Quantitative Determination of Cd in Soil Using Laser-Induced Breakdown Spectroscopy in Air and Ar Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Samples
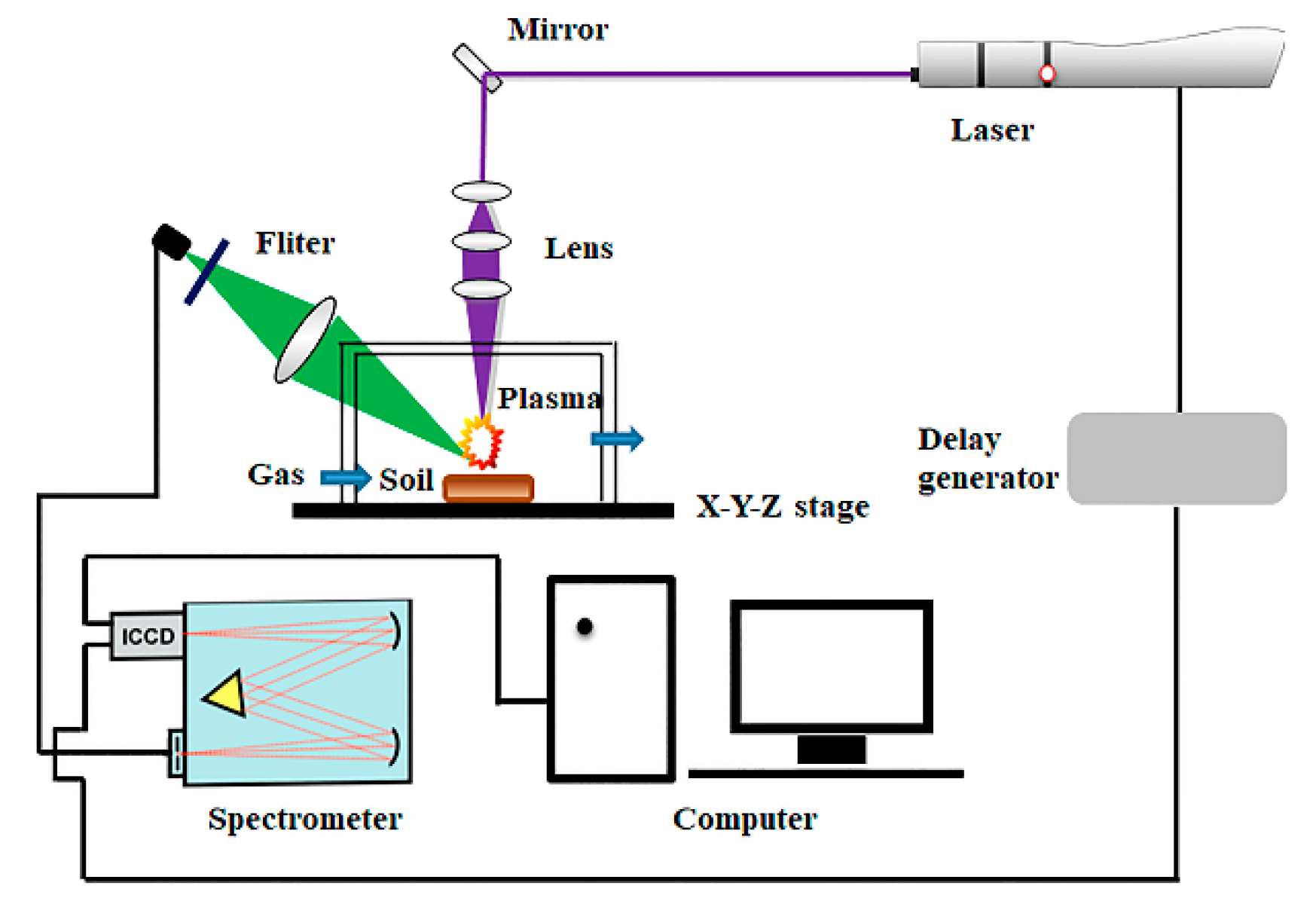
2.2. Spectral Acquisition
2.3. Data Analysis
2.3.1. Data Preprocessing
2.3.2. Quantitative Analysis Methods
2.4. Software Tools
3. Results and Discussion
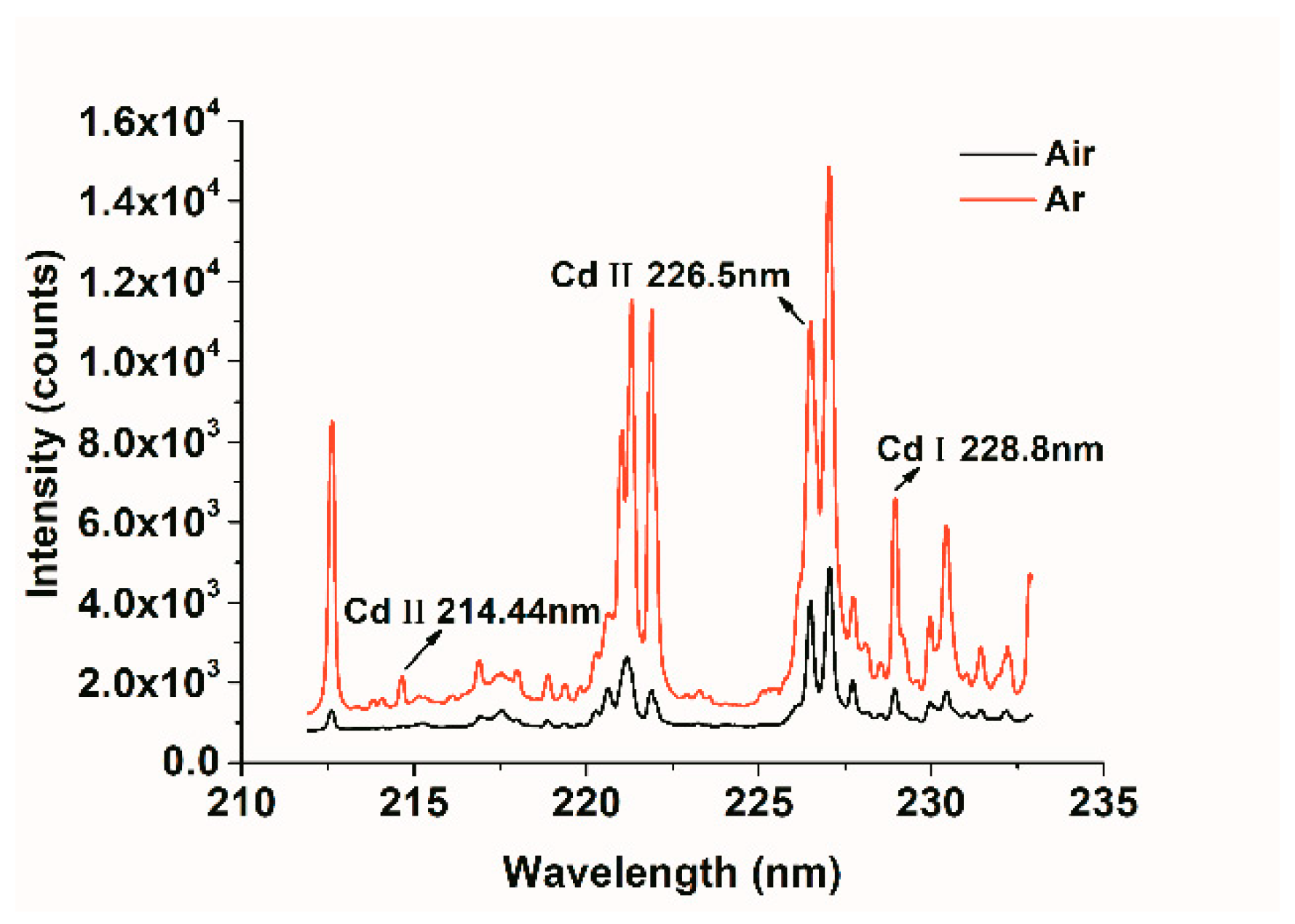
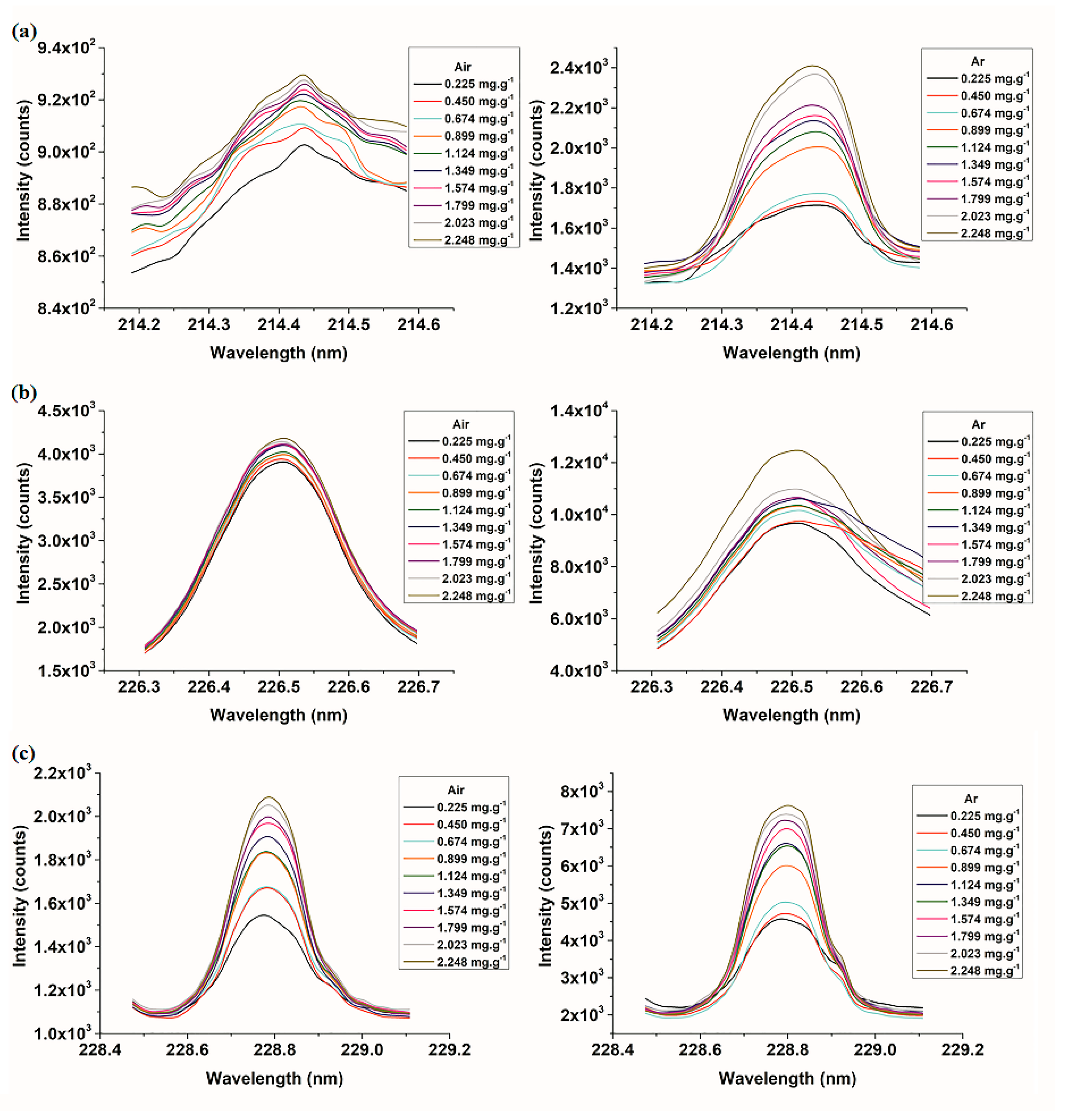
3.1. Spectral Analysis
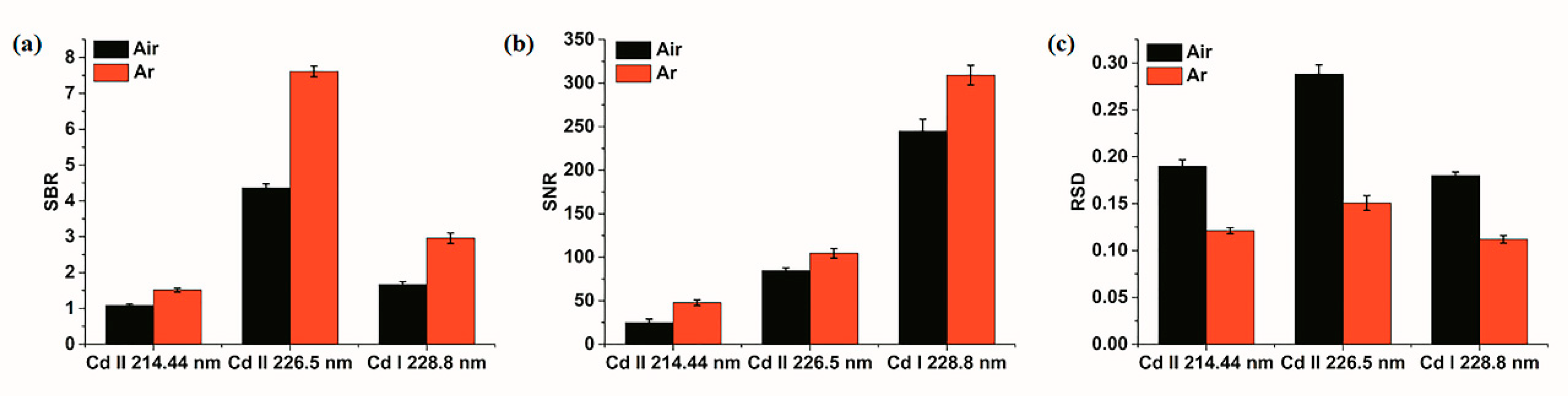
3.2. System Performance Analysis
3.3. Quantitative Determination of Cd in Soil Samples
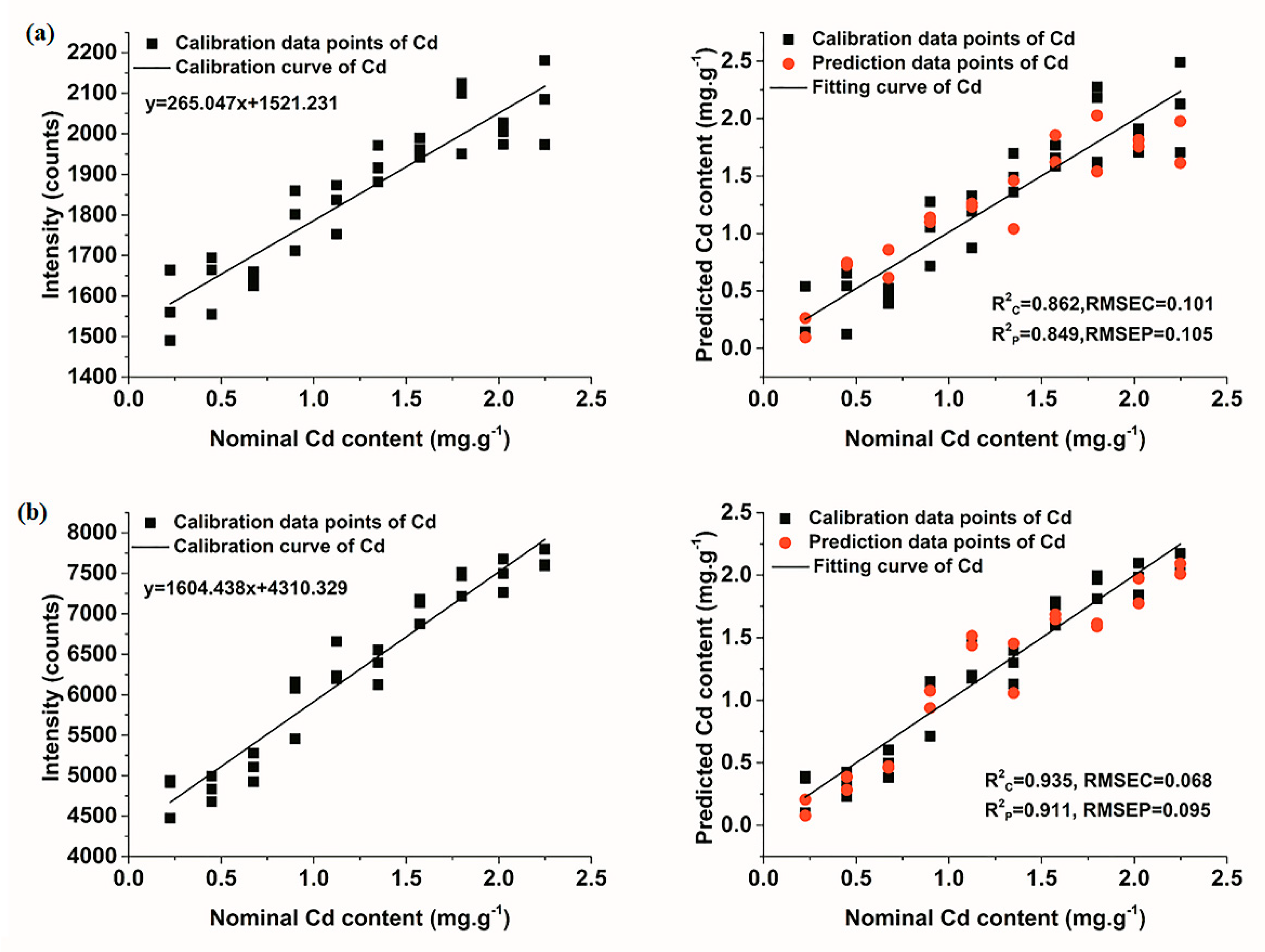
3.3.1. Quantitative Determination of Cd Based on Univariate Models
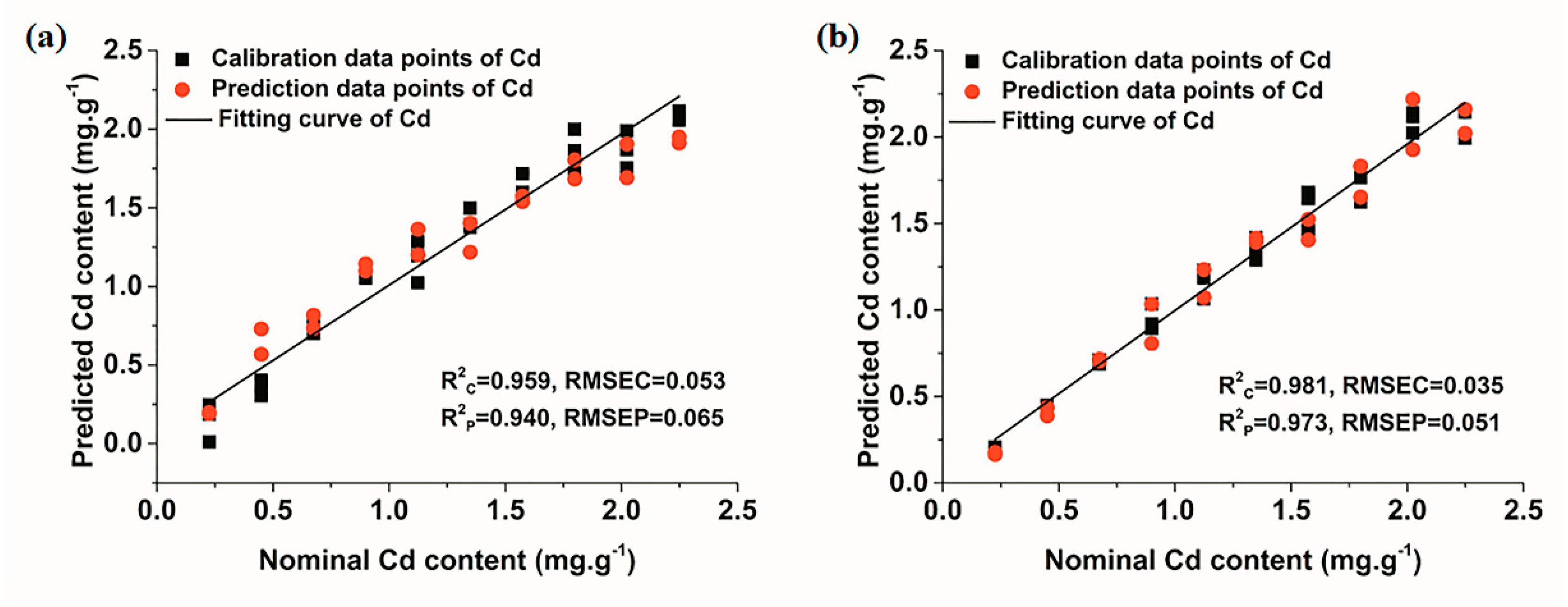
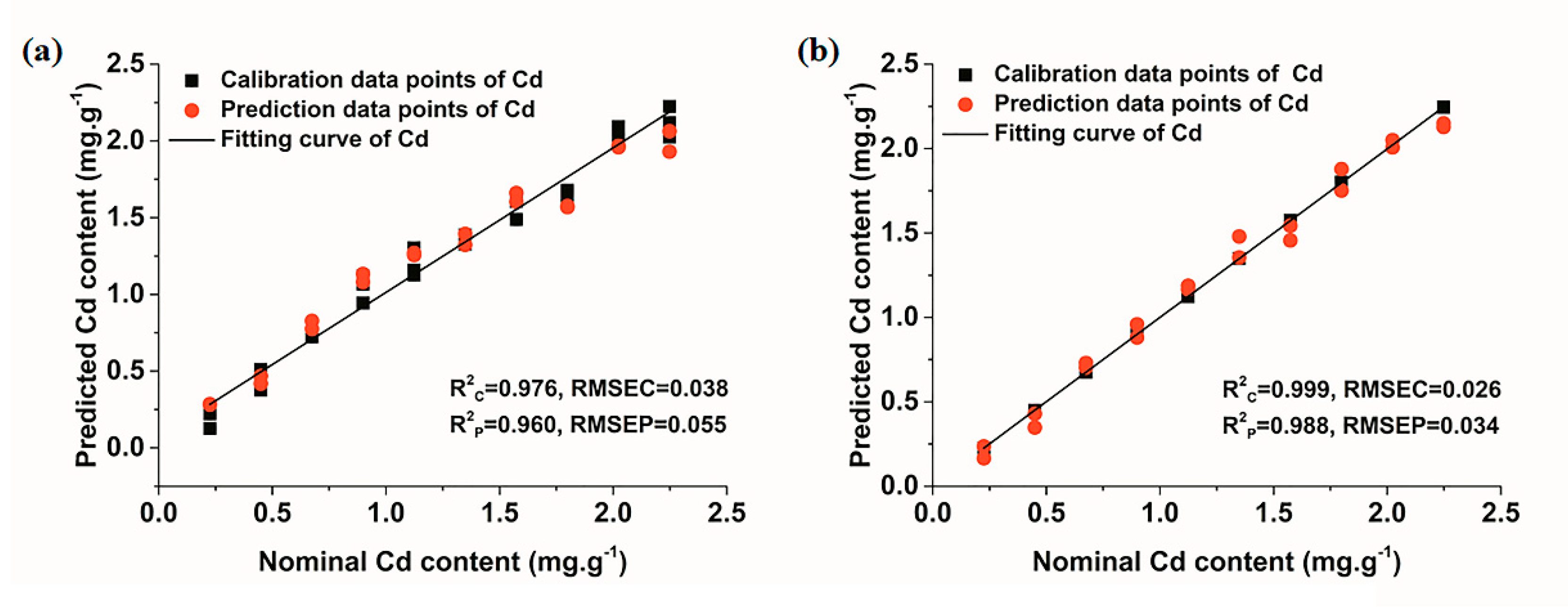
3.3.2. Quantitative Determination of Cd Based on Multivariate Models
3.3.3. Comparison of Univariate and Multivariate Analysis Models
3.4. The Influence Principle of Ar on LIBS Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liess, M.; Schmidt, J.; Glaser, B. Improving the spatial prediction of soil organic carbon stocks in a complex tropical mountain landscape by methodological specifications in machine learning approaches. PLoS ONE 2016, 11, e0153673. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Dong, T.; He, Y.; Qu, F. Detection of soil nitrogen using near infrared sensors based on soil pretreatment and algorithms. Sensors 2017, 17, 1102. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, R.P. Spatial distribution of soil nutrients in a watershed of Himalayan landscape using terrain attributes and geostatistical methods. Environ. Earth Sci. 2016, 75, 473. [Google Scholar] [CrossRef]
- Fu, J.; Zhou, Q.; Liu, J.; Liu, W.; Wang, T.; Zhang, Q.; Jiang, G. High levels of heavy metals in rice (Oryza sativa L.) from a typical e-waste recycling area in southeast China and its potential risk to human health. Chemosphere 2008, 71, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.W.; Lan, C.Y.; Wang, H.B.; Zhuang, P.; Shu, W.S. Cadmium in soil–rice system and health risk associated with the use of untreated mining wastewater for irrigation in Lechang, China. Agric. Water Mgmt. 2006, 84, 147–152. [Google Scholar] [CrossRef]
- Santos, D.; Nunes, L.C.; Trevizan, L.C.; Godoi, Q.; Leme, F.O.; Braga, J.W.B.; Krug, F.J. Evaluation of laser induced breakdown spectroscopy for cadmium determination in soils. Spectrochim. Acta B 2009, 64, 1073–1078. [Google Scholar] [CrossRef]
- Zuckerman, J.A. IARC monographs on the evaluation of carcinogenic risks to humans. J. Clin. Pathol. 1995, 48, 691. [Google Scholar] [CrossRef]
- Johri, N.; Jacquillet, G.; Unwin, R. Heavy metal poisoning: The effects of cadmium on the kidney. Biometals 2010, 23, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Basiri, S.; Moinfar, S.; Hosseini, M.R.M. Determination of as(iii) using developed dispersive liquid–liquid microextraction and flame atomic absorption spectrometry. Int. J. Environ. Anal. Chem. 2011, 91, 1453–1465. [Google Scholar] [CrossRef]
- Momen, A.A.; Zachariadis, G.A.; Anthemidis, A.N.; Stratis, J.A. Use of fractional factorial design for optimization of digestion procedures followed by multi-element determination of essential and non-essential elements in nuts using icp-oes technique. Talanta 2007, 71, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liu, Y.; Yang, Y.; Hu, Z. Calibration and correction of LA-ICP-MS and LA-MC-ICP-MS analyses for element contents and isotopic ratios. Solid Earth Sci. 2016, 1, 5–27. [Google Scholar] [CrossRef]
- Yu, K.; Zhao, Y.; Liu, F.; He, Y. Laser-induced breakdown spectroscopy coupled with multivariate chemometrics for variety discrimination of soil. Sci. Rep. 2016, 6, 27574. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liu, F.; Zhou, F.; Song, K.; Zhang, C.; Ye, L.; He, Y. Challenging applications for multi-element analysis by laser-induced breakdown spectroscopy in agriculture: A review. TrAC Trends Anal. Chem. 2016, 85, 260–272. [Google Scholar] [CrossRef]
- Peng, J.; He, Y.; Ye, L.; Shen, T.; Liu, F.; Kong, W.; Liu, X.; Zhao, Y. Moisture influence reducing method for heavy metals detection in plant materials using laser-induced breakdown spectroscopy: A case study for chromium content detection in rice leaves. Anal. Chem. 2017, 89, 7593–7600. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Y.; Gu, X.; Bao, J.; Yang, H.; Sun, L. Laser-induced breakdown spectroscopy application in environmental monitoring of water quality: A review. Environ. Monit. Assess. 2014, 186, 8969–8980. [Google Scholar] [CrossRef] [PubMed]
- Canel, T.; Demir, P.; Kacar, E.; Genc Oztoprak, B.; Akman, E.; Gunes, M.; Demir, A. Optimization of parameters for depth resolution of galvanized steel by libs technique. Opt. Laser Technol. 2013, 54, 257–264. [Google Scholar] [CrossRef]
- Rehse, S.J.; Salimnia, H.; Miziolek, A.W. Laser-induced breakdown spectroscopy (LIBS): An overview of recent progress and future potential for biomedical applications. J. Med. Eng. Technol. 2012, 36, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Allegrini, F.; Olivieri, A.C. Iupac-consistent approach to the limit of detection in partial least-squares calibration. Anal. Chem. 2014, 86, 7858–7866. [Google Scholar] [CrossRef] [PubMed]
- Nicolodelli, G.; Senesi, G.S.; Romano, R.A.; de Oliveira Perazzoli, I.L.; Milori, D.M.B.P. Signal enhancement in collinear double-pulse laser-induced breakdown spectroscopy applied to different soils. Spectrochim. Acta B 2015, 111, 23–29. [Google Scholar] [CrossRef]
- Tognoni, E.; Cristoforetti, G. Basic mechanisms of signal enhancement in ns double-pulse laser-induced breakdown spectroscopy in a gas environment. J. Anal. At. Spectrom. 2014, 29, 1318–1338. [Google Scholar] [CrossRef]
- Li, Y.; Tian, D.; Ding, Y.; Yang, G.; Wang, C.; Han, X. A review of laser-induced breakdown spectroscopy signal enhancement. Appl. Spectrosc. Rev. 2017, 53, 1–35. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; Alrifai, R.; De Giacomo, A. Nanoparticle enhanced laser induced breakdown spectroscopy (nelibs), a first review. Spectrochim. Acta B 2018, 148, 105–112. [Google Scholar] [CrossRef]
- De Giacomo, A.; Dell’Aglio, M.; Gaudiuso, R.; Koral, C.; Valenza, G. Perspective on the use of nanoparticles to improve libs analytical performance: Nanoparticle enhanced laser induced breakdown spectroscopy (nelibs). J. Anal. Atom. Spectrom. 2016, 31, 1566–1573. [Google Scholar] [CrossRef]
- Popov, A.M.; Colao, F.; Fantoni, R. Spatial confinement of laser-induced plasma to enhance LIBS sensitivity for trace elements determination in soils. J. Anal. Atom. Spectrom. 2010, 25, 2491–2494. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, B.; He, X.; Li, C.; Zhou, Y.; Wu, T.; Park, J.B.; Zeng, X.; Lu, Y. Optimally enhanced optical emission in laser-induced breakdown spectroscopy by combining spatial confinement and dual-pulse irradiation. Opt. Express 2012, 20, 1436–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yip, W.L.; Cheung, N.H. Analysis of aluminum alloys by resonance-enhanced laser-induced breakdown spectroscopy: How the beam profile of the ablation laser and the energy of the dye laser affect analytical performance. Spectrochim. Acta B 2009, 64, 315–322. [Google Scholar] [CrossRef]
- Goueguel, C.; Laville, S.; Vidal, F.; Sabsabi, M.; Chaker, M. Investigation of resonance-enhanced laser-induced breakdown spectroscopy for analysis of aluminium alloys. J. Anal. Atom. Spectrom. 2010, 25, 246–253. [Google Scholar] [CrossRef]
- Chen, K.; Lu, J.; Li, J. Real-time, quantitative analysis of multi-elements in liquid steel by LIBS. Spectrosc. Spectr. Anal. 2011, 31, 823–826. [Google Scholar]
- Kim, C.-K.; In, J.-H.; Lee, S.-H.; Jeong, S. Influence of ar buffer gas on the libs signal of thin cigs films. J. Anal. At. Spectrom. 2013, 28, 460–467. [Google Scholar] [CrossRef]
- Sarkar, A.; Mishra, R.K.; Kaushik, C.P.; Wattal, P.K.; Alamelu, D.; Aggarwal, S.K. Analysis of barium borosilicate glass matrix for uranium determination by using NS-IR-LIBS in air and Ar atmosphere. Radiochim. Acta 2014, 102, 805–812. [Google Scholar] [CrossRef]
- Cheri, M.S.; Tavassoli, S.H. Quantitative analysis of toxic metals lead and cadmium in water jet by laser-induced breakdown spectroscopy. Appl. Opt. 2011, 50, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Yang, H.; Huang, L.; Chen, T.; Rao, G.; Liu, M. Detection of heavy metal Cd in polluted fresh leafy vegetables by laser-induced breakdown spectroscopy. Appl. Opt. 2017, 56, 4070–4075. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.-J.; Guo, Z.; Ma, M.-J.; Fang, L.; Yang, M.; Li, S.-S.; Liu, J.-H.; Zhao, N.-J.; Huang, X.-J.; Liu, W.-Q. Electrochemical laser induced breakdown spectroscopy for enhanced detection of cd(ii) without interference in rice on layer-by-layer assembly of graphene oxides. Electrochim. Acta 2016, 216, 188–195. [Google Scholar] [CrossRef]
- Nunes, L.C.; Júnior, D.S.; Krug, F.J. Determination of Cd, Cr and Pb in phosphate fertilizers by laser-induced breakdown spectroscopy. Spectrochim. Acta B 2014, 97, 42–48. [Google Scholar] [CrossRef]
- Yu, K.; Zhao, Y.; Liu, F.; He, Y. Laser-induced breakdown spectroscopy for determining content of Pb and Cd in soil. Trans. CSAE 2016, 32, 197–203. [Google Scholar]
- Pan, A.; Liu, L.; Lin, Z. Analysis of the Cd element in soil by laser-induced breakdown spectroscopy. Appl. Laser 2012, 32, 124–127. [Google Scholar]
- Wang, J.; Wang, J.; Craig, R. Reducing carrier phase errors with EMD-wavelet for precise GPS positioning. In Proceedings of the National Technical Meeting of the Institute of Navigation NTM, San Diego, CA, USA, 22–24 January 2007; The Institute of Navigation: Manassas, VA, USA, 2017; pp. 919–928. [Google Scholar]
- Chen, P.; Tian, D.; Qiao, S.; Yang, G. An automatic peak detection method for LIBS spectrum based on continuous wavelet transform. Spectrosc. Spectr. Anal. 2014, 34, 1969–1972. [Google Scholar]
- Sobron, P.; Wang, A.; Sobron, F. Extraction of compositional and hydration information of sulfates from laser-induced plasma spectra recorded under mars atmospheric conditions—Implications for chemcam investigations on curiosity rover. Spectrochim. Acta B 2012, 68, 1–16. [Google Scholar] [CrossRef]
- Leys, C.; Ley, C.; Klein, O.; Bernard, P.; Licata, L. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 2013, 49, 764–766. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Liu, F.; Shen, T.; Ye, L.; Kong, W.; Wang, W.; Liu, X.; He, Y. Comparative study of the detection of chromium content in rice leaves by 532 nm and 1064 nm laser-induced breakdown spectroscopy. Sensors 2018, 18, 621. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Dong, T.; He, Y.; Xiao, S. Research on the effects of drying temperature on nitrogen detection of different soil types by near infrared sensors. Sensors 2018, 18, 391. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xiao, S.; Nie, P.; Dong, T.; Qu, F.; Lin, L. Research on the optimum water content of detecting soil nitrogen using near infrared sensor. Sensors 2017, 17, 2045. [Google Scholar] [CrossRef] [PubMed]
- Martı́n, M.E.; Hernández, O.M.; Jiménez, A.I.; Arias, J.J.; Jiménez, F. Partial least-squares method in analysis by differential pulse polarography. Simultaneous determination of amiloride and hydrochlorothiazide in pharmaceutical preparations. Anal. Chim. Acta 1999, 381, 247–256. [Google Scholar] [CrossRef]
- Sjöström, M.; Wold, S.; Lindberg, W.; Persson, J. Å.; Martens, H. A multivariate calibration problem in analytical chemistry solved by partial least-squares models in latent variables. Anal. Chim. Acta 1983, 150, 61–70. [Google Scholar] [CrossRef]
- Geladi, P.; Kowalski, B.R. An example of 2-block predictive partial least-squares regression with simulated data. Anal. Chim. Acta 1986, 185, 19–32. [Google Scholar] [CrossRef]
- Peng, J.; Liu, F.; Kong, W.; Zhang, C.; Yu, K.; He, Y. Rapid identification of varieties of walnut powder based on laser-induced breakdown spectroscopy. Trans. ASABE 2017, 60, 19–28. [Google Scholar]
- Boqué, R.; Rius, F.X. Multivariate detection limits estimators. Chemom. Intell. Lab. 1996, 32, 11–23. [Google Scholar] [CrossRef]
- Olivieri, A.C.; Faber, N.M. Standard error of prediction in parallel factor analysis of three-way data. Chemom. Intell. Lab. 2004, 70, 75–82. [Google Scholar] [CrossRef]
- Braga, J.W.; Poppi, R.J. Figures of merit for the determination of the polymorphic purity of carbamazepine by infrared spectroscopy and multivariate calibration. J. Pharm. Sci. 2004, 93, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.P.; Pereirafilho, E.R. Twelve different types of data normalization for the proposition of classification, univariate and multivariate regression models for the direct analyses of alloys by laser-induced breakdown spectroscopy (LIBS). J. Anal. Atom. Spectrom. 2016, 31, 2005–2014. [Google Scholar] [CrossRef]
- Valderrama, P.; Braga, J.W.; Poppi, R.J. Variable selection, outlier detection, and figures of merit estimation in a partial least-squares regression multivariate calibration model. A case study for the determination of quality parameters in the alcohol industry by near-infrared spectroscopy. J. Agric. Food Chem. 2007, 55, 8331–8338. [Google Scholar] [CrossRef] [PubMed]
- Braga, J.W.B.; Trevizan, L.C.; Nunes, L.C.; Rufini, I.A.; Santos, D., Jr.; Krug, F.J. Comparison of univariate and multivariate calibration for the determination of micronutrients in pellets of plant materials by laser induced breakdown spectrometry. Spectrochim. Acta B 2010, 65, 66–74. [Google Scholar] [CrossRef]
- Yin, W.; Zhang, C.; Zhu, H.; Zhao, Y.; He, Y. Application of near-infrared hyperspectral imaging to discriminate different geographical origins of Chinese wolfberries. PLoS ONE 2017, 12, e0180534. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; He, Y.; Feng, S.; Sun, D.-W. Study on infrared spectroscopy technique for fast measurement of protein content in milk powder based on ls-svm. J. Food Eng. 2008, 84, 124–131. [Google Scholar] [CrossRef]
- Devos, O.; Ruckebusch, C.; Durand, A.; Duponchel, L.; Huvenne, J.-P. Support vector machines (SVM) in near infrared (NIR) spectroscopy: Focus on parameters optimization and model interpretation. Chemom. Intell. Lab. 2009, 96, 27–33. [Google Scholar] [CrossRef]
- Li, S.X.; Zhang, Y.J.; Zeng, Q.Y.; Li, L.F.; Guo, Z.Y.; Liu, Z.M.; Xiong, H.L.; Liu, S.H. Potential of cancer screening with serum surface-enhanced Raman spectroscopy and a support vector machine. Laser Phys. Lett. 2014, 11, 065603. [Google Scholar] [CrossRef]
- Liu, F.; Nie, P.; Huang, M.; Kong, W.; He, Y. Nondestructive determination of nutritional information in oilseed rape leaves using visible/near infrared spectroscopy and multivariate calibrations. Sci. China Inf. Sci. 2011, 54, 598–608. [Google Scholar] [CrossRef]
- Liu, F.; He, Y.; Sun, G. Determination of protein content of auricularia auricula using near infrared spectroscopy combined with linear and nonlinear calibrations. J. Agric. Food Chem. 2009, 57, 4520–4527. [Google Scholar] [CrossRef] [PubMed]
- Balabin, R.M.; Lomakina, E.I. Support vector machine regression (SVR/LS-SVM)—An alternative to neural networks (ANN) for analytical chemistry? Comparison of nonlinear methods on near infrared (NIR) spectroscopy data. Analyst 2011, 136, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Chauchard, F.; Cogdill, R.; Roussel, S.; Roger, J.M.; Bellon-Maurel, V. Application of LS-SVM to non-linear phenomena in NIR spectroscopy: Development of a robust and portable sensor for acidity prediction in grapes. Chemom. Intell. Lab. 2004, 71, 141–150. [Google Scholar] [CrossRef]
- Haider, Z.; Munajat, Y.B.; Kamarulzaman, R.; Shahami, N. Comparison of single pulse and double simultaneous pulse laser induced breakdown spectroscopy. Anal. Lett. 2014, 48, 308–317. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Fu, Y.; Li, Z.; Liu, J.; Ni, W. Application of a spectrum standardization method for carbon analysis in coal using laser-induced breakdown spectroscopy (LIBS). Appl. Spectrosc. 2014, 68, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Fu, Y.; Li, Z.; Ni, W. Wavelength dependence in the analysis of carbon content in coal by nanosecond 266 nm and 1064 nm laser induced breakdown spectroscopy. Plasma Sci. Technol. 2015, 17, 621–624. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liu, J.T.; Hirak, D.M.; Weckman, D.C.; Kerr, H.W. Determining the spot size and gaussian distribution coefficient of pulsed laser beams using kapton films. J. Laser Appl. 1993, 5, 5–12. [Google Scholar] [CrossRef]
- Liu, D. Investigations of the Process of Laser Ablation on Titannium and the Properties of the Plasma. Ph.D. Thesis, Shandong Normal University, Jinan, China, June 2016. [Google Scholar]
- Lida, Y. Atomic emission characteristics of laser-induced plasmas in an argon atmosphere at reduced pressure. Appl. Spectrosc. 1989, 43, 229–234. [Google Scholar]
- Son, J.G.; Choi, S.C.; Oh, M.K.; Kang, H.; Suk, H.; Lee, Y. Application of pulsed buffer gas jets for the signal enhancement of laser-induced breakdown spectroscopy. Appl. Spectrosc. 2010, 64, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Li, H. DP-LIBS for the Research and Implementation of Carbon Content Detection in Iron-Based Alloy. Master’s Thesis, Changchun University of Technology, Changchun, China, April 2015. [Google Scholar]
Sample Availability: Samples of the soil are not available from the authors. |

| Data | Model | Parameter | R2C | RMSEC | R2P | RMSEP |
|---|---|---|---|---|---|---|
| Univariate | - | 0.862 | 0.101 | 0.849 | 0.105 | |
| Air | PLS-DA | 3 | 0.959 | 0.053 | 0.940 | 0.065 |
| LS-SVM | (4.359 × 107, 2.857 × 107) | 0.976 | 0.038 | 0.960 | 0.055 | |
| Univariate | - | 0.935 | 0.068 | 0.911 | 0.095 | |
| Ar | PLS-DA | 2 | 0.981 | 0.035 | 0.973 | 0.051 |
| LS-SVM | (3.922 × 104, 753.284) | 0.999 | 0.026 | 0.988 | 0.034 |
| Condition | Volume/μm3 | Cross-Sectional Area/μm2 | Maximum Depth/μm |
|---|---|---|---|
| Air | 2.15628 × 106 ± 1 × 10 | 1.9626 × 105 ± 1 × 10 | 22.0 ± 0.1 |
| Ar | 3.89284 × 106 ± 2 × 10 | 1.5568 × 105 ± 1 × 10 | 36.5 ± 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Liu, F.; Huang, W.; Peng, J.; Shen, T.; He, Y. Quantitative Determination of Cd in Soil Using Laser-Induced Breakdown Spectroscopy in Air and Ar Conditions. Molecules 2018, 23, 2492. https://doi.org/10.3390/molecules23102492
Liu X, Liu F, Huang W, Peng J, Shen T, He Y. Quantitative Determination of Cd in Soil Using Laser-Induced Breakdown Spectroscopy in Air and Ar Conditions. Molecules. 2018; 23(10):2492. https://doi.org/10.3390/molecules23102492
Chicago/Turabian StyleLiu, Xiaodan, Fei Liu, Weihao Huang, Jiyu Peng, Tingting Shen, and Yong He. 2018. "Quantitative Determination of Cd in Soil Using Laser-Induced Breakdown Spectroscopy in Air and Ar Conditions" Molecules 23, no. 10: 2492. https://doi.org/10.3390/molecules23102492






