Microwave-Assisted Extraction of Phenolic Compounds from Melastoma sanguineum Fruit: Optimization and Identification
Abstract
:1. Introduction
2. Results and Discussion
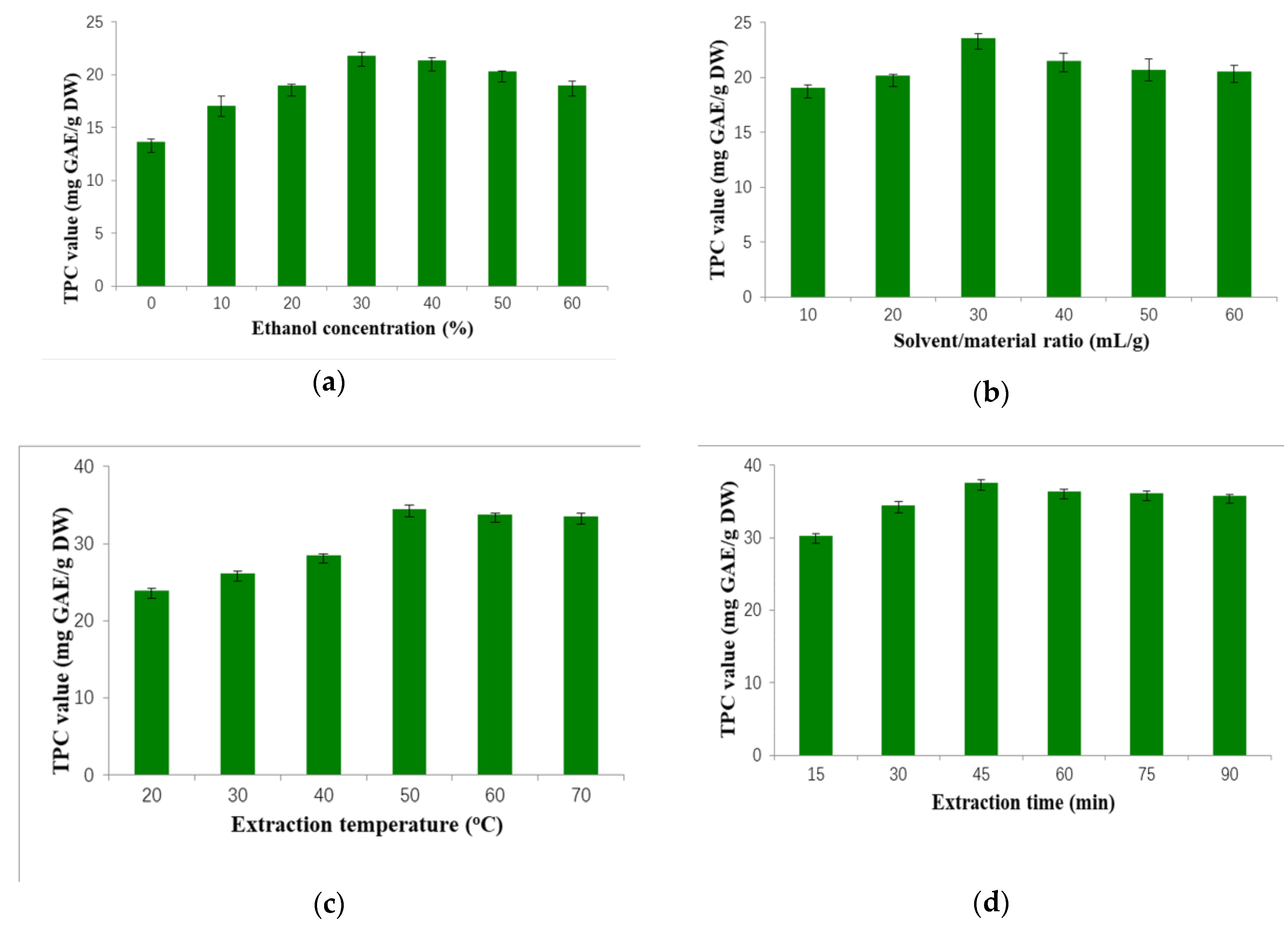
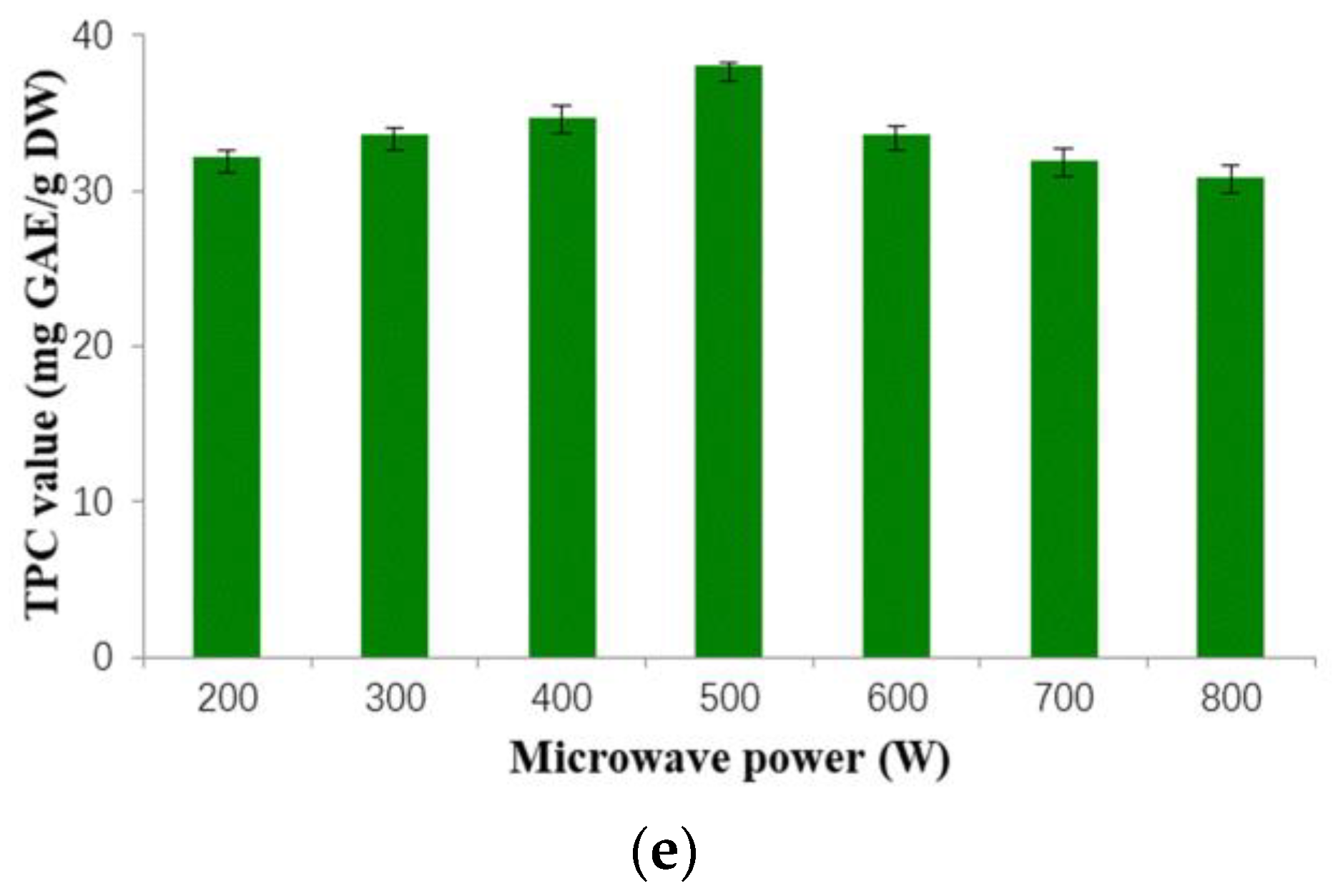
2.1. Results of Single-Factor Tests
2.2. Results of Response Surface Methodology Optimization
2.2.1. Results of Central Composite Design
2.2.2. Model Fitting
2.2.3. Graphical Analysis
2.2.4. Verification of the Model
2.3. Comparison of MAE with Maceration and Soxhlet Extraction
2.4. Qualitative and Quantitative Measurement of Phenolic Compounds by UPLC-MS/MS
3. Materials and Methods
3.1. Sample Preparation
3.2. Standards and Reagents
3.3. Microwave-Assisted Extraction
3.4. Maceration Extraction
3.5. Soxhlet Extraction
3.6. Measurement of Total Phenolic and Flavonoid Contents, and Antioxidant Capacity
3.7. Experimental Design and Statistical Analysis
3.8. UPLC-MS/MS Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Goulas, V.; Vicente, A.R.; Terry, L.A. Berry antioxidants: Small fruits providing large benefits. J. Sci. Food Agric. 2014, 94, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Kang, X.; He, X.; Dong, M.; Zhang, Q.; Liu, R.H. Cellular antioxidant activity of common fruits. J. Agric. Food Chem. 2008, 56, 8418–8426. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.N.; Meng, X.; Li, Y.; Li, S.; Liu, Q.; Tang, G.Y.; Li, H.B. Fruits for prevention and treatment of cardiovascular diseases. Nutrients 2017, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, Y.; Chao, L.; Wang, S.; Wu, W.; Dai, S.; Wang, F.; Fan, Q.; Zhou, R. Extensive hybridization and introgression between Melastoma candidum and M-sanguineum. PLoS ONE 2014, 9, e96680. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.T.; Xu, X.R.; Qin, X.S.; Gan, R.Y.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 wild fruits from south China. Molecules 2010, 15, 8602–8617. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Kim, I.S.; Lee, Y.M.; Lee, Y.; Kim, J.; Kim, J.S. 2″,4″-O-diacetylquercitrin, a novel advanced glycation end-product formation and aldose reductase inhibitor from Melastoma sanguineum. Chem. Pharm. Bull. 2013, 61, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Cujic, N.; Savikin, K.; Jankovic, T.; Pljevljakusic, D.; Zdunic, G.; Ibric, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Diz, P.; Prieto, M.A.; Barros, L.; Rodrigues, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Optimization of ultrasound-assisted extraction to obtain mycosterols from Agaricus bisporus L. by response surface methodology and comparison with conventional Soxhlet extraction. Food Chem. 2016, 197, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.; Meullemiestre, A.; Fabiano-Tixier, A.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.B. Ultrasound-assisted extraction of natural antioxidants from the flower of Limonium sinuatum: Optimization and comparison with conventional methods. Food Chem. 2017, 217, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Pourmortazavi, S.M.; Hajimirsadeghi, S.S. Supercritical fluid extraction in plant essential and volatile oil analysis. J. Chromatogr. A 2007, 1163, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mendoza, M.D.P.; Espinosa-Pardo, F.A.; Baseggio, A.M.; Fernandez Barbero, G.; Marostica Junior, M.R.; Rostagno, M.A.; Martinez, J. Extraction of phenolic compounds and anthocyanins from jucara (Euterpe edulis Mart.) residues using pressurized liquids and supercritical fluids. J. Supercrit. Fluids 2017, 119, 9–16. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Lin, S.J.; Zhang, J.J.; Zhao, C.N.; Li, H.B. Microwave-assisted extraction of natural antioxidants from the exotic Gordonia axillaris fruit: Optimization and identification of phenolic compounds. Molecules 2017, 22, 1481. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, D.J.; Quan, V.V.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Microwave-assisted extraction of Eucalyptus robusta leaf for the optimal yield of total phenolic compounds. Ind. Crop. Prod. 2015, 69, 290–299. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.; Gong, X. Microwave-assisted extraction used for the isolation of total triterpenoid saponins from Ganoderma atrum. J. Food Eng. 2007, 81, 162–170. [Google Scholar] [CrossRef]
- Dahmoune, F.; Spigno, G.; Moussi, K.; Remini, H.; Cherbal, A.; Madani, K. Pistacia lentiscus leaves as a source of phenolic compounds: Microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Ind. Crop. Prod. 2014, 61, 31–40. [Google Scholar] [CrossRef]
- Kala, H.K.; Mehta, R.; Sen, K.K.; Tandey, R.; Mandal, V. Critical analysis of research trends and issues in microwave assisted extraction of phenolics: Have we really done enough. Trac-Trends Anal. Chem. 2016, 85, 140–152. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-assisted extraction of flavonoids: A review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, J.; Hao, J.; Li, X.; Guo, N. Simultaneous extraction, identification and quantification of phenolic compounds in Eclipta prostrata using microwave-assisted extraction combined with HPLC-DAD-ESI-MS/MS. Food Chem. 2015, 188, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Deng, Z.; Wu, T.; Liu, R.; Loewen, S.; Tsao, R. Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chem. 2012, 130, 928–936. [Google Scholar] [CrossRef]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Shao, Q.; Ye, S.; Li, S.; Wu, M.; Ding, M.; Li, Y. Simultaneous extraction and identification of phenolic compounds in Anoectochilus roxburghii using microwave-assisted extraction combined with UPLC-Q-TOF-MS/MS and their antioxidant activities. Front. Plant Sci. 2017, 8, 1474. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chiou, Y.; Wang, Y.; Ho, C.; Lin, J. Multistage carcinogenesis process as molecular targets in cancer chemoprevention by epicatechin-3-gallate. Food Funct. 2011, 2, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lee, M.; Wang, C.; Hsu, C.; Lin, H. Autophagic effects of Hibiscus sabdariffa leaf polyphenols and epicatechin gallate (ECG) against oxidized LDL-induced injury of human endothelial cells. Eur. J. Nutr. 2017, 56, 1963–1981. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of Melastoma sanguineum fruit and several phenolic standards are available from the authors. |

| Run | X1 (Ethanol Concentration, %) | X2 (Solvent/Material Ratio, mL/g) | X3 (Temperature, °C) | Y (TPC Value, mg GAE/g DW) | |
|---|---|---|---|---|---|
| Actual | Predicted | ||||
| 1 | 20 (−1) | 40 (1) | 40 (−1) | 28.71 | 27.70 |
| 2 | 40 (1) | 40 (1) | 60 (1) | 30.32 | 30.32 |
| 3 | 30 (0) | 30 (0) | 50 (0) | 39.30 | 38.68 |
| 4 | 30 (0) | 46.82 (1.68) | 50 (0) | 30.33 | 30.50 |
| 5 | 40 (1) | 20 (−1) | 60 (1) | 28.11 | 29.60 |
| 6 | 20 (−1) | 20 (-1) | 60 (1) | 26.93 | 27.47 |
| 7 | 30 (0) | 30 (0) | 50 (0) | 39.42 | 38.68 |
| 8 | 40 (1) | 20 (−1) | 40 (−1) | 23.11 | 22.42 |
| 9 | 30 (0) | 30 (0) | 66.82 (1.68) | 32.63 | 30.96 |
| 10 | 30 (0) | 30 (0) | 50 (0) | 38.17 | 38.68 |
| 11 | 20 (−1) | 40 (1) | 60 (1) | 28.11 | 29.28 |
| 12 | 13.18 (−1.68) | 30 (0) | 50 (0) | 27.72 | 27.25 |
| 13 | 40 (1) | 40 (1) | 40 (−1) | 29.75 | 29.69 |
| 14 | 30 (0) | 30 (0) | 50 (0) | 38.65 | 38.68 |
| 15 | 30 (0) | 30 (0) | 33.18 (−1.68) | 22.61 | 23.60 |
| 16 | 20 (−1) | 20 (−1) | 40 (−1) | 18.87 | 19.35 |
| 17 | 30 (0) | 30 (0) | 50 (0) | 37.51 | 38.68 |
| 18 | 30 (0) | 13.18 (−1.68) | 50 (0) | 23.72 | 22.87 |
| 19 | 46.82 (1.68) | 30 (0) | 50 (0) | 30.91 | 30.70 |
| 20 | 30 (0) | 30 (0) | 50 (0) | 38.90 | 38.68 |
| Source | Sum of Squares | df | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| Model | 726.77 | 9 | 80.75 | 62.17 | <0.0001 |
| X1 | 14.42 | 1 | 14.42 | 11.10 | 0.0076 |
| X2 | 70.31 | 1 | 70.31 | 54.13 | <0.0001 |
| X3 | 65.38 | 1 | 65.38 | 50.34 | <0.0001 |
| X1X2 | 0.59 | 1 | 0.59 | 0.45 | 0.5161 |
| X1X3 | 0.45 | 1 | 0.45 | 0.34 | 0.5707 |
| X2X3 | 21.42 | 1 | 21.42 | 16.49 | 0.0023 |
| X12 | 169.56 | 1 | 169.56 | 130.54 | <0.0001 |
| X22 | 259.05 | 1 | 259.05 | 199.44 | <0.0001 |
| X32 | 233.98 | 1 | 233.98 | 180.14 | <0.0001 |
| Residual | 12.99 | 10 | 1.30 | ||
| Lack of Fit | 10.38 | 5 | 2.08 | 3.98 | 0.0778 |
| Pure Error | 2.61 | 5 | 0.52 | ||
| Cor. Total | 739.76 | 19 | |||
| R2 | 0.9824 | ||||
| Adjusted R2 | 0.9666 |
| Extraction Methods | Ethanol Concentration (%) | Time | Temperature (°C) | TPC (mg GAE/g DW) | TEAC (μmol Trolox/g DW) | TFC (mg QE/g DW) |
|---|---|---|---|---|---|---|
| Maceration extraction | 31.33 | 24 h | 25 | 25.79 ± 1.03 | 380.66 ± 1.09 | 1.11 ± 0.28 |
| Soxhlet extraction | 31.33 | 4 h | 95 | 18.40 ± 1.34 | 309.10 ± 1.32 | 1.19 ± 0.23 |
| MAE | 31.33 | 45 min | 52.24 | 39.02 ± 0.73 | 480.58 ± 1.23 | 1.33 ± 0.31 |
| Phenolic Compounds | Classification | Retention Time (tR, min) | Parent Ion (m/z, [M − H]−) | Product Ion (m/z) | Contents (µg/g DW) |
|---|---|---|---|---|---|
| Epicatechin gallate | Flavonoid | 6.87 | 441 | 169 | 256.14 ± 18.42 |
| Epicatechin | Flavonoid | 5.4 | 289 | 203 | 22.57 ± 1.78 |
| Rutin | Flavonoid | 9.67 | 609 | 300 | 17.24 ± 1.52 |
| Epigallocatechin | Flavonoid | 3.03 | 305 | 137 | 7.84 ± 0.67 |
| Protocatechuic acid | Phenolic acid | 3.09 | 152.9 | 107.8 | 0.74 ± 0.14 |
| Chlorogenic acid | Phenolic acid | 4.13 | 353 | 191 | 0.65 ± 0.08 |
| Quercetin | Flavonoid | 11.8 | 301 | 179 | 0.35 ± 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.-N.; Zhang, J.-J.; Li, Y.; Meng, X.; Li, H.-B. Microwave-Assisted Extraction of Phenolic Compounds from Melastoma sanguineum Fruit: Optimization and Identification. Molecules 2018, 23, 2498. https://doi.org/10.3390/molecules23102498
Zhao C-N, Zhang J-J, Li Y, Meng X, Li H-B. Microwave-Assisted Extraction of Phenolic Compounds from Melastoma sanguineum Fruit: Optimization and Identification. Molecules. 2018; 23(10):2498. https://doi.org/10.3390/molecules23102498
Chicago/Turabian StyleZhao, Cai-Ning, Jiao-Jiao Zhang, Ya Li, Xiao Meng, and Hua-Bin Li. 2018. "Microwave-Assisted Extraction of Phenolic Compounds from Melastoma sanguineum Fruit: Optimization and Identification" Molecules 23, no. 10: 2498. https://doi.org/10.3390/molecules23102498







