead accumulation in Westlake Longjing tea: Non-edaphic genesis as revealed by regional scale estimate. J. Soils Sediments 2010, 10, 933–942. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, J.K.; Lee, K.J.; Lee, K.J.; Park, S.K.; Lee, J.H.; Son, N.Y.; Jin, Y.I.; Kim, S.H. Geographic authentication of Asian rice (Oryza sativa L.) using multi-elemental and stable isotopic data combined with multivariate analysis. Food Chem. 2018, 240, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Kara, D. Evaluation of trace metal concentrations in some herbs and herbal teas by principal component analysis. Food Chem. 2009, 114, 347–354. [Google Scholar] [CrossRef]
- Bertoldi, D.; Barbero, A.; Camin, F.; Caligiani, A.; Larcher, R. Multielemental fingerprinting and geographic traceability of Theobroma cacao beans and cocoa products. Food Control 2016, 65, 46–53. [Google Scholar] [CrossRef]
- Fragni, R.; Trifirò, A.; Nucci, A. Towards the development of a multi-element analysis by ICP-oa-TOF-MS for tracing the geographical origin of processed tomato products. Food Control 2015, 48, 96–101. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (OPLS). J. Chemometrics 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Jumtee, K.; Bamba, T.; Fukusaki, E. Fast GC-FID based metabolic fingerprinting of Japanese green tea leaf for its quality ranking prediction. J. Separat. Sci. 2009, 32, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Kim, J.K.; Lee, J.H.; An, M.J.; Lee, K.J.; Park, S.K.; Kim, J.U.; Kim, M.J.; Kim, S.H. C/N/O/S stable isotopic and chemometric analyses for determining the geographical origin of Panax ginseng cultivated in Korea. J. Ginseng Res. 2018, 42, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Han, J.G.; Kong, W.S.; Kim, J.K.; An, M.J.; Lee, J.H.; An, Y.J.; Jung, M.Y.; Kim, S.H. Regional discrimination of Agaricus bisporus mushroom using the natural stable isotope ratios. Food Chem. 2018, 264, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Guo, B.L.; Wei, Y.M.; Zhang, B. Effects of wheat origin, genotype, and their interaction on multielement fingerprints for geographical traceability. J. Agric. Food Chem. 2012, 60, 10957–10962. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Yu, C.D.; Li, M. Effects of geographical origin, variety, season and their interactions on minerals in tea for traceability. J. Food Compos. Anal. 2017, 63, 15–20. [Google Scholar] [CrossRef]
- Pilgrim, T.S.; Watling, R.J.; Grice, K. Application of trace element and stable isotope signatures to determine the provenance of tea (Camellia sinensis) samples. Food Chem. 2010, 118, 921–926. [Google Scholar] [CrossRef]
- Canizoa, B.V.; Escuderoa, L.B.; Péreza, M.B.; Pelleranob, R.G.; Wuillouda, R.G. Intra-regional classification of grape seeds produced in Mendoza province (Argentina) by multi-elemental analysis and chemometrics tools. Food Chem. 2018, 242, 272–278. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |





| Element | AS (n = 19) | LS (n = 24) | MTFG (n = 44) | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | ||
| Al b | 270 | 540 | 371 | 72 | 240 | 530 | 416 | 78 | 200 | 630 | 420 | 100 | 0.126 c |
| As b | 0.073 | 0.221 | 0.128 | 0.039 | 0.020 | 0.112 | 0.050 | 0.024 | 0.028 | 0.099 | 0.059 | 0.018 | 0.000 d |
| Ba b | 7.3 | 31.2 | 12.7 | 5.6 | 7.4 | 19.7 | 12.8 | 3.5 | 2.7 | 28.4 | 9.7 | 5.1 | 0.018 d |
| Bi b | 0.006 | 0.022 | 0.013 | 0.004 | 0.002 | 0.010 | 0.005 | 0.002 | 0.002 | 0.013 | 0.006 | 0.002 | 0.000 d |
| Ca b | 0.29 | 0.52 | 0.39 | 0.07 | 0.20 | 0.31 | 0.25 | 0.03 | 0.22 | 0.42 | 0.31 | 0.05 | 0.000 d |
| Cd b | 0.028 | 0.088 | 0.051 | 0.019 | 0.016 | 0.243 | 0.053 | 0.046 | 0.022 | 0.180 | 0.081 | 0.034 | 0.001 d |
| Ce b | 0.071 | 0.345 | 0.156 | 0.082 | 0.037 | 0.162 | 0.096 | 0.038 | 0.047 | 0.828 | 0.215 | 0.163 | 0.001 d |
| Co b | 0.068 | 1.530 | 0.419 | 0.443 | 0.065 | 0.676 | 0.320 | 0.171 | 0.152 | 2.610 | 0.628 | 0.530 | 0.019 d |
| Cr b | 0.49 | 1.85 | 0.91 | 0.35 | 0.13 | 0.73 | 0.41 | 0.19 | 0.19 | 1.16 | 0.53 | 0.25 | 0.000 d |
| Cs b | 0.090 | 1.740 | 0.480 | 0.438 | 0.052 | 0.592 | 0.220 | 0.148 | 0.018 | 0.752 | 0.162 | 0.179 | 0.000 d |
| Cu b | 15.10 | 26.10 | 18.50 | 2.55 | 10.80 | 24.10 | 17.28 | 3.36 | 11.40 | 22.10 | 16.94 | 2.35 | 0.115 c |
| Dy a | 4.5 | 23.9 | 10.2 | 5.6 | 1.9 | 22.6 | 6.0 | 4.5 | 3.0 | 24.5 | 9.7 | 5.4 | 0.011 d |
| Er a | 2.4 | 17.8 | 5.8 | 3.5 | 0.8 | 13.9 | 3.1 | 3.2 | 1.3 | 17.6 | 5.2 | 3.6 | 0.028 d |
| Fe b | 57 | 275 | 115 | 50 | 66 | 189 | 111 | 28 | 83 | 173 | 114 | 20 | 0.934 c |
| Gd a | 5.0 | 35.7 | 12.4 | 7.7 | 2.1 | 28.5 | 8.2 | 6.2 | 4.3 | 27.9 | 12.0 | 6.8 | 0.065 c |
| Ho a | 0.8 | 6.4 | 2.0 | 1.4 | 0.1 | 4.9 | 1.1 | 1.0 | 0.4 | 4.9 | 1.8 | 1.1 | 0.025 d |
| K b | 1.38 | 2.29 | 1.63 | 0.21 | 1.63 | 2.23 | 1.94 | 0.17 | 1.72 | 2.39 | 2.06 | 0.16 | 0.000 d |
| La b | 0.036 | 0.181 | 0.079 | 0.044 | 0.016 | 0.178 | 0.059 | 0.039 | 0.029 | 0.295 | 0.100 | 0.070 | 0.021 d |
| Li b | 0.05 | 0.22 | 0.10 | 0.04 | 0.02 | 0.13 | 0.06 | 0.03 | 0.04 | 0.17 | 0.08 | 0.03 | 0.001 d |
| Mg b | 0.15 | 0.28 | 0.19 | 0.03 | 0.15 | 0.23 | 0.19 | 0.02 | 0.15 | 0.24 | 0.20 | 0.02 | 0.185 c |
| Mn b | 159 | 2050 | 921 | 570 | 273 | 1555 | 743 | 331 | 205 | 2290 | 836 | 450 | 0.436 c |
| Na b | 20 | 70 | 47 | 13 | 30 | 100 | 73 | 19 | 30 | 80 | 52 | 15 | 0.000 d |
| Nb a | 2.6 | 12.2 | 5.3 | 2.3 | 0.5 | 5.4 | 2.6 | 1.3 | 1.3 | 7.8 | 3.6 | 1.5 | 0.000 d |
| Nd b | 0.022 | 0.149 | 0.057 | 0.034 | 0.009 | 0.122 | 0.041 | 0.029 | 0.021 | 0.188 | 0.067 | 0.043 | 0.027 d |
| Ni b | 11.55 | 34.50 | 18.78 | 6.28 | 5.55 | 19.65 | 11.58 | 4.03 | 6.63 | 33.00 | 13.24 | 4.88 | 0.000 d |
| P b | 0.476 | 0.620 | 0.541 | 0.040 | 0.406 | 0.551 | 0.482 | 0.042 | 0.427 | 0.639 | 0.559 | 0.049 | 0.000 d |
| Pb b | 0.413 | 0.937 | 0.574 | 0.153 | 0.106 | 0.476 | 0.268 | 0.092 | 0.093 | 0.463 | 0.218 | 0.073 | 0.000 d |
| Pr b | 0.006 | 0.037 | 0.015 | 0.008 | 0.003 | 0.035 | 0.011 | 0.008 | 0.006 | 0.053 | 0.019 | 0.012 | 0.019 d |
| Rb b | 16.7 | 83.0 | 53.0 | 19.0 | 21.7 | 135.0 | 66.8 | 25.2 | 16.7 | 161.0 | 53.0 | 31.3 | 0.116 c |
| S b | 0.271 | 0.390 | 0.322 | 0.029 | 0.252 | 0.316 | 0.289 | 0.017 | 0.282 | 0.383 | 0.333 | 0.024 | 0.000 d |
| Sb b | 0.021 | 0.070 | 0.038 | 0.010 | 0.022 | 0.071 | 0.045 | 0.014 | 0.009 | 0.032 | 0.019 | 0.006 | 0.000 d |
| Se b | 0.07 | 0.18 | 0.11 | 0.03 | 0.04 | 0.17 | 0.09 | 0.03 | 0.05 | 0.21 | 0.11 | 0.04 | 0.114 c |
| Sm b | 0.003 | 0.034 | 0.011 | 0.007 | 0.002 | 0.025 | 0.008 | 0.006 | 0.003 | 0.040 | 0.013 | 0.009 | 0.054 c |
| Sr b | 5.28 | 12.85 | 7.89 | 2.23 | 2.51 | 11.15 | 5.88 | 1.81 | 3.30 | 18.60 | 6.92 | 3.21 | 0.055 c |
| Th b | 0.003 | 0.037 | 0.008 | 0.007 | 0.002 | 0.010 | 0.005 | 0.002 | 0.002 | 0.012 | 0.006 | 0.002 | 0.006 d |
| Tl b | 0.009 | 0.044 | 0.024 | 0.012 | 0.003 | 0.030 | 0.012 | 0.006 | 0.004 | 0.139 | 0.021 | 0.022 | 0.058 c |
| U a | 2.8 | 8.6 | 4.8 | 1.5 | 1.0 | 4.3 | 2.3 | 0.7 | 1.8 | 6.6 | 4.4 | 1.2 | 0.000 d |
| W b | 0.010 | 0.235 | 0.060 | 0.069 | 0.007 | 0.193 | 0.033 | 0.037 | 0.011 | 0.096 | 0.033 | 0.018 | 0.033 d |
| Y b | 0.033 | 0.191 | 0.074 | 0.045 | 0.008 | 0.113 | 0.032 | 0.024 | 0.024 | 0.174 | 0.067 | 0.033 | 0.000 d |
| Zn b | 43.8 | 62.2 | 52.4 | 5.0 | 41.1 | 59.8 | 48.3 | 5.5 | 40.6 | 75.7 | 50.9 | 6.0 | 0.058 c |
| Tea region | Model | Verification Samples | Predicted Group Membership | Correctly Classified (%) | ||
|---|---|---|---|---|---|---|
| AS | LS | MTFG | ||||
| AS | LDA | 19 | 19 | 0 | 0 | 100 |
| LS | 24 | 0 | 23 | 1 | 95.8 | |
| MTFG | 44 | 0 | 0 | 44 | 100 | |
| Total | 87 | 19 | 23 | 45 | 98.9 | |
| AS | OPLS-DA | 19 | 19 | 0 | 0 | 100 |
| LS | 24 | 0 | 24 | 0 | 100 | |
| MTFG | 44 | 0 | 0 | 44 | 100 | |
| Total | 87 | 19 | 24 | 44 | 100 | |
| Elements | LOD (μg·g−1) | LOQ (μg·g−1) | Recoveries of CRMs (%) | RDDS (%) |
|---|---|---|---|---|
| Al | 10 | 25,000 | 77.78−128.57 | 0.00−7.84 |
| As | 0.005 | 10,000 | 88.74−108.53 | 1.35−18.75 |
| Ba | 0.1 | 9000 | 87.88−103.03 | 0.00−2.13 |
| Bi | 0.001 | 9000 | 83.33−117.39 | 0.00−33.33 |
| Ca | 10 | 40,000 | 93.25−109.68 | 0.00−3.85 |
| Cd | 0.001 | 2000 | 85.71−128.57 | 0.00−9.23 |
| Ce | 0.001 | 500 | 90.46−105.88 | 0.72−6.90 |
| Co | 0.002 | 9000 | 91.12−109.52 | 0.17−5.64 |
| Cr | 0.05 | 10,000 | 90.13−113.92 | 2.00−34.18 |
| Cs | 0.001 | 500 | 95.00−115.00 | 0.84−12.50 |
| Cu | 0.01 | 9000 | 92.34−109.91 | 0.23−5.61 |
| Dy | 0.0005 | 1000 | 85.00−110.00 | 2.08−23.53 |
| Er | 0.0005 | 1000 | 81.40−125.00 | 0.00−17.24 |
| Fe | 1 | 50,000 | 89.76−109.34 | 0.38−5.22 |
| Gd | 0.0005 | 1000 | 84.92−103.70 | 0.70−17.33 |
| Ho | 0.0001 | 1000 | 87.50−106.25 | 0.00−33.33 |
| K | 10 | 100,000 | 93.68−108.84 | 0.27−2.20 |
| La | 0.001 | 9000 | 90.65−107.41 | 0.00−8.33 |
| Li | 0.02 | 10,000 | 86.67−113.33 | 0.00−11.11 |
| Mg | 10 | 300,000 | 91.11−110.34 | 0.23−2.36 |
| Mn | 0.1 | 50,000 | 91.05−110.31 | 0.45−3.09 |
| Na | 10 | 100,000 | 80.00−120.00 | 0.00−25.00 |
| Nb | 0.0005 | 500 | 82.50−112.80 | 3.23−20.00 |
| Nd | 0.001 | 1000 | 84.14−105.00 | 0.00−18.52 |
| Ni | 0.02 | 9000 | 92.44−109.00 | 0.00−5.57 |
| P | 5 | 50,000 | 93.22−108.55 | 0.21−2.52 |
| Pb | 0.005 | 9000 | 89.05−109.52 | 0.11−7.64 |
| Pr | 0.001 | 1000 | 83.33−112.90 | 0.00−11.11 |
| Rb | 0.01 | 9000 | 97.99−108.43 | 0.39−8.14 |
| S | 10 | 100,000 | 93.10−110.10 | 0.00−2.48 |
| Sb | 0.002 | 9000 | 82.47−107.10 | 1.82−22.08 |
| Se | 0.02 | 1000 | 96.23−117.39 | 0.00−12.50 |
| Sm | 0.001 | 1000 | 82.61−114.81 | 0.00−25.00 |
| Sr | 0.02 | 10,000 | 96.49−107.84 | 0.25−6.32 |
| Th | 0.001 | 1000 | 87.50−106.45 | 0.00−23.08 |
| Tl | 0.001 | 1000 | 94.87−115.38 | 0.00−5.75 |
| U | 0.0005 | 9000 | 87.97−112.78 | 0.00−32.08 |
| W | 0.002 | 9000 | 86.21−115.25 | 0.00−20.55 |
| Y | 0.001 | 500 | 89.55−110.45 | 0.65−8.40 |
| Zn | 0.1 | 9000 | 93.62−109.12 | 0.20−5.23 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yang, R.; Chen, R.; Li, Y.C.; Peng, Y.; Liu, C. Multielemental Analysis Associated with Chemometric Techniques for Geographical Origin Discrimination of Tea Leaves (Camelia sinensis) in Guizhou Province, SW China. Molecules 2018, 23, 3013. https://doi.org/10.3390/molecules23113013
Zhang J, Yang R, Chen R, Li YC, Peng Y, Liu C. Multielemental Analysis Associated with Chemometric Techniques for Geographical Origin Discrimination of Tea Leaves (Camelia sinensis) in Guizhou Province, SW China. Molecules. 2018; 23(11):3013. https://doi.org/10.3390/molecules23113013
Chicago/Turabian StyleZhang, Jian, Ruidong Yang, Rong Chen, Yuncong C. Li, Yishu Peng, and Chunlin Liu. 2018. "Multielemental Analysis Associated with Chemometric Techniques for Geographical Origin Discrimination of Tea Leaves (Camelia sinensis) in Guizhou Province, SW China" Molecules 23, no. 11: 3013. https://doi.org/10.3390/molecules23113013