One-Pot Synthesis of 3-Functionalized 4-Hydroxycoumarin under Catalyst-Free Conditions
Abstract
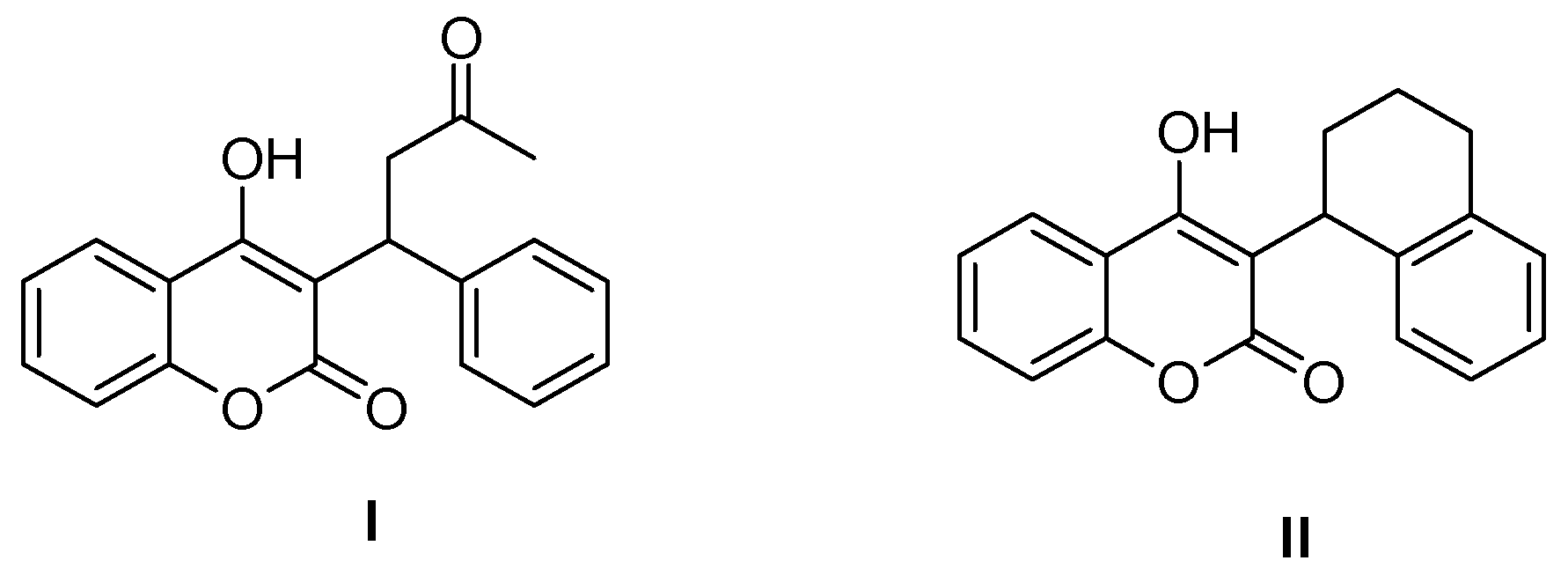
:1. Introduction
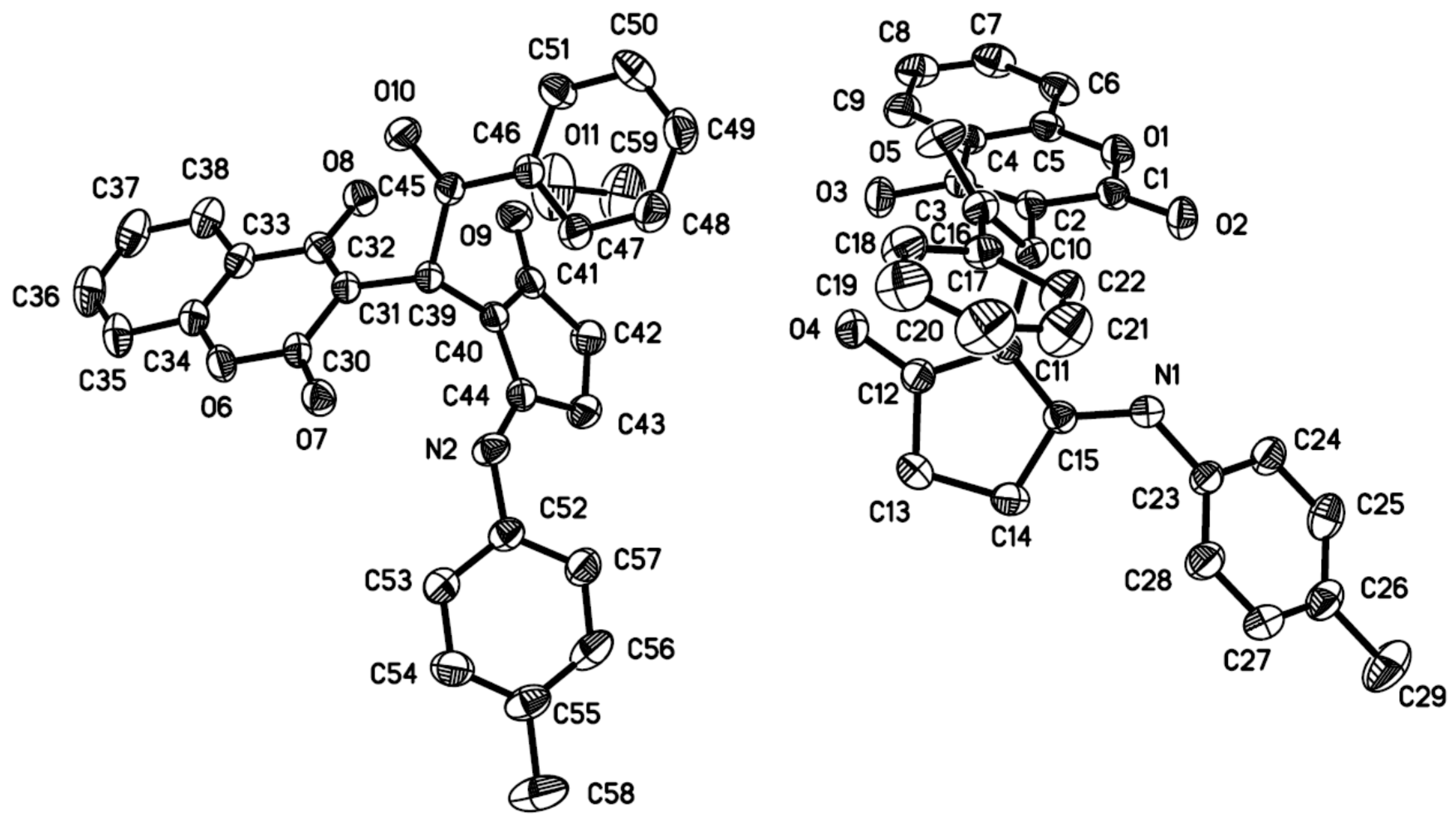
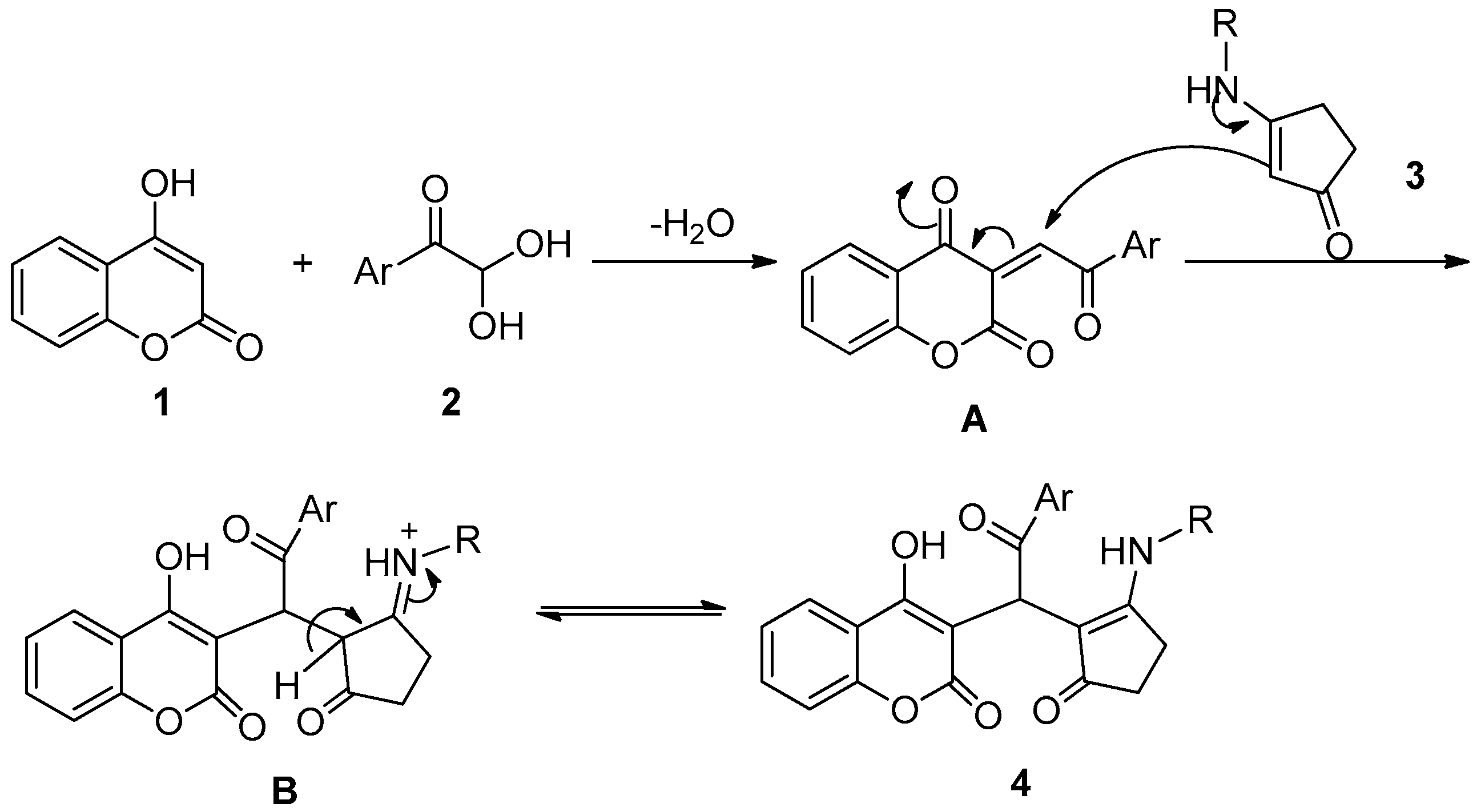
2. Results and Discussion
3. Experimental
3.1. General
3.2. General Procedure for the Synthesis of 3-Functionalized 4-Hydroxycoumarin Derivatives 4 and 6
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Boger, D.L.; Boyce, C.W.; Labroli, M.A.; Sehon, C.A.; Jin, Q. Total Synthesis of Ningalin A, Lamellarin O, Lukianol A, and Permethyl Storniamide A Utilizing Heterocyclic Azediene Diels-Alder Reactions. J. Am. Chem. Soc. 1999, 121, 54–62. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kurazono, M. A new class of anti-MRSA and anti-VRE agents: Preparation and antibacterial activities of indole-containing compounds. Bioorg. Med. Chem. Lett. 2007, 17, 1626–1628. [Google Scholar] [CrossRef] [PubMed]
- Fylaktakidou, K.C.; Hadjipavloi-Litina, D.J.; Litinas, K.E.; Nicolaides, D.N. Natural and Synthetic Coumarin Derivatives with Anti-Inflammatory/Antioxidant Activities. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef] [PubMed]
- Thaisivongs, S.; Watenpaugh, K.D.; Howe, W.J.; Tomich, P.K.; Dolak, L.A.; Chong, K.T.; Tomich, C.S.C.; Tomasselli, A.G.; Tuner, S.R.; Strohbach, J.W.; et al. Structure-based design of novel HIV protease inhibitors: Carboxamide-containing 4-hydroxycoumarins and 4-hydroxy-2-pyrones as potent nonpeptidic inhibitors. J. Med. Chem. 1995, 38, 3624–3637. [Google Scholar] [CrossRef]
- Stanchev, S.; Momekov, G.; Jensen, F.; Manolov, I. Synthesis, computational study and cytotoxic activity of new 4-hydroxycoumarin derivatives. Eur. J. Med. Chem. 2008, 43, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafez, O.M.; Amin, K.M.; Batran, R.Z.; Maher, T.J.; Nada, S.A.; Sethumadhavan, S. Synthesis, anticoagulant and PIVKA-II induced by new 4-hydroxycoumarin derivatives. Bioorg. Med. Chem. 2010, 18, 3371–3378. [Google Scholar] [CrossRef] [PubMed]
- Stanchev, S.; Hadjimitova, V.; Traykov, T.; Boyanov, T.; Manolova, I. Investigation of the antioxidant properties of some new 4-hydroxycoumarin derivatives. Eur. J. Med. Chem. 2009, 44, 3077–3082. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, R.A. The Stereoselective Interaction of Warfarin and Metronidazole in Man. N. Engl. J. Med. 1976, 295, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Dömling, A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Feng, X.; Xun, Z.; Shi, D.Q.; Huang, Z.B. Multicomponent Strategy to Pyrazolo[3,4-e]indolizine Derivatives under Microwave Irradiation. J. Org. Chem. 2015, 80, 8435–8442. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gupta, M.K.; Kumar, M. An efficient non-ionic surfactant catalyzed multicomponent synthesis of novel benzylamino coumarin derivative via Mannich type reaction in aqueous media. Tetrahedron Lett. 2011, 52, 4521–4525. [Google Scholar] [CrossRef]
- Ghosh, P.P.; Das, A.R. Nano crystalline ZnO: A competent and reusable catalyst for one pot synthesis of novel benzylamino coumarin derivatives in aqueous media. Tetrahedron Lett. 2012, 53, 3140–3143. [Google Scholar] [CrossRef]
- Wang, H.Y.; Liu, X.C.; Feng, X.; Huang, Z.B.; Shi, D.Q. GAP chenistry for pyrrolyl coumarin derivatives: A highly efficient one-pot synthesis under catalyst-free conditions. Green Chem. 2013, 15, 3307–3311. [Google Scholar] [CrossRef]
- Khodabakhshi, S.; Karami, B. Tungstate sulfuric acid catalyzed one-pot synthesis of a new class of aroylamido coumarins under solvent-free conditions. Tetrahedron Lett. 2014, 55, 7136–7139. [Google Scholar] [CrossRef]
- Yaragola, S.; Pareek, A.; Dada, R. Ca(II)-catalyzed, one-pot four-component synthesis of functionally embellished benzyl pyrazolyl coumarins in water. Tetrahedron Lett. 2015, 56, 4770–4774. [Google Scholar] [CrossRef]
- Fu, L.; Feng, X.; Zhang, J.J.; Hu, J.D.; Xun, Z.; Wang, J.J.; Huang, Z.B.; Shi, D.Q. Highly efficient construction of a bridged pentacyclic skeleton via a six-component domino reaction under microwave irradiation. Green Chem. 2015, 17, 1535–1545. [Google Scholar] [CrossRef]
- Cao, C.P.; Xu, C.L.; Lin, W.; Li, X.M.; Hu, M.H.; Wang, J.X.; Huang, Z.B.; Shi, D.Q.; Wang, Y.C. Microwave-assisted improved synthesis of pyrrolo[2,3,4-kl]acridine and dihydropyrrolo[2,3,4-kl]acridine derivatives catalyzed by silica sulfuric acid. Molecules 2013, 18, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Narumi, T.; Nomura, W.; Tamamura, H. Microwave-assisted synthesis of azacoumarin fluorophores and the fluorescene characterization. J. Org. Chem. 2017, 82, 2739–2744. [Google Scholar] [CrossRef] [PubMed]
- Bouasla, S.; Amaro-Gahete, J.; Esquivel, D.; López, M.I.; Jiménez-Sanchidrián, C.; Teguiche, M.; Romero-Salguero, F.J. Coumarin derivatives solvent-free synthesis under microwave irradiation over heterogeneous solid catalysts. Molecules 2017, 22, 2072. [Google Scholar] [CrossRef] [PubMed]
- Kattuboina, A.; Kaur, P.; Nguyen, T.; Li, G. Chiral N-phosphonyl imine chemistry: Asymmetric 1,2-additions of allylmagnesium bromides. Tetrahedron Lett. 2008, 49, 3722–3724. [Google Scholar] [CrossRef]
- An, G.; Seifert, C.; Li, G. N-Phosphonyl/phosphinyl imines and group-assisted purification (GAP) chemistry/technology. Org. Biomol. Chem. 2015, 13, 1600–1617. [Google Scholar] [CrossRef] [PubMed]
- Kattamuri, P.V.; Xiong, Y.; Pan, Y.; Li, G.N. N-Diisopropyl-N-phosphonyl imines lead to efficient asymmetric synthesis of aziridine-2-carboxylic esters. Org. Biomol. Chem. 2013, 11, 3400–3408. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Wever, W.; Pindi, S.; Milles, R.; Gu, P.; Shi, M.; Li, G. The GAP chemistry for chiral N-phosphonyl imine-based Strecker reaction. Green Chem. 2011, 13, 1288–1292. [Google Scholar] [CrossRef]
- Pindi, S.; Wu, J.; Li, G. Design, Synthesis, and Applications of Chiral N-2-phenyl-2-propyl Sulfinyl Imines for Group-Assisted Purification (GAP) Asymmetric Synthesis. J. Org. Chem. 2013, 78, 4006–4012. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Rezvanian, A.; Zhu, L.G. Synthesis of Heterocyclic [3.3.3]Propellanes via a Sequential Four-Component Reaction. J. Org. Chem. 2012, 77, 4385–4390. [Google Scholar] [CrossRef] [PubMed]
- Chennapuram, M.; Emmadi, N.R.; Bingi, C.; Nanubolu, J.B.; Atmakur, K. Group-assisted pueification (GAP) chemistry for dihydrofurans: Water as a medium for catalyst free synthesis in a one pot four component reaction. Green Chem. 2014, 16, 3237–3246. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.R.; Lin, X.R.; Yan, S.J.; Lin, J. Catalyst-free cascade reaction of heterocyclic ketene aminals with N-substituted maleimide to synthesise bicyclic pyrrolidine derivatives. RSC Adv. 2014, 4, 27582–27590. [Google Scholar] [CrossRef]
- Wang, J.X.; Bai, X.G.; Xu, C.L.; Wang, Y.C.; Lin, W.; Zou, Y.; Shi, D.Q. Ultrasound-Promoted One-Pot, Three-Component Synthesis of Spiro[indoline-3,1’-pyrazolo[1,2-b]phthalazine] derivatives. Molecules 2012, 17, 8674–8686. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Li, Q.; Zhang, J.J.; Lin, W.; Wang, J.X.; Wang, Y.C.; Huang, Z.B.; Shi, D.Q. Ultrasound-Promoted One-Pot, Four-Component Synthesis of Pyridine-2(1H)-One Derivatives. Molecules 2013, 18, 14519–14528. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Lin, W.; Liu, H.T.; Hu, M.H.; Feng, X.; Ren, J.F.; Huang, Z.B.; Shi, D.Q. An efficient synthesis coumarino[4,3-d]pyrazolo[3,4-b]pyridine derivatives catalyzed by silica sulfuric acid under microwave irradiation. Chin. J. Org. Chem. 2015, 35, 927–933. [Google Scholar] [CrossRef]
- Wang, J.X.; Gao, Y.; Zhang, J.J.; Zhang, G.N.; Ren, J.F.; Zhao, Y.; Wang, Y.C.; Shi, D.Q. An efficient and multi-component synthesis of 3-imino-3,5-dihydro-2H-chromeno[3,4-e]pyridine-2-one derivatives. Heterocycles 2017, 96, 1143–1151. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 4a–4k and 6a–6i are available from the authors. |
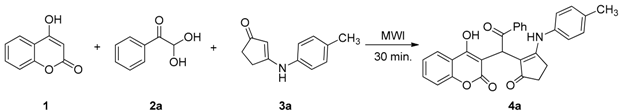
| Entry | Catalyst (mol %) | Solvent (v/v) | Temperature (°C) | Time (min) | Yield (%) a |
|---|---|---|---|---|---|
| 1 | No | EtOH | 100 | 30 | 89 |
| 2 | No | H2O | 100 | 30 | 36 |
| 3 | No | DMF | 100 | 30 | 70 |
| 4 | No | CH3CN | 100 | 30 | 80 |
| 5 | No | EtOH-H2O (1:1) | 100 | 30 | 54 |
| 6 | No | EtOH:H2O (3:1) | 100 | 30 | 72 |
| 7 | NaOH (20) | EtOH | 100 | 30 | 33 |
| 8 | Et2NH (20) | EtOH | 100 | 30 | 50 |
| 9 | p-TSA (20) | EtOH | 100 | 30 | 80 |
| 10 | Benzoic Acid (20) | EtOH | 100 | 30 | 51 |
| 11 | L-Proline (20) | EtOH | 100 | 30 | 81 |
| 12 | No | EtOH | 80 | 30 | 69 |
| 13 | No | EtOH | 90 | 30 | 76 |
| 14 | No | EtOH | 110 | 30 | 87 |
| 15 | No | EtOH | 100 | 10 | 57 |
| 16 | No | EtOH | 100 | 20 | 65 |
| 17 | No | EtOH | 100 | 40 | 86 |
| 18 | No | EtOH | Reflux (absence of microwave) | 240 | 60 |

| Entry | R1 | R2 | Product | Isolated Yield (%) |
|---|---|---|---|---|
| 1 | H | CH3 | 4a | 86 |
| 2 | CH3 | Br | 4b | 90 |
| 3 | CH3 | Cl | 4c | 79 |
| 4 | CH3O | Br | 4d | 85 |
| 5 | CH3O | CH3 | 4e | 86 |
| 6 | CH3O | CH3O | 4f | 81 |
| 7 | Cl | Br | 4g | 95 |
| 8 | Cl | CH3 | 4h | 72 |
| 9 | Cl | CH3O | 4i | 83 |
| 10 | Br | Br | 4j | 70 |
| 11 | Br | CH3 | 4k | 77 |
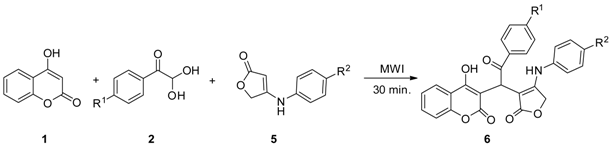
| Entry | R1 | R2 | Product | Isolated Yield (%) |
|---|---|---|---|---|
| 1 | H | Br | 6a | 89 |
| 2 | H | CH3O | 6b | 84 |
| 3 | Cl | CH3 | 6c | 92 |
| 4 | Cl | Cl | 6d | 70 |
| 5 | Cl | Br | 6e | 91 |
| 6 | Br | CH3 | 6f | 91 |
| 7 | Br | CH3O | 6g | 77 |
| 8 | CH3O | CH3O | 6h | 91 |
| 9 | CH3O | Cl | 6i | 72 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Zhang, G.-N.; Wang, J.; Bai, X.; Li, Y.; Wang, Y. One-Pot Synthesis of 3-Functionalized 4-Hydroxycoumarin under Catalyst-Free Conditions. Molecules 2018, 23, 235. https://doi.org/10.3390/molecules23010235
Gao Y, Zhang G-N, Wang J, Bai X, Li Y, Wang Y. One-Pot Synthesis of 3-Functionalized 4-Hydroxycoumarin under Catalyst-Free Conditions. Molecules. 2018; 23(1):235. https://doi.org/10.3390/molecules23010235
Chicago/Turabian StyleGao, Yang, Guo-Ning Zhang, Juxian Wang, Xiaoguang Bai, Yiliang Li, and Yucheng Wang. 2018. "One-Pot Synthesis of 3-Functionalized 4-Hydroxycoumarin under Catalyst-Free Conditions" Molecules 23, no. 1: 235. https://doi.org/10.3390/molecules23010235





