Determination of Enantiomeric Excess by Solid-Phase Extraction Using a Chiral Metal-Organic Framework as Sorbent
Abstract
:1. Introduction
2. Results and Discussion
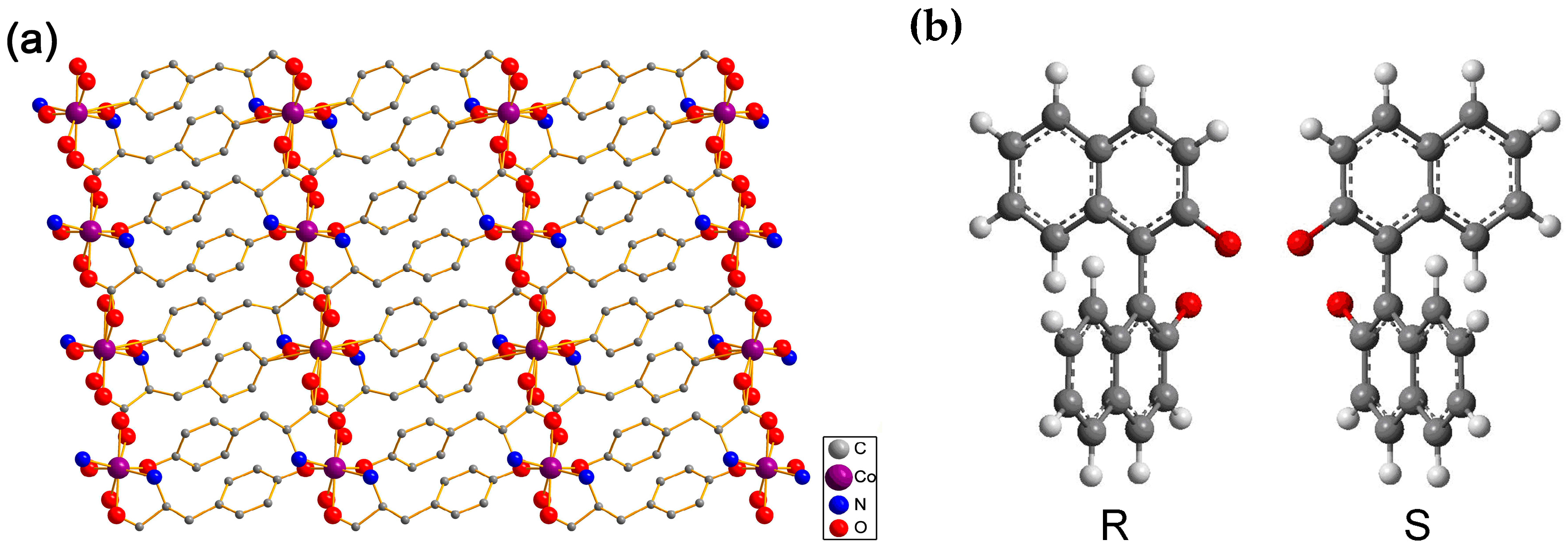
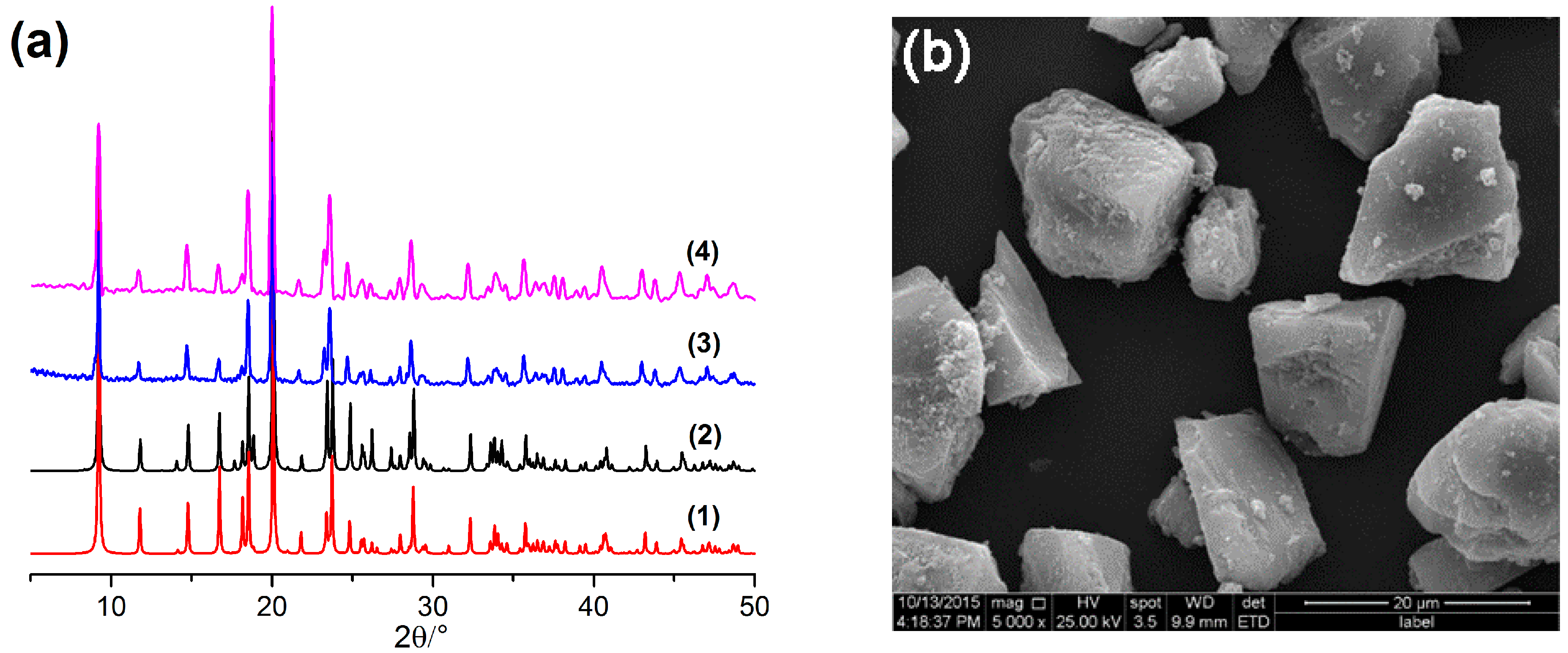
2.1. Characterization of the Synthesized [Co(l-tyr)]n(l-tyrCo)
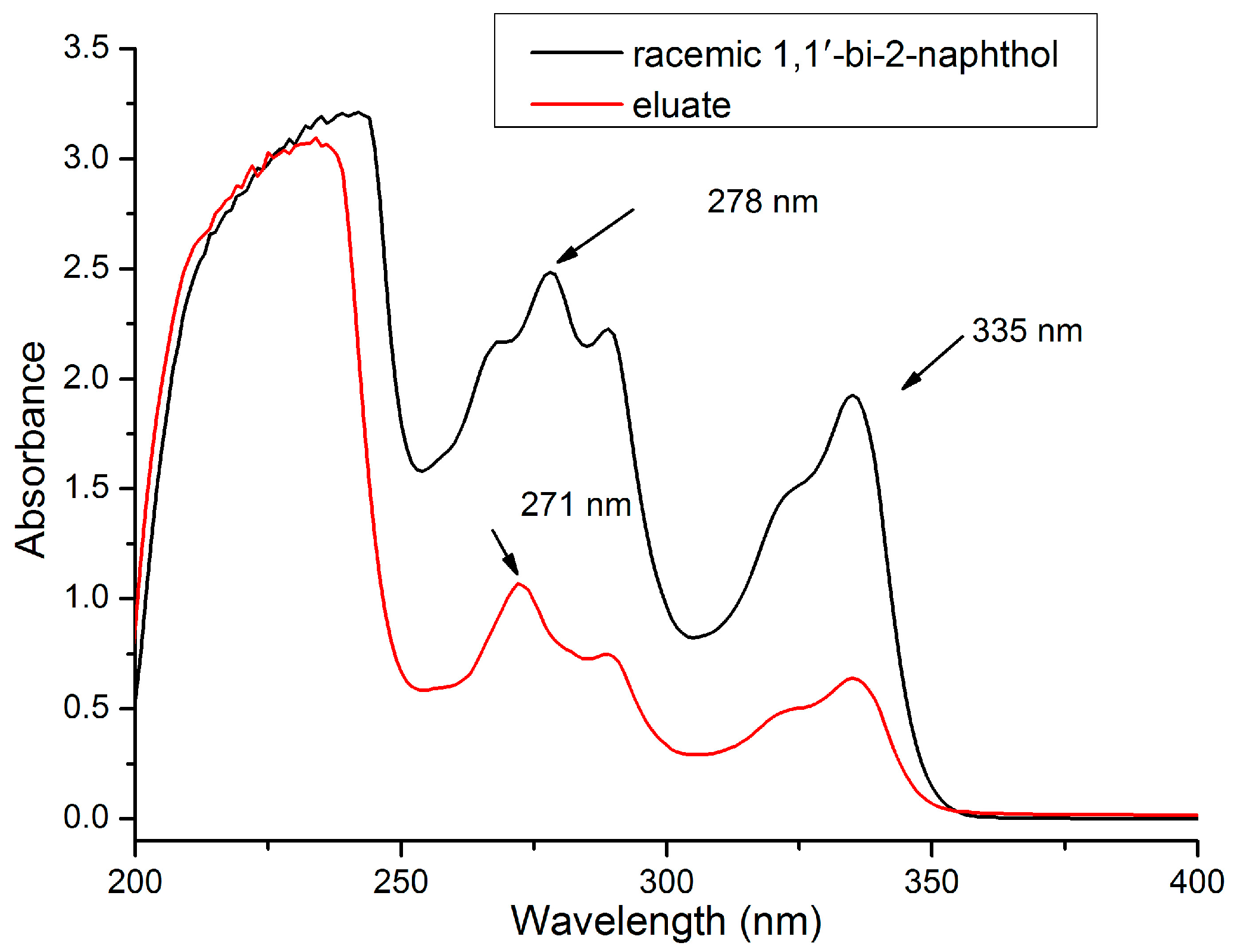
2.2. Selection of UV Wavelength for Analysis of Eluate
2.3. Optimization of SPE Conditions
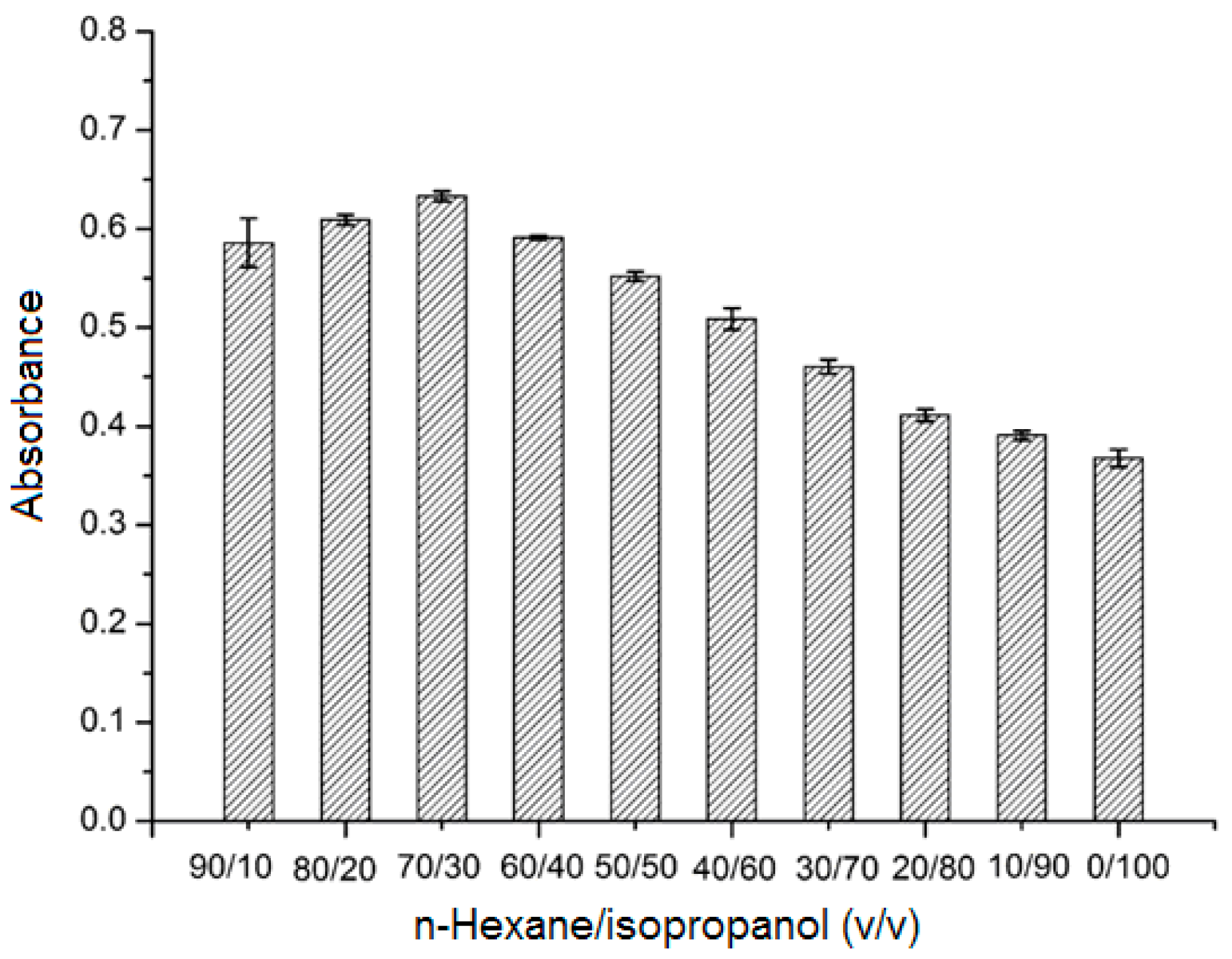
2.3.1. Effect of Sample Solvent Composition
2.3.2. Effect of Sample Solution Volume
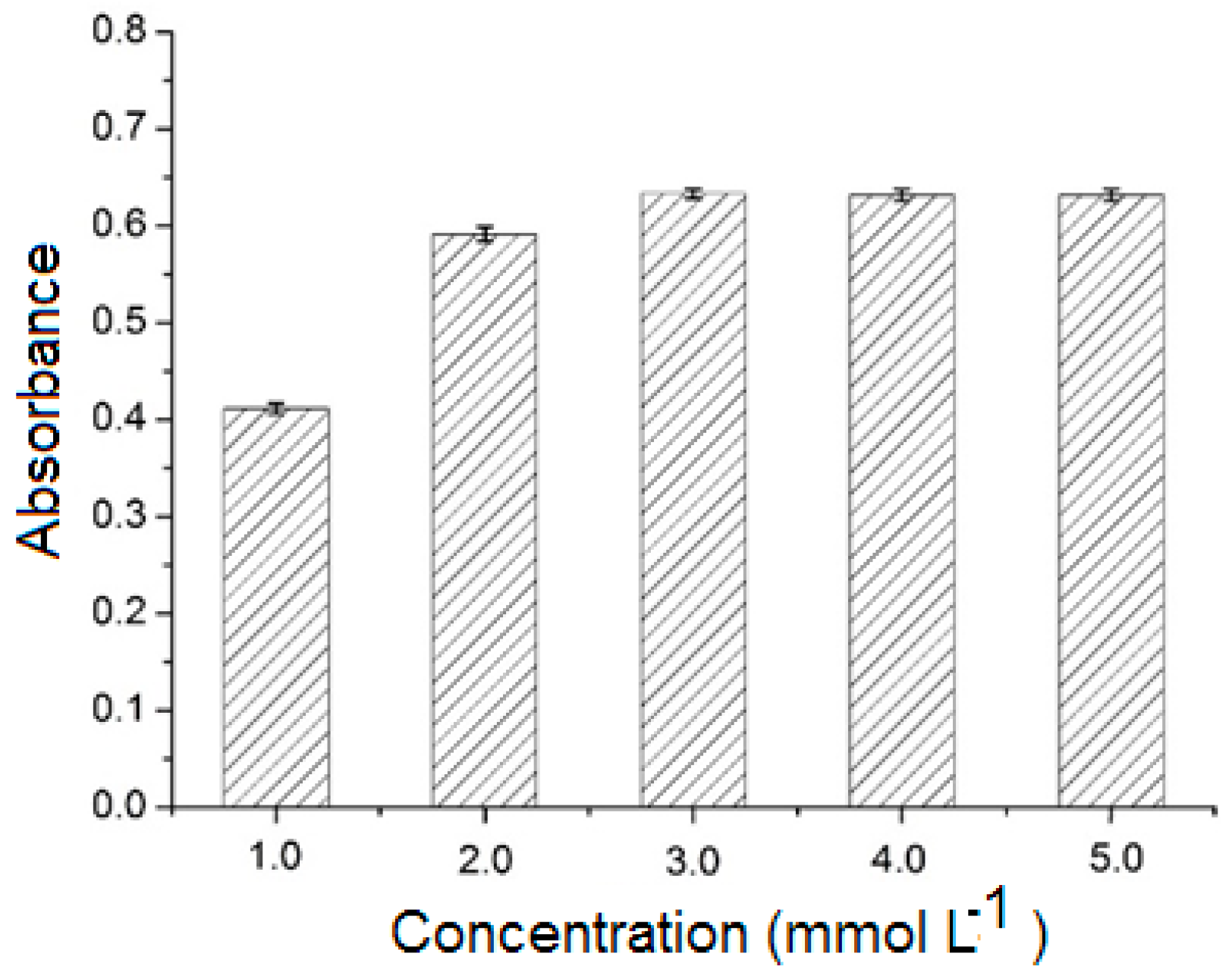
2.3.3. Effect of Analyte Concentration
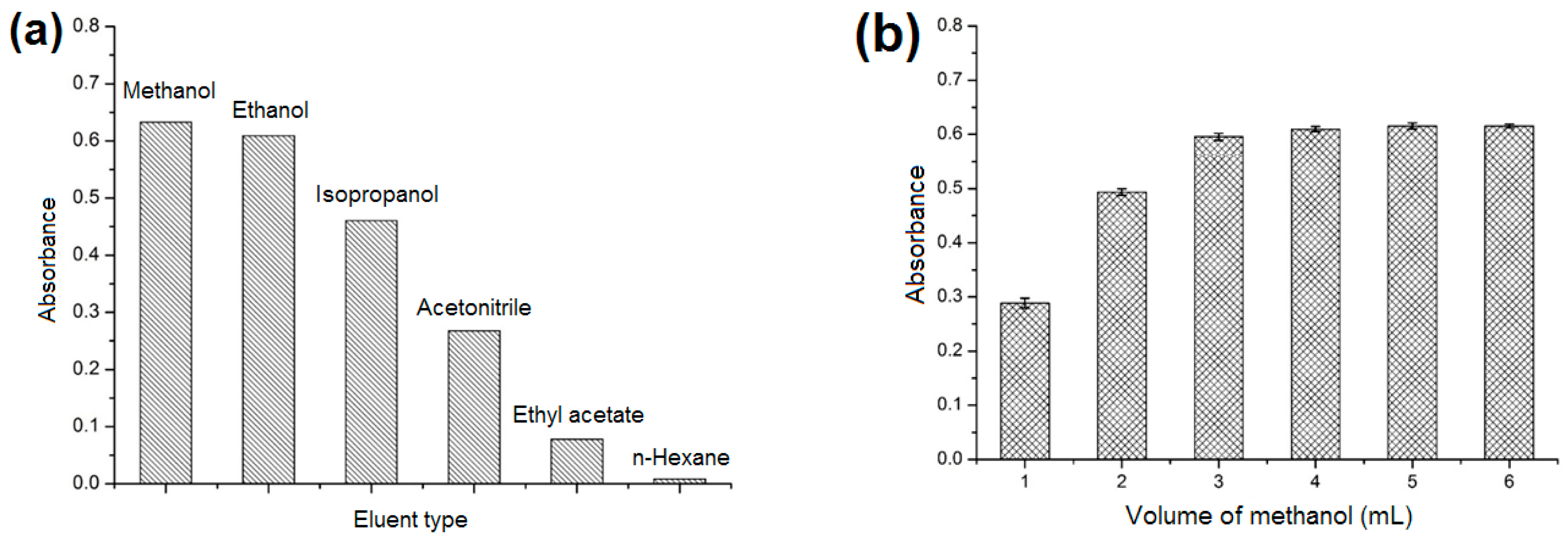
2.3.4. Effect of Type and Volume of Elution Solvent
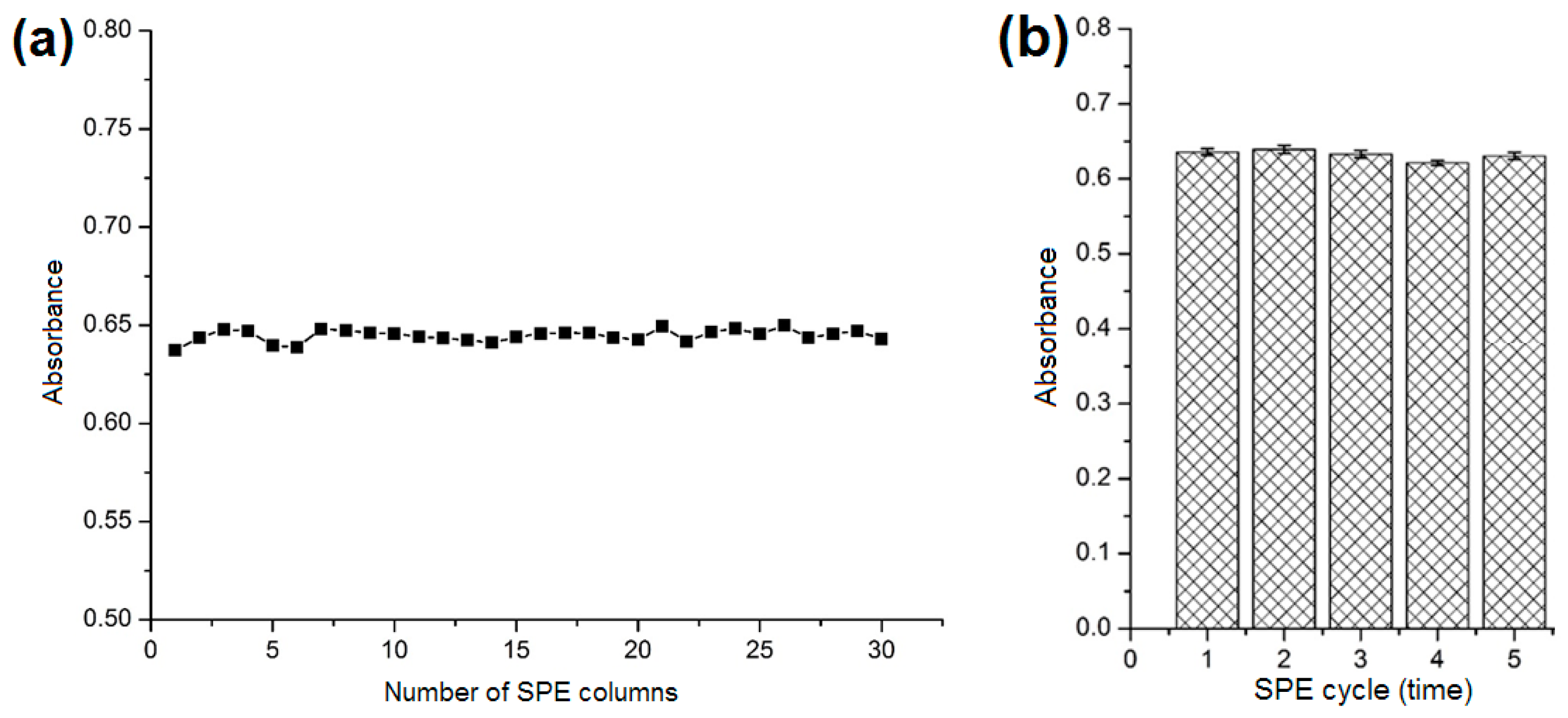
2.4. Reproducibility and Reusability of the SPE Column
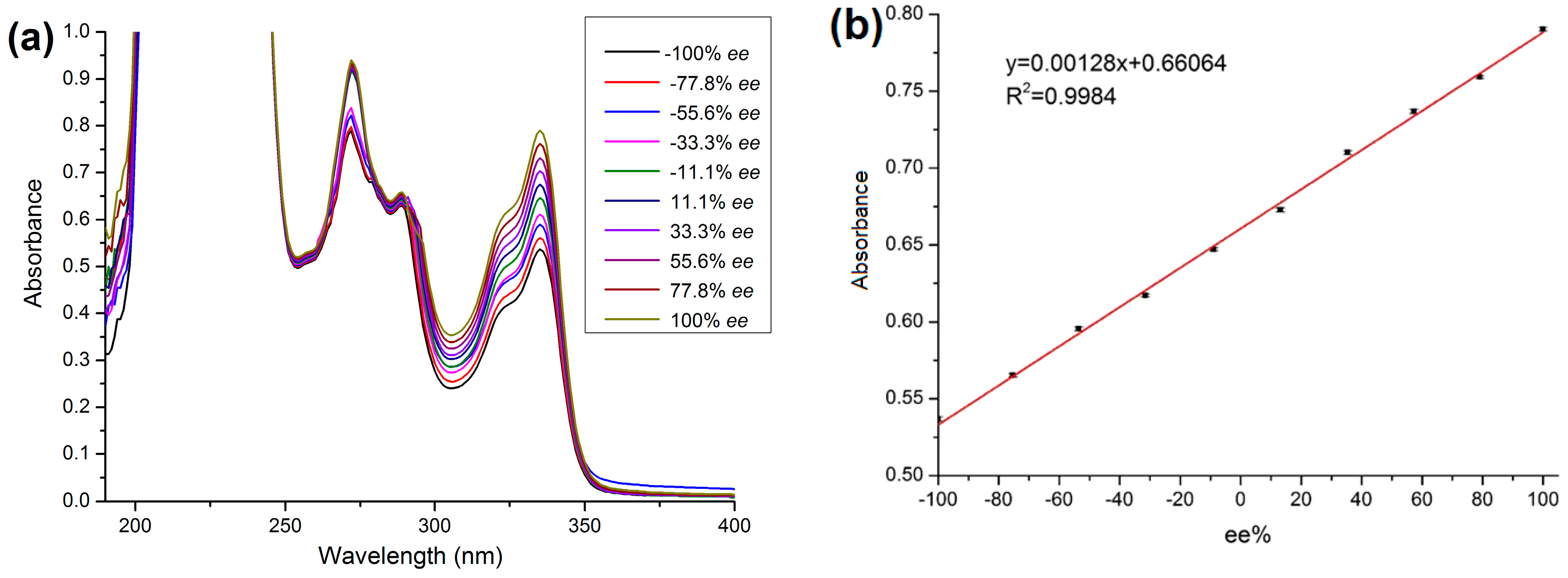
2.5. Method Validation
3. Materials and Methods
3.1. Reagents and Materials
3.2. Instrumentations
3.3. Synthesis of [Co(l-tyr)]n(l-tyrCo)
3.4. Preparation of SPE Columns
3.5. SPE Procedures
3.6. Calculation of ee Value
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maier, N.; Franco, P.; Lindner, W. Separation of enantiomers: Needs, challenges, perspectives. J. Chromatogr. A 2001, 906, 3–33. [Google Scholar] [CrossRef]
- Jo, H.H.; Lin, C.Y.; Anslyn, E.V. Rapid optical methods for enantiomeric excess analysis: From enantioselective indicator displacement assays to exciton-coupled circular dichroism. Acc. Chem. Res. 2014, 47, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Dragna, J.M.; Gade, A.M.; Tran, L.; Lynch, V.M.; Anslyn, E.V. Chiral amine enantiomeric excess determination using self-assembled octahedral Fe(II)-imine complexes. Chirality 2015, 27, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Folmer-Andersen, J.F.; Lynch, V.M.; Anslyn, E.V. Colorimetric enantiodiscrimination of α-amino acids in protic media. J. Am. Chem. Soc. 2005, 127, 7986–7987. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Wolf, C. Determination of enantiomeric excess and concentration of unprotected amino acids, amines, amino alcohols, and carboxylic acids by competitive binding assays with a chiral scandium complex. J. Am. Chem. Soc. 2006, 128, 13326–13327. [Google Scholar] [CrossRef] [PubMed]
- Korbel, G.A.; Lalic, G.; Shair, M.D. Reaction microarrays: A method for rapidly determining the enantiomeric excess of thousands of samples. J. Am. Chem. Soc. 2001, 123, 361–362. [Google Scholar] [CrossRef] [PubMed]
- Li, P. Fluorescence of organic molecules in chiral recognition. Chem. Rev. 2004, 104, 1687–1716. [Google Scholar]
- Guo, J.; Wu, J.; Siuzdak, G.; Finn, M.G. Measurement of enantiomeric excess by kinetic resolution and mass spectrometry. Angew. Chem. Int. Ed. 1999, 38, 1755–1758. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Shimizu, K.D. Measurement of enantiomeric excess using molecularly imprinted polymers. Org. Lett. 2002, 4, 2937–2940. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, P.; Cheng, J.; Zhu, X.; Guo, W.; Liu, L.; Wang, Q.; He, C.; Liu, S. Novel microporous β-cyclodextrin polymer as sorbent for solid-phase extraction of bisphenols in water samples and orange juice. Talanta 2018, 187, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Modern trends in solid phase extraction: New sorbent media. TrAC Trends Anal. Chem. 2016, 77, 23–43. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.Y.; Yang, C.X.; Chang, N.; Yan, X.P. Metal-organic frameworks for analytical chemistry: From sample collection to chromatographic separation. Acc. Chem. Res. 2012, 45, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.J.; Dinca, M.; Long, J.R. Hydrogen storage in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xuan, W.M.; Cui, Y. Engineering homochiral metal-organic frameworks for heterogeneous asymmetric catalysis and enantioselective separation. Adv. Mater. 2010, 22, 4112–4135. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.J.; Yue, Y.F.; Qian, G.D.; Chen, B.L. Luminescent functional metal-organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, J.; Liu, D.M.; Lin, W.B. Nanoscale metal-organic frameworks for biomedical imaging and drug delivery. Acc. Chem. Res. 2011, 44, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.Y.; Chen, Y.J.; Jiang, J.Q.; Yan, X.P. Metal-organic frameworks for efficient enrichment of peptides with simultaneous exclusion of proteins from complex biological samples. Chem. Commun. 2011, 47, 4787–4789. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.F.; Zang, H.; Wang, X.; Cheng, J.G.; Zhao, R.S.; Cheng, C.G.; Lu, X.Q. Metal-organic framework MIL-53(Al) as a solid-phase microextraction adsorbent for the determination of 16 polycyclic aromatic hydrocarbons in water samples by gas chromatography-tandem mass spectrometry. Analyst 2012, 137, 5411–5419. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.J.; Liu, S.Q.; Han, Z.B.; Jiang, R.F.; Liu, H.; Zhu, F.; Zeng, F.; Su, C.Y.; Ouyang, G.F. Preparation and characterization of metal-organic framework MIL-101(Cr)-coated solid-phase microextraction fiber. Anal. Chim. Acta 2015, 853, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.H.; Yan, X.P. Facile magnetization of metal-organic framework MIL-101 for magnetic solid-phase extraction of polycyclic aromatic hydrocarbons in environmental water samples. Analyst 2012, 137, 3445–3451. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.Y.; Gu, Z.Y.; Jiang, D.Q.; Li, Y.; Wang, H.F.; Yan, X.P. In situ hydrothermal growth of metal-organic framework 199 films on stainless steel fibers for solid-phase microextraction of gaseous benzene homologues. Anal. Chem. 2009, 81, 9771–9777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Huang, Y.C.; Ding, W.W.; Li, G.K. Multilayer interparticle linking hybrid MOF-199 for non-invasive enrichment and analysis of plant hormone ethylene. Anal. Chem. 2014, 86, 3533–3540. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Lee, H.K. Water stability of zeolite imidazolate framework 8 and application to porous membrane-protected micro-solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. J. Chromatogr. A 2011, 1218, 8490–8495. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.; Gu, Z.Y.; Wang, H.F.; Yan, X.P. Metal-organic framework-based tandem molecular sieves as a dual platform for selective microextraction and high-resolution gas chromatographic separation of n-alkanes in complex matrixes. Anal. Chem. 2011, 83, 7094–7101. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhu, L.; Xia, X.; Tang, H. The water-resistant zeolite imidazolate framework 67 is a viable solid phase sorbent for fluoroquinolones while efficiently excluding macromolecules. Microchim. Acta 2016, 183, 1839–1846. [Google Scholar] [CrossRef]
- Yu, L.Q.; Yan, X.P. Covalent bonding of zeolitic imidazolate framework-90 to functionalized silica fibers for solid-phase microextraction. Chem. Commun. 2013, 49, 2142–2144. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Yang, C.X.; Yan, X.P. Fabrication of metal-organic framework MIL-88B films on stainless steel fibers for solid-phase microextraction of polychlorinated biphenyls. J. Chromatogr. A 2014, 1334, 1–8. [Google Scholar] [CrossRef] [PubMed]
- He, C.T.; Tian, J.Y.; Liu, S.Y.; Ouyang, G.; Zhang, J.P.; Chen, X.M. A porous coordination framework for highly sensitive and selective solid-phase microextraction of non-polar volatile organic compounds. Chem. Sci. 2013, 4, 351–356. [Google Scholar] [CrossRef]
- Hu, Y.L.; Lian, H.X.; Zhou, L.J.; Li, G.K. In situ solvothermal growth of metal-organic framework-5 supported on porous copper foam for noninvasive sampling of plant volatile sulfides. Anal. Chem. 2015, 87, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Huang, C.; Lin, Y.; Tong, P.; Zhang, L. In situ solvothermal synthesis of metal-organic framework coated fiber for highly sensitive solid-phase microextraction of polycyclic aromatic hydrocarbons. J. Chromatogr. A 2016, 1436, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Zhang, X.; Chang, Q.; Li, S.; Wang, C.; Wang, Z. Metal-organic framework UiO-67-coated fiber for the solid-phase microextraction of nitrobenzene compounds from water. J. Sep. Sci. 2016, 39, 2770–2776. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rui, M.; Lu, G.H. Recent applications of metal-organic frameworks in sample pretreatment. J. Sep. Sci. 2018, 41, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhang, J.H.; Zi, M.; Chen, X.X.; Yuan, L.M. Solid-phase extraction with metal-organic frameworks for the analysis of chiral compounds. Chirality 2016, 28, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Silva, N.J.O.; Shi, F.N.; Palacio, F.; Mafra, L.; Rocha, J. CoII/ZnII-(L-Tyrosine) magnetic metal-organic frameworks. Eur. J. Inorg. Chem. 2012, 32, 5259–5268. [Google Scholar] [CrossRef]
Sample Availability: Samples of (±)-1,1′-bi-2-naphthol, (R)-1,1′-bi-2-naphthol and (S)-1,1′-bi-2-naphthol are available from the authors. |

| Sample | Absorbance | Experimental ee (%) | Actual ee (%) | Absolute Error (%) | Average Error (%) |
|---|---|---|---|---|---|
| 1 | 0.5566 | −81.25 | −84.41 | 3.16 | 2.26 |
| 2 | 0.6022 | −45.67 | −43.34 | 2.33 | |
| 3 | 0.6582 | −1.93 | −0.87 | 1.06 | |
| 4 | 0.7098 | 38.41 | 41.79 | 3.38 | |
| 5 | 0.7621 | 79.24 | 77.89 | 1.35 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.-H.; Tang, B.; Xie, S.-M.; Wang, B.-J.; Zhang, M.; Chen, X.-L.; Zi, M.; Yuan, L.-M. Determination of Enantiomeric Excess by Solid-Phase Extraction Using a Chiral Metal-Organic Framework as Sorbent. Molecules 2018, 23, 2802. https://doi.org/10.3390/molecules23112802
Zhang J-H, Tang B, Xie S-M, Wang B-J, Zhang M, Chen X-L, Zi M, Yuan L-M. Determination of Enantiomeric Excess by Solid-Phase Extraction Using a Chiral Metal-Organic Framework as Sorbent. Molecules. 2018; 23(11):2802. https://doi.org/10.3390/molecules23112802
Chicago/Turabian StyleZhang, Jun-Hui, Bo Tang, Sheng-Ming Xie, Bang-Jin Wang, Mei Zhang, Xing-Lian Chen, Min Zi, and Li-Ming Yuan. 2018. "Determination of Enantiomeric Excess by Solid-Phase Extraction Using a Chiral Metal-Organic Framework as Sorbent" Molecules 23, no. 11: 2802. https://doi.org/10.3390/molecules23112802






