Theoretical Investigations on the Reactivity of Hydrogen Peroxide toward 2,3,7,8-Tetrachlorodibenzo-p-dioxin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Reaction of Neutral H2O2 with TCDD
2.1.1. Direct Reaction of the Neutral H2O2 with TCDD
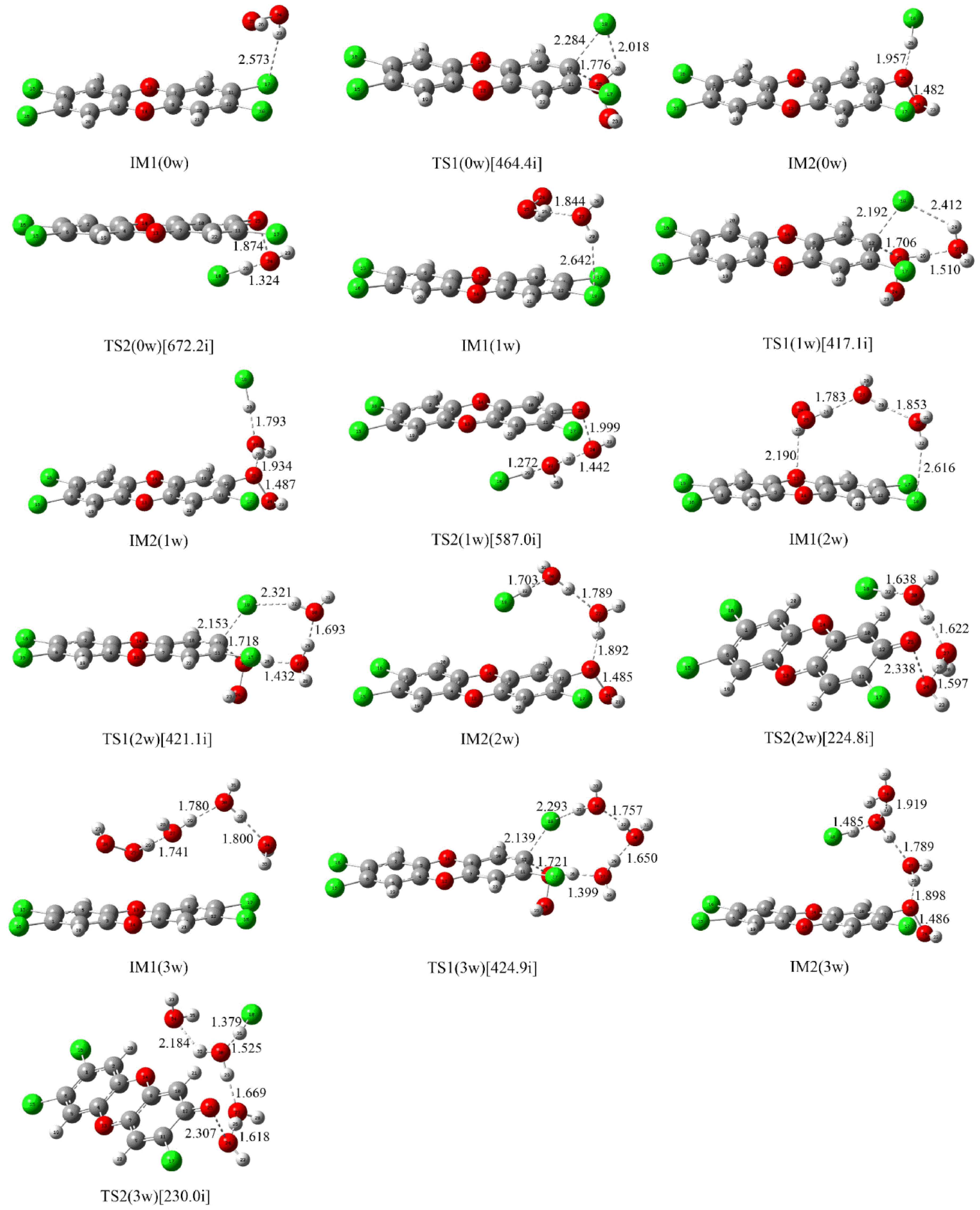
2.1.2. Water-Assisted Reaction of Neutral H2O2 with TCDD
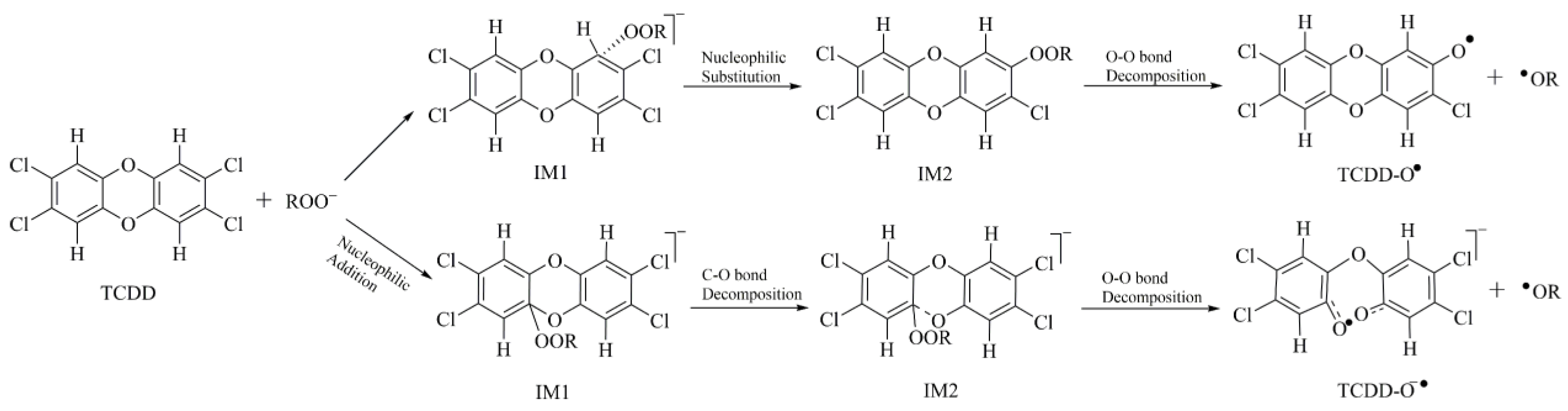
Formation of the Initial Intermediate
Nucleophilic Substitution Process
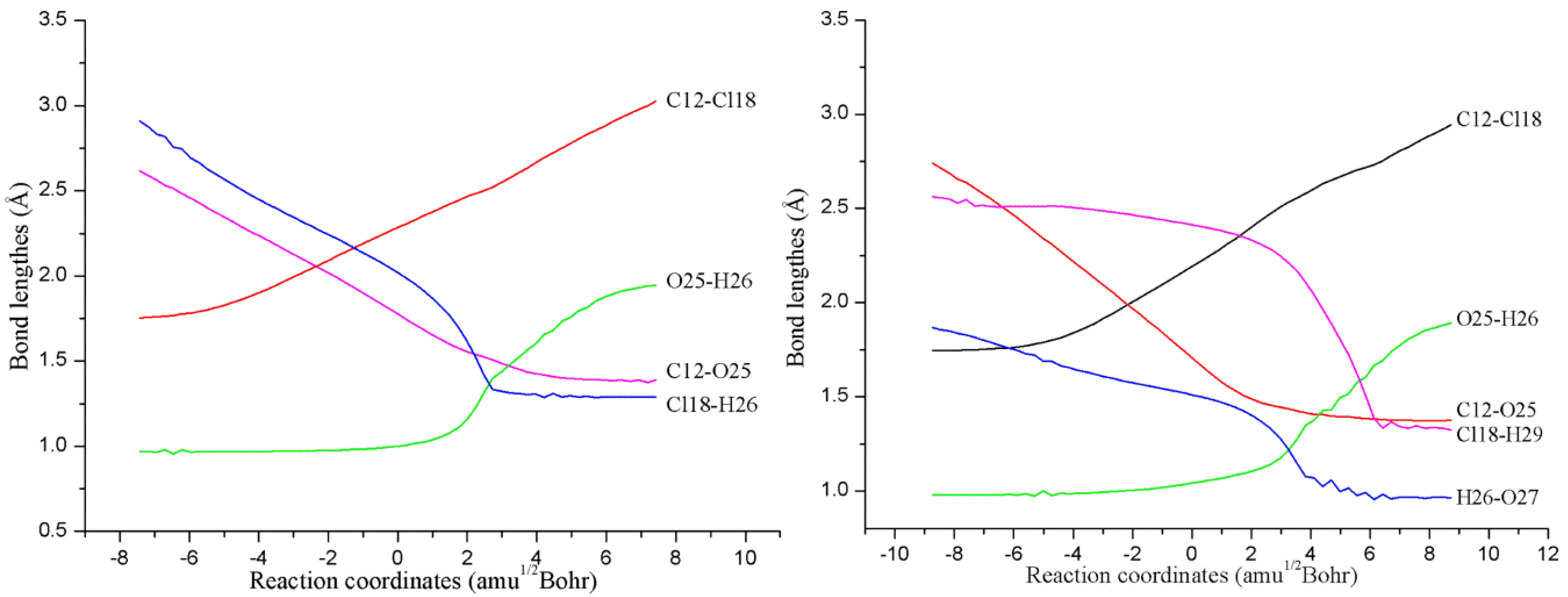
Cleavage of the O-O Bond
2.2. Reaction of TCDD with HO2− Anion
2.2.1. Nucleophilic Substitution Process
2.2.2. Nucleophilic Addition Process
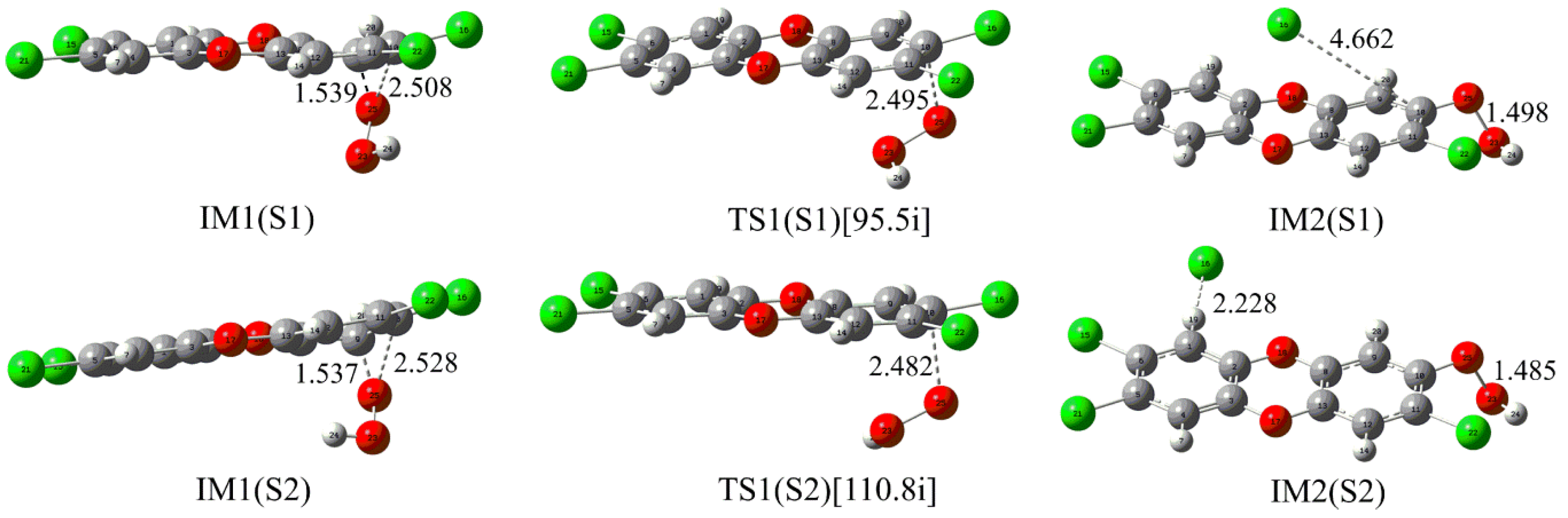
2.3. Substitution Effects
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zou, L.L.; Ni, Y.W.; Gao, Y.; Tang, F.M.; Jin, J.; Chen, J.P. Spatial variation of PCDD/F and PCB emissions and their composition profiles in stack flue gas from the typical cement plants in China. Chemosphere 2018, 195, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Sappington, E.N.; Balasubramani, A.; Rifai, H.S. Polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (PCDD/Fs) in municipal and industrial effluents. Chemosphere 2015, 133, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Ni, Y.W.; Zhang, H.J.; Zhang, X.P.; Chen, J.P. Formation and emission of PCDD/Fs in Chinese non-wood pulp and paper mills. Environ. Sci. Technol. 2012, 46, 12234–12240. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Ni, Y.; Du, Q.; Zhang, X.; Chen, J. Kinetics of PCDD/Fs formation from non-wood pulp bleaching with chlorine. Environ. Sci. Technol. 2014, 48, 4361–4367. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, M.; Chandia, C.; Hernandez, A. Impact of forest fires on the concentrations of polychlorinated dibenzo-p-dioxin and dibenzofurans in coastal waters of central Chile. Sci. Total Environ. 2016, 573, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, H.; Themelis, N.J. Inventory of US 2012 dioxin emissions to atmosphere. Waste Manag. 2015, 46, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Heeb, N.V.; Rey, M.D.; Zennegg, M.; Haag, R.; Wichser, A.; Schmid, P.; Seiler, C.; Honegger, P.; Zeyer, K.; Mohn, J.; et al. Biofuel-promoted polychlorinated dibenzodioxin/furan formation in an iron-catalyzed diesel particle filter. Environ. Sci. Technol. 2015, 49, 9273–9279. [Google Scholar] [CrossRef] [PubMed]
- Heeb, N.V.; Zennegg, M.; Haag, R.; Wichser, A.; Schmid, P.; Seiler, C.; Ulrich, A.; Honegger, P.; Zeyer, K.; Emmenegger, L.; et al. PCDD/F formation in an iron/potassium-catalyzed diesel particle filter. Environ. Sci. Technol. 2013, 47, 6510–6517. [Google Scholar] [CrossRef] [PubMed]
- Altarawneh, M.; Dlugogorski, B.Z.; Kennedy, E.M.; Mackie, J.C. Mechanisms for formation, chlorination, dechlorination and destruction of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs). Prog. Energy Combust. Sci. 2009, 35, 245–274. [Google Scholar] [CrossRef]
- Hidayat, A.; Tachibana, S. Degradation of 2,4,8-trichlorodibenzofuran by a new isolate of Cerrena sp. F0607. Int. Biodeter. Biodegr. 2013, 77, 51–55. [Google Scholar] [CrossRef]
- Govindan, M.; Moon, I.S. Expeditious removal of PCDD/Fs from industrial waste incinerator fly ash using electrogenerated homogeneous Ag (II) ions. Chem. Eng. J. 2015, 272, 145–150. [Google Scholar] [CrossRef]
- Palanisami, N.; Chung, S.J.; Moon, I.S. Cerium (IV)-mediated electrochemical oxidation process for removal of polychlorinated dibenzo-p-dioxins and dibenzofurans. J. Ind. Eng. Chem. 2015, 28, 28–31. [Google Scholar] [CrossRef]
- Liljelind, P.; Unsworth, J.; Maaskant, O.; Marklund, S. Removal of dioxins and related aromatic hydrocarbons from flue gas streams by adsorption and catalytic destruction. Chemosphere 2001, 42, 615–623. [Google Scholar] [CrossRef]
- Finocchio, E.; Busca, G.; Notaro, M. A review of catalytic processes for the destruction of PCDD and PCDF from waste gases. Appl. Catal. B 2006, 62, 12–20. [Google Scholar] [CrossRef]
- Daikoku, T.; Takemoto, M.; Yoshida, Y.; Okuda, T.; Takahashi, Y.; Ota, K.; Tokuoka, F.; Kawaguchi, A.T.; Shiraki, K. Decomposition of organic chemicals in the air and inactivation of aerosol-associated influenza infectivity by photocatalysis. Aerosol Air Qual. Res. 2015, 15, 1469–1484. [Google Scholar] [CrossRef]
- Huang, L.Y.; Su, G.J.; Liu, Y.X.; Li, L.W.; Liu, S.; Lu, H.J.; Zheng, M.H. Effect of NiFe2O4 on PCDF byproducts formation during thermal degradation of decachlorobiphenyl. RSC Adv. 2014, 4, 25453–25460. [Google Scholar] [CrossRef]
- Yu, M.F.; Lin, X.Q.; Li, X.D.; Chen, T.; Yan, J.H. Catalytic decomposition of PCDD/Fs over nano-TiO2 based V2O5/CeO2 catalyst at low temperature. Aerosol Air Qual. Res. 2016, 16, 2011–2022. [Google Scholar] [CrossRef]
- Hung, P.C.; Chang, S.H.; Ou-Yang, C.C.; Chang, M.B. Simultaneous removal of PCDD/Fs, pentachlorophenol and mercury from contaminated soil. Chemosphere 2016, 144, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, Y.; Onwudili, J.A.; Williams, P.T. Removal potential of toxic 2,3,7,8-substituted PCDD/F from incinerator flue gases by waste-derived activated carbons. Waste Manag. 2011, 31, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, A.; Katayama, M.; Matsumoto, N.; Honda, K. Physicochemical characteristics of carbonaceous adsorbent for dioxin-like polychlorinated biphenyl adsorption. Chemosphere 2011, 83, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Sun, S.M.; Zhang, H. Mechanism and kinetic study on the ring-opening degradation of 2,3,7,8-tetrachlorinated dibenzofuran initiated by OH radicals in waste incineration. RSC Adv. 2015, 5, 81153–81161. [Google Scholar] [CrossRef]
- Sun, X.M.; Zhang, C.X.; Zhao, Y.Y.; Bai, J.; Zhang, Q.Z.; Wang, W.X. Atmospheric chemical reactions of 2,3,7,8-tetrachlorinated dibenzofuran initiated by an OH radical: Mechanism and kinetics study. Environ. Sci. Technol. 2012, 46, 8148–8155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Sun, X.M.; Xu, Y.S.; Qi, C.S.; Zhang, J.H. Atmospheric chemistry of 2,3,7,8-TCDD/F: Mechanism and kinetics study. J. Environ. Chem. Eng. 2014, 2, 1098–1103. [Google Scholar] [CrossRef]
- Pan, W.X.; Qi, Y.Y.; Wang, R.X.; Han, Z.; Zhang, D.J.; Zhan, J.H. Adsorption of TCDD with 1-butyl-3-methylimidazolium dicyanamide ionic liquid: A combined molecular dynamics simulation and quantum chemistry study. Chemosphere 2013, 91, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.X.; Yong, Y.L.; Ju, W.W.; Su, X.Y.; Li, X.H.; Wang, C.Y.; Fu, Z.B. DFT study of the adsorption of 2,3,7,8-tetrachlorodibenzofuran (TCDF) on vacancy-defected graphene doped with Mn and Fe. Curr. Appl. Phys. 2018, 18, 61–67. [Google Scholar] [CrossRef]
- Weber, R.; Sakurai, T.; Hagenmaier, H. Formation and destruction of PCDD/F during heat treat treatment of fly ash samples from fluidized bed incinerators. Chemosphere 1999, 38, 2633–2642. [Google Scholar] [CrossRef]
- Lundin, L.; Marklund, S. Thermal degradation of PCDD/F in municipal solid waste ashes in sealed glass ampules. Environ. Sci. Technol. 2005, 39, 3872–3877. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.Z.; Kalyanaraman, B.; Jiang, G.B. Molecular mechanism for metal-independent production of hydroxyl radicals by hydrogen peroxide and halogenated quinines. Proc. Natl. Acad. Sci. USA 2007, 104, 17575–17578. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.Z.; Zhao, H.T.; Kalyanaraman, B.; Liu, J.; Shan, G.Q.; Du, Y.G.; Frei, B. Mechanism of metal-independent decomposition of organic hydroperoxides and formation of alkoxyl radicals by halogenated quinones. Proc. Natl. Acad. Sci. USA 2007, 104, 3698–3702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, B.Z.; Shan, G.Q.; Huang, C.H.; Kalyanaraman, B.; Mao, L.; Du, Y.G. Metal-independent decomposition of hydroperoxides by halogenated quinones: Detection and identification of a quinone ketoxy radical. Proc. Natl. Acad. Sci. USA 2009, 106, 11466–11471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.H.; Ren, F.R.; Shan, G.Q.; Qin, H.; Mao, L.; Zhu, B.Z. Molecular mechanism of metal-independent decomposition of organic hydroperoxides by halogenated quinoid carcinogens and the potential biological implications. Chem. Res. Toxicol. 2015, 28, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Zhang, D.; Song, Y.; Zhu, B.Z.; Wang, H. Potent DNA damage by polyhalogenated quinones and H2O2 via a metal-independent and intercalation-enhanced oxidation mechanism. Sci. Rep. 2013, 3, 1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Wang, W.H.; Sun, Q.; Li, Z.; Du, A.J.; Bi, S.W.; Zhao, Y. Insights into the mechanism of the reaction between tetrachloro-p-benzoquinone and hydrogen peroxide and their implications in the catalytic role of water molecules in producing the hydroxyl radical. Chem. Phys. Chem. 2013, 14, 2737–2743. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Guo, C.; Feng, W.L.; Sun, Q.; Wang, W.H. A DFT study on the reaction mechanism between tetrachloro-o-benzoquinone and H2O2 and an alternative reaction approach to produce the hydroxyl radical. RSC Adv. 2017, 7, 22919–22926. [Google Scholar] [CrossRef]
- Arulmozhiraja, S.; Morita, M. Electron affinities and reductive dechlorination of toxic polychlorinated dibenzofurans: A density functional theory study. J. Phys. Chem. A 2004, 108, 3499–3508. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Tao, F.M.; Zeng, E.Y. Structures, reductive dechlorination, and electron affinities of selected polychlorinated dibenzo-p-dioxins: Density functional theory study. J. Phys. Chem. A 2007, 111, 11638–11644. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, W.H.; Feng, W.L.; Li, P. Insights into the one-electron reduction behavior of tetrachloro-o-benzoquinone: A DFT and molecular dynamics study. RSC Adv. 2017, 7, 12775–12782. [Google Scholar] [CrossRef]
- Li, P.; Wang, W.H.; Sun, H.T.; Bi, S.W. A DFT study on the electron affinity of tetrachloro-p-benzoquinone: Toward to understanding its electron-accepting ability in solution. Comput. Theor. Chem. 2013, 1006, 127–132. [Google Scholar] [CrossRef]
- Wei, W.J.; Wang, W.H.; Xu, K.N.; Feng, W.L.; Li, X.P.; Li, P. Theoretical insights into the reaction mechanisms between 2,3,7,8-tetrachlorodibenzofuran and the methylidyne radical. RSC Adv. 2018, 8, 21150–21163. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.H.; Feng, W.L.; Wang, W.L.; Li, P. Theoretical insights into the interaction mechanisms between nitric acid and nitrous oxide initiated by an excess electron. J. Phys. Chem. A 2018, 122, 7312–7319. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Feng, W.L.; Wang, W.L.; Li, P. Theoretical investigations on the reactivity of methylidyne radical toward 2,3,7,8-tetrachlorodibenzo-p-dioxin: A DFT and molecular dynamics study. Molecules 2018, 23, 2685. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Feng, W.L.; Wang, W.L.; Li, P. Theoretical insights into the electron capture behavior of H2SO4···N2O complex: A DFT and molecular dynamics study. Molecules 2018, 23, 2349. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Shi, X.; Zhang, Q.; Wang, W. Mechanism for the growth of polycyclic aromatic hydrocarbons from the reactions of naphthalene with cyclopentadienyl and indenyl. Chemosphere 2016, 162, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Lv, X.; Pan, L.; Lu, L.; Ge, Z.; Wang, J.; Wang, J.; Liu, J.; Liu, W.; Zhang, C. Benzotriazole UV 328 and UV-P showed distinct antiandrogenic activity upon human CYP3A4-mediated biotransformation. Environ. Pollut. 2017, 220, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shi, X.; Sun, Y.; Zhang, Q.; Wang, W. The growth mechanism of polycyclic aromatic hydrocarbons from the reactions of anthracene and phenanthrene with cyclopentadienyl and indenyl. Chemosphere 2017, 189, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, W.; Wei, W.; Feng, W.; Sun, Q.; Li, P. Insights into the reaction mechanism of Criegee intermediate CH2OO with methane and implications for the formation of methanol. J. Phys. Chem. A 2017, 121, 7236–7245. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Zhang, X.X.; Li, P.; Sun, Q.; Li, Z.; Ren, C.; Guo, C. CO2 capture and separation from N2/CH4 mixtures by Co@B8/Co@B8− and M@B9/M@B9− (M = Ir, Rh, Ru) clusters: A theoretical study. J. Phys. Chem. A 2015, 119, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.L.; Ren, C.; Wang, W.H.; Guo, C.; Sun, Q.; Li, P. An identification of the C–C bonding spin adduct in the spin trapping of N-methyl benzohydroxamic acid radical by 5,5-dimethyl-1-pyrroline-N-oxide. Theor. Chem. Acc. 2016, 135, 190. [Google Scholar] [CrossRef]
- Feng, W.L.; Ren, C.; Wang, W.H.; Guo, C.; Sun, Q.; Li, P. Theoretical studies on the spin trapping of the 2-chloro-5-hydroxy-1,4-benzoquinone radical by 5,5-dimethyl-1-pyrroline N-oxide (DMPO): The identification of the C–O bonding spin adduct. RSC Adv. 2016, 6, 48099–48108. [Google Scholar] [CrossRef]
- Wang, W.H.; Guo, C.; Feng, W.L.; Sun, Q.; Li, P. Theoretical insights into the reaction mechanism between tetrachloro-o-benzoquinone and N-methyl benzohydroxamic acid. RSC Adv. 2017, 7, 32419–32426. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Bu, Y.X. Multiwater-assisted proton transfer study in glycinamide using density functional theory. J. Phys. Chem. B 2004, 108, 18088–18097. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009.
Sample Availability: Samples of the compounds are not available from the authors. |
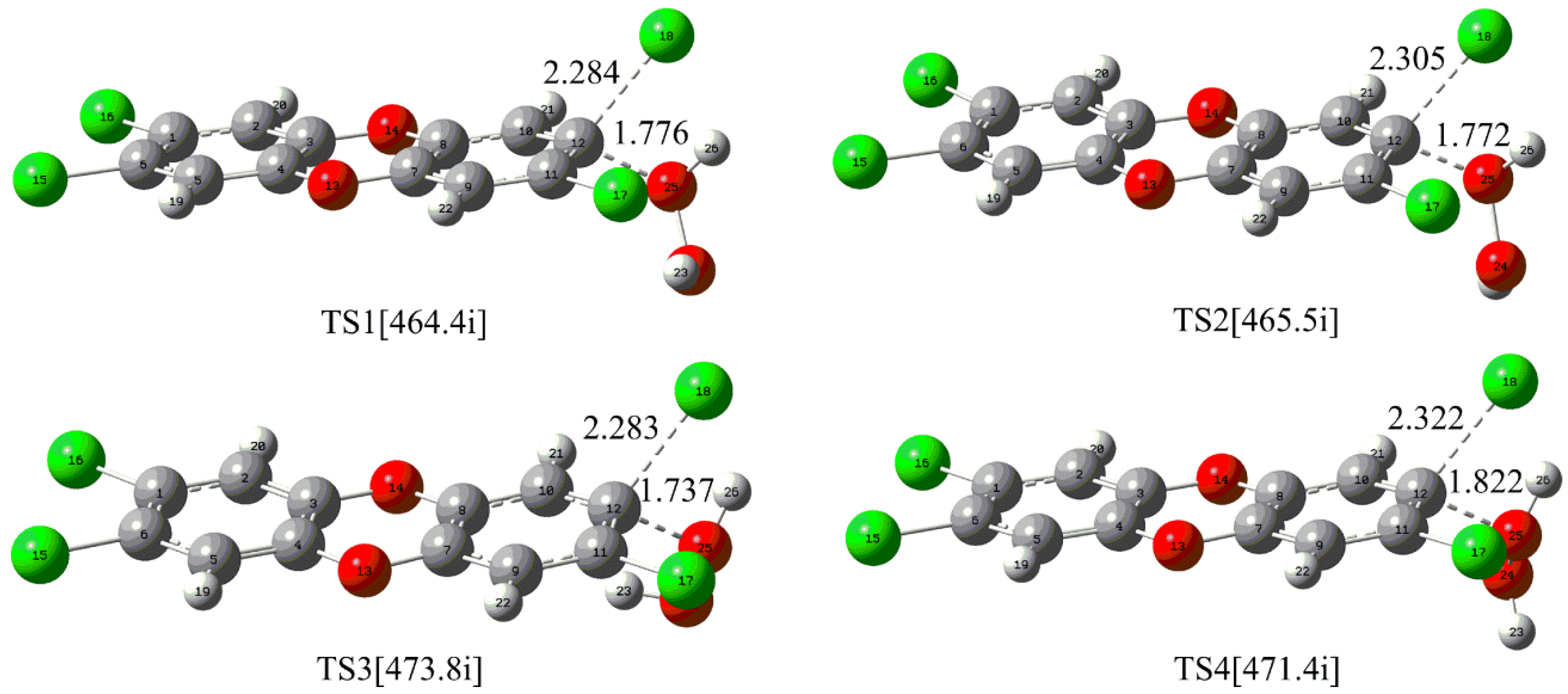

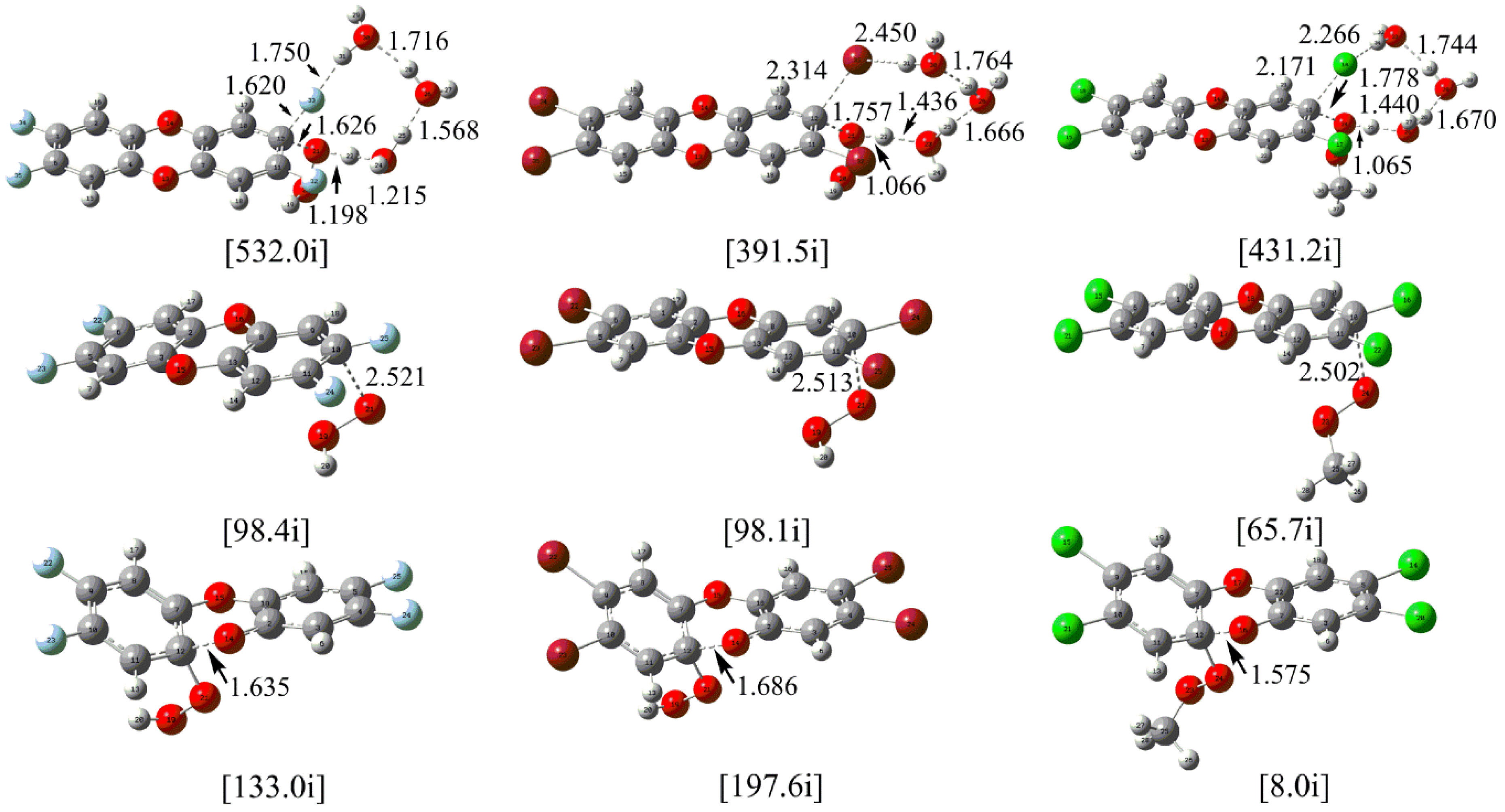
| Attack Modes | ΔG*1 | ΔG*2 | ΔG*2 − ΔG*1 |
|---|---|---|---|
| Mode 1 | 62.43/62.22 | 63.63 | 1.20 |
| Mode 2 | 62.39/62.77 | 61.47 | −0.91 |
| Mode 3 | 64.04/64.33 | 62.97 | −1.07 |
| Mode 4 | 61.63/62.89 | 61.27 | −0.36 |
| Pathways | Parameters | IM1 | TS1 | IM2 | TS2 | Pro |
|---|---|---|---|---|---|---|
| A | ΔE | −1.52 | 52.10 | −7.82 | −2.93(4.89) | −2.43 |
| ΔH | −0.83 | 51.47 | −7.61 | −3.37 | −1.48 | |
| ΔG | 4.26 | 62.43 | −0.33 | 5.94 | −3.75 | |
| B | ΔE | −6.83 | 41.75 | −13.74 | −7.92(5.82) | −9.46 |
| ΔH | −6.55 | 40.64 | −14.06 | −9.14 | −9.03 | |
| ΔG | 7.93 | 60.94 | 1.82 | 10.35 | −2.58 | |
| C | ΔE | −13.82 | 31.84 | −20.31 | −9.04(11.27) | −15.90 |
| ΔH | −14.16 | 29.92 | −21.07 | −10.49 | −15.94 | |
| ΔG | 10.27 | 60.08 | 2.70 | 16.79 | −1.16 | |
| D | ΔE | −21.31 | 23.72 | −25.79 | −12.01(13.78) | −21.46 |
| ΔH | −22.00 | 21.37 | −27.13 | −13.89 | −22.83 | |
| ΔG | 10.26 | 59.91 | 5.88 | 22.25 | 3.11 |
| Species | H2O2 | IM2(0w) | IM2(1w) | IM2(2w) | IM2(3w) |
|---|---|---|---|---|---|
| BDE | 48.62/44.08 | 20.32/6.14 | 19.81/5.03 | 18.89/5.14 | 18.96/4.96 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Wang, Y.; Feng, W.; Wang, W.; Li, P. Theoretical Investigations on the Reactivity of Hydrogen Peroxide toward 2,3,7,8-Tetrachlorodibenzo-p-dioxin. Molecules 2018, 23, 2826. https://doi.org/10.3390/molecules23112826
Wang W, Wang Y, Feng W, Wang W, Li P. Theoretical Investigations on the Reactivity of Hydrogen Peroxide toward 2,3,7,8-Tetrachlorodibenzo-p-dioxin. Molecules. 2018; 23(11):2826. https://doi.org/10.3390/molecules23112826
Chicago/Turabian StyleWang, Weihua, Yuhua Wang, Wenling Feng, Wenliang Wang, and Ping Li. 2018. "Theoretical Investigations on the Reactivity of Hydrogen Peroxide toward 2,3,7,8-Tetrachlorodibenzo-p-dioxin" Molecules 23, no. 11: 2826. https://doi.org/10.3390/molecules23112826





