A Semi-Pilot Photocatalytic Rotating Reactor (RFR) with Supported TiO2/Ag Catalysts for Water Treatment
Abstract
:1. Introduction
Importance of the Investigation
2. Materials and Methods
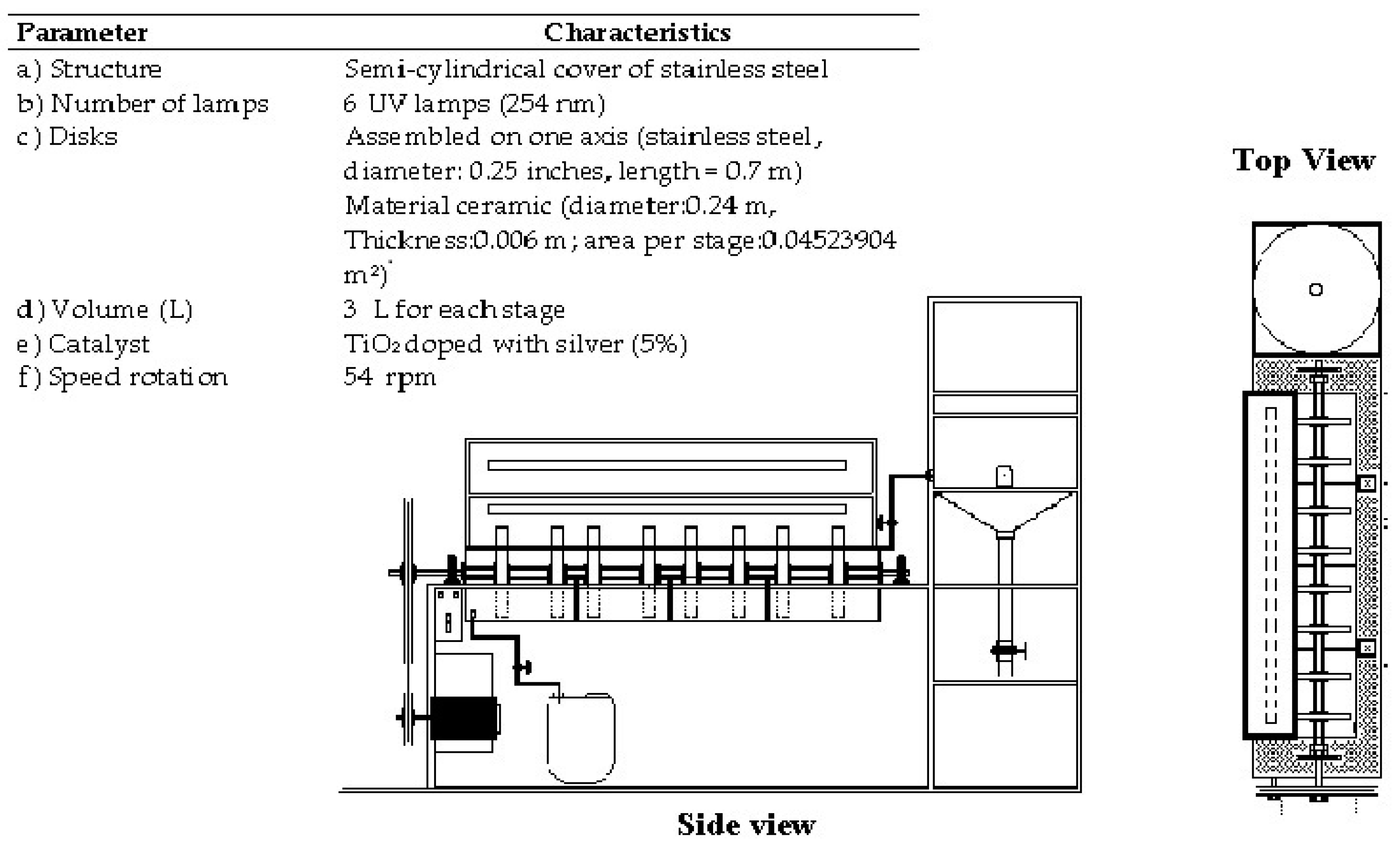
2.1. Characteristics of RFR
2.2. Synthesis and Coupling of the Catalyst TiO2/Ag to the RFR
2.3. Characterization of the Catalyst
2.4. Photoactivity Tests
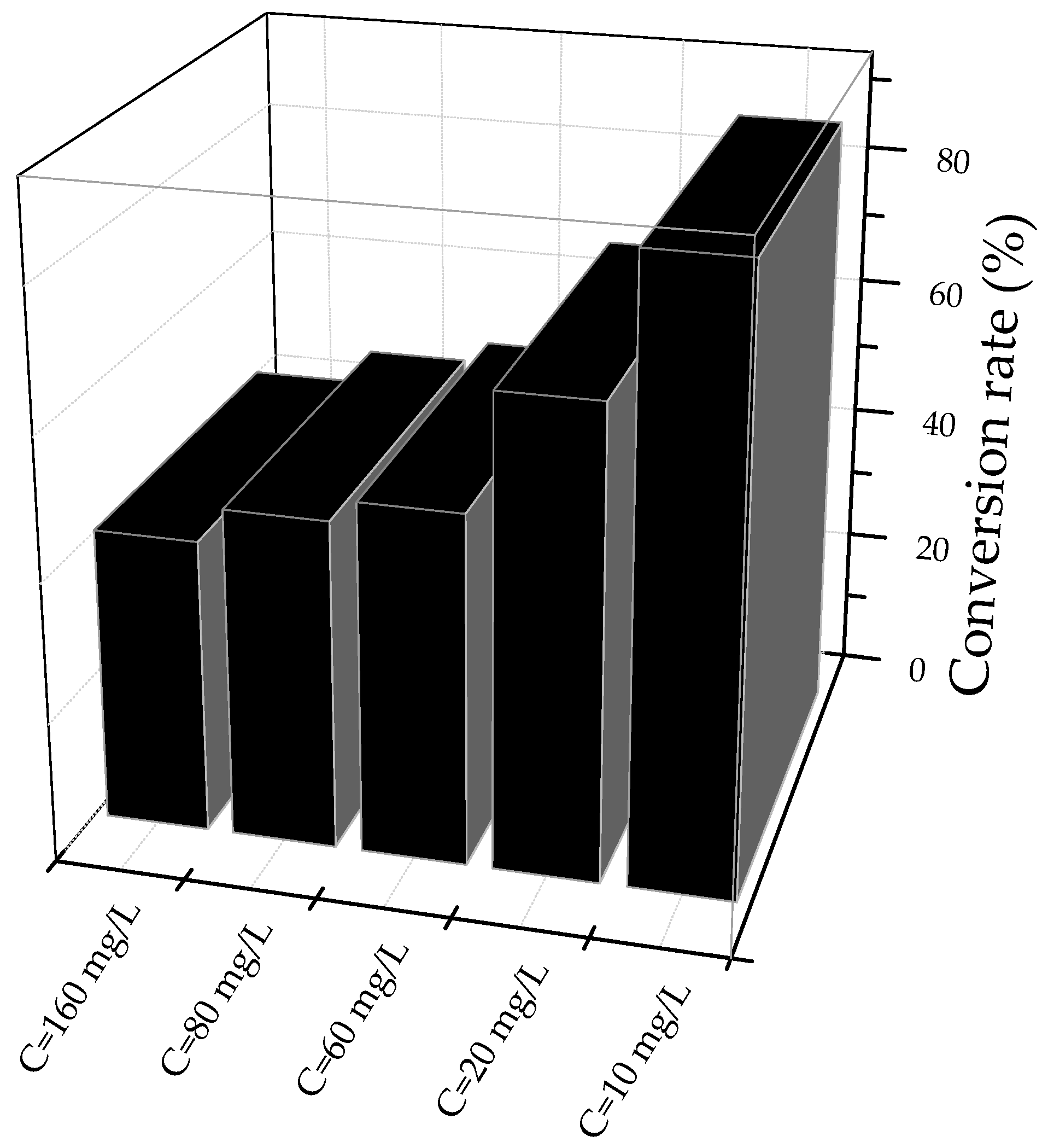
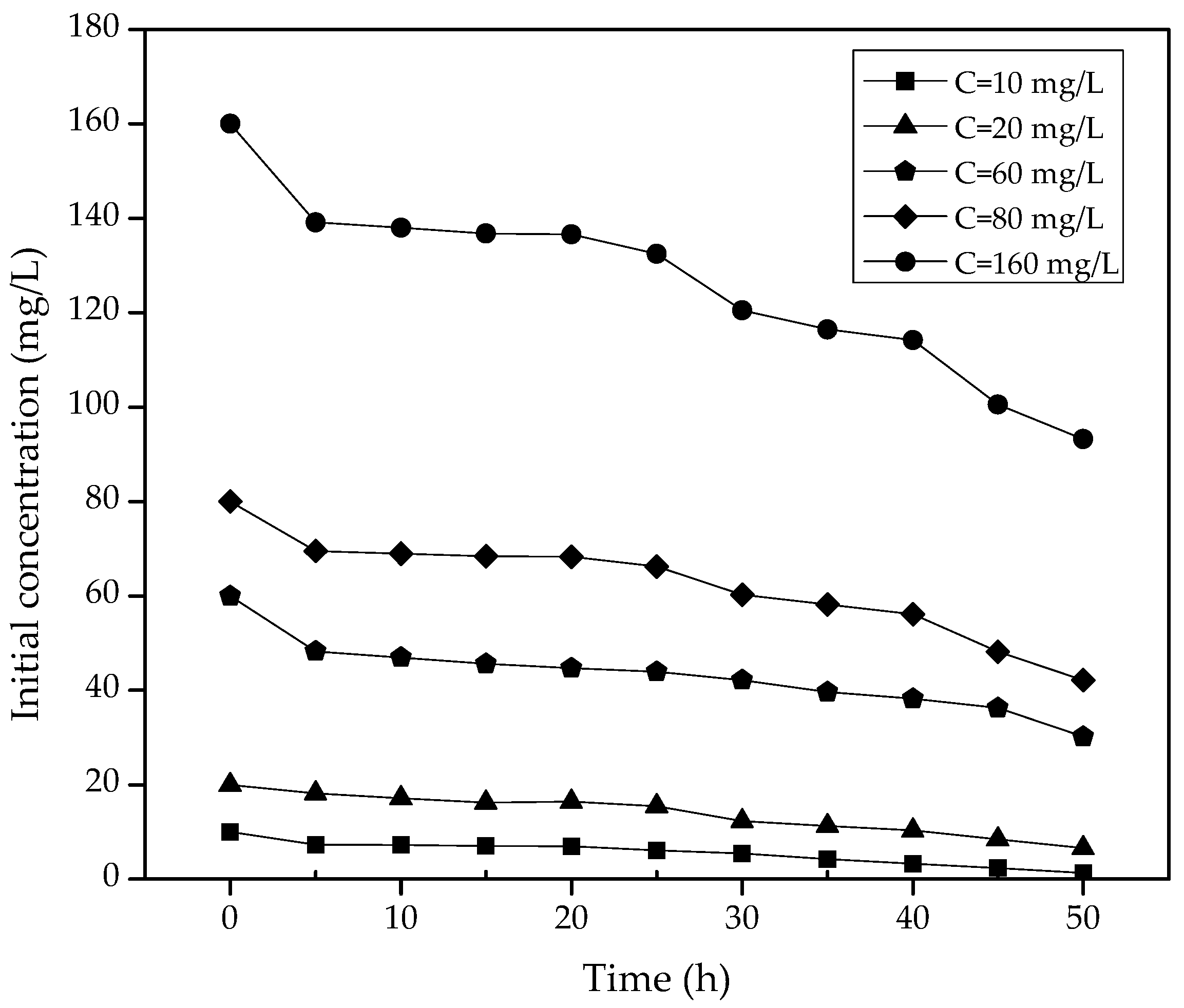
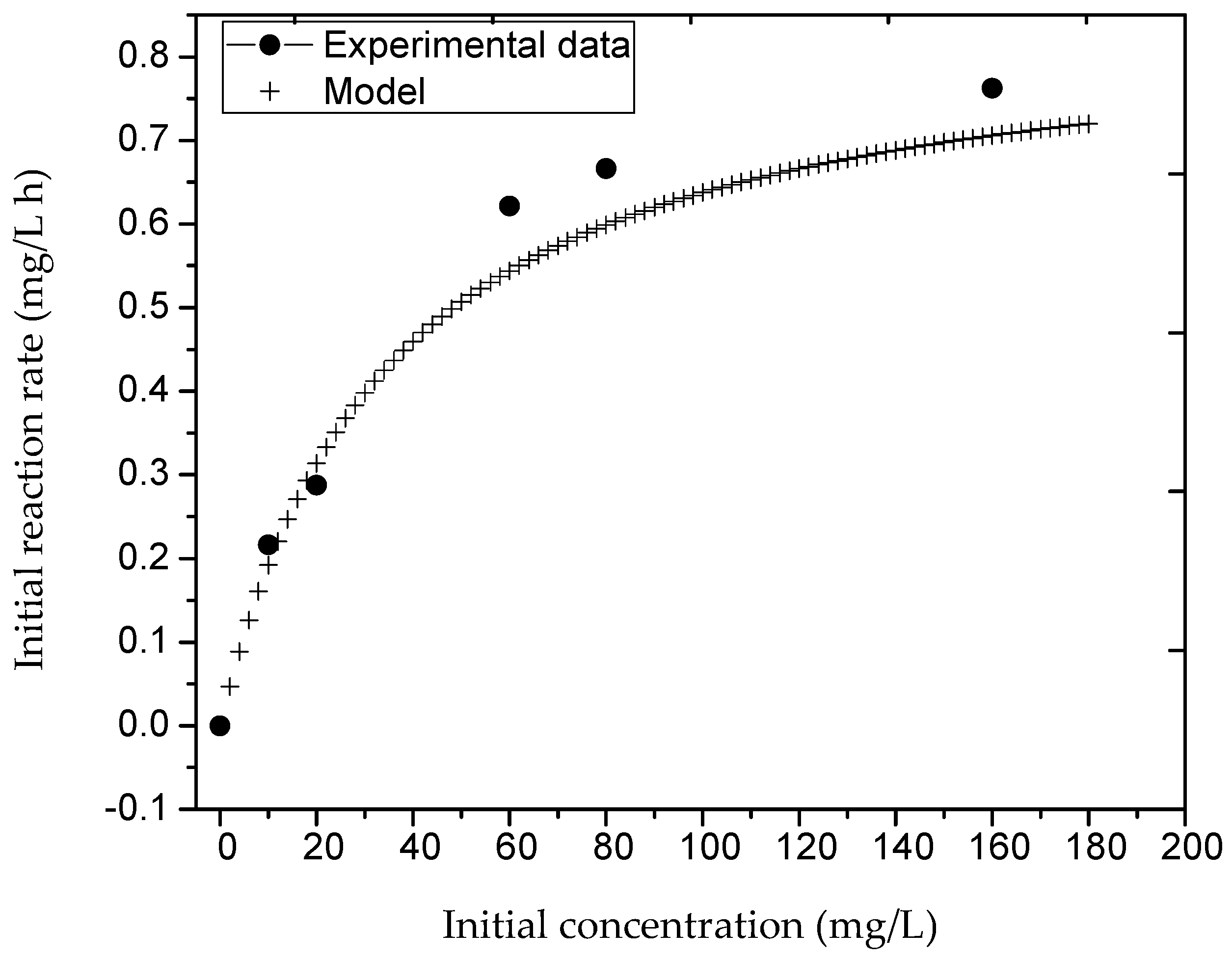
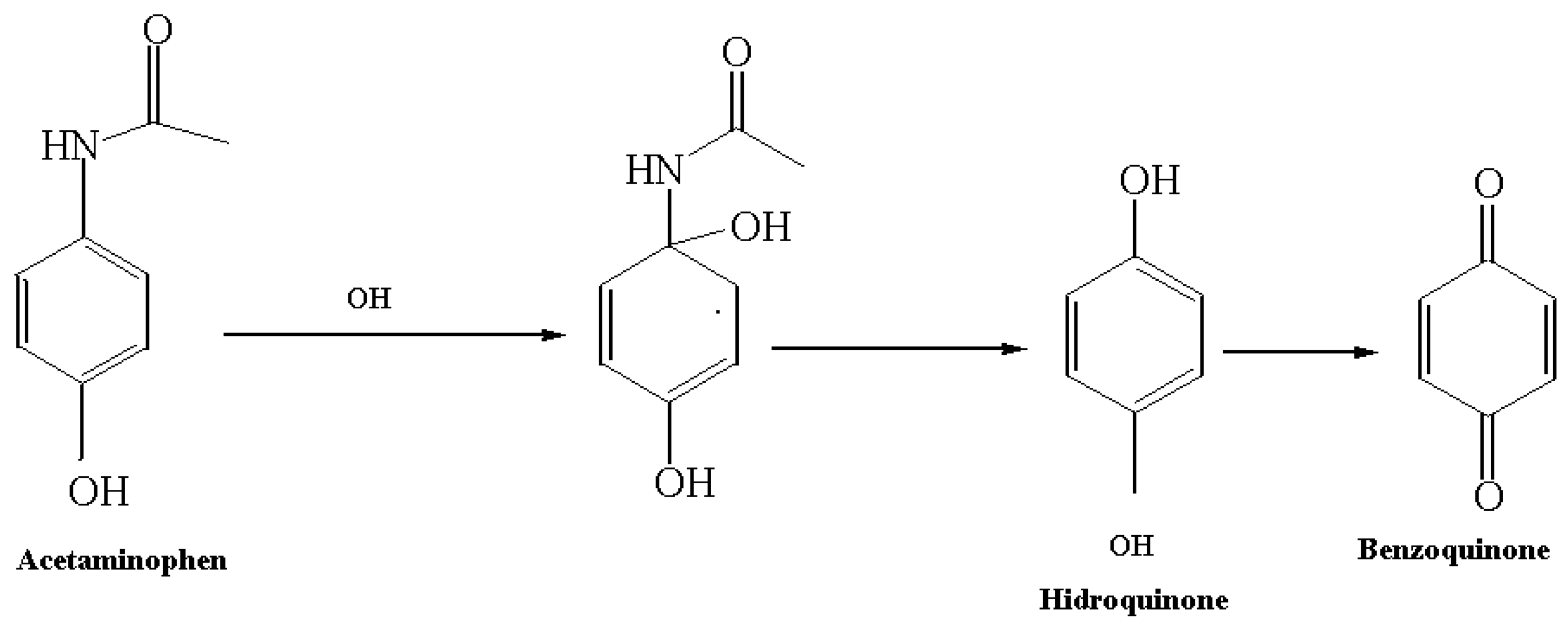
3. Results
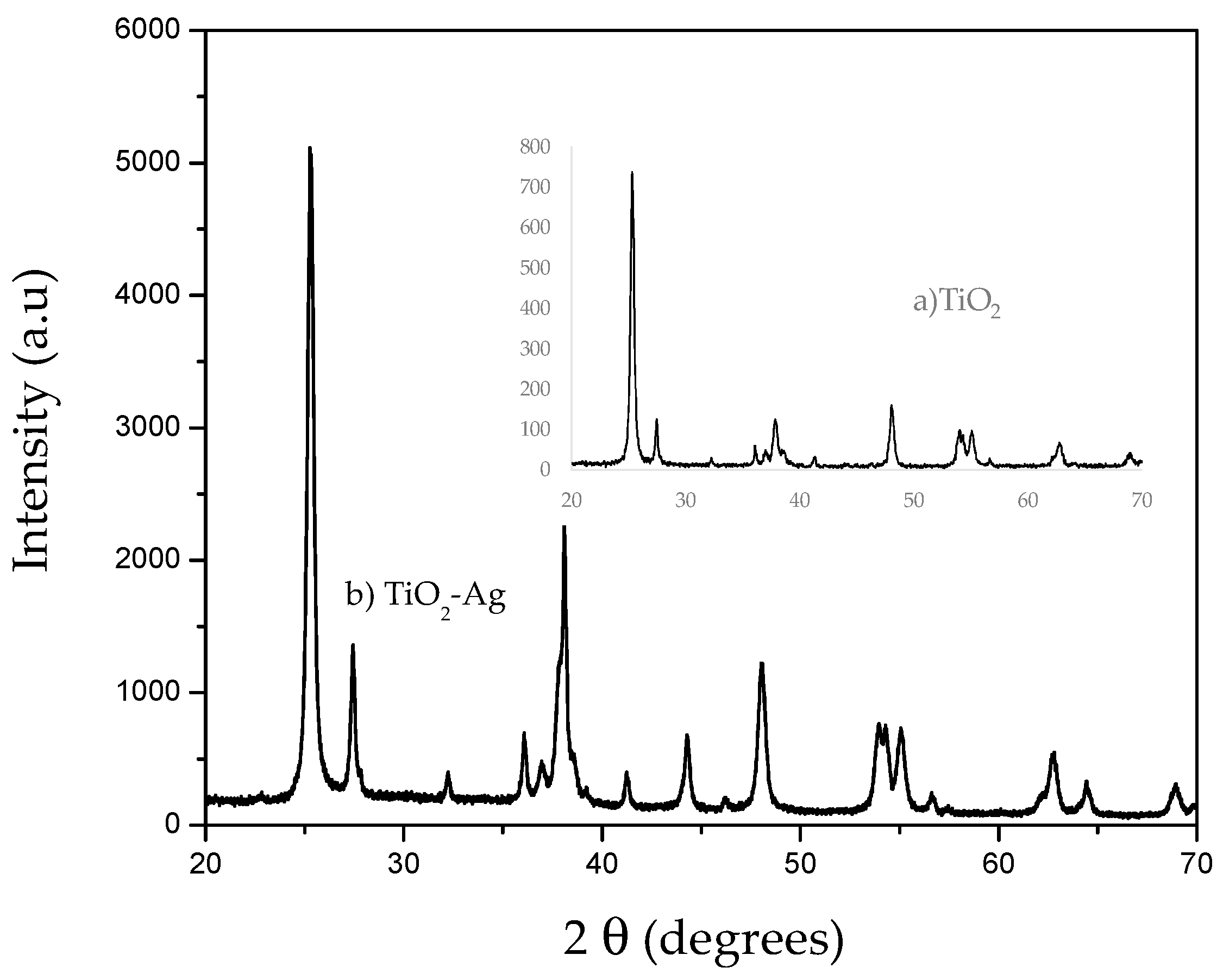
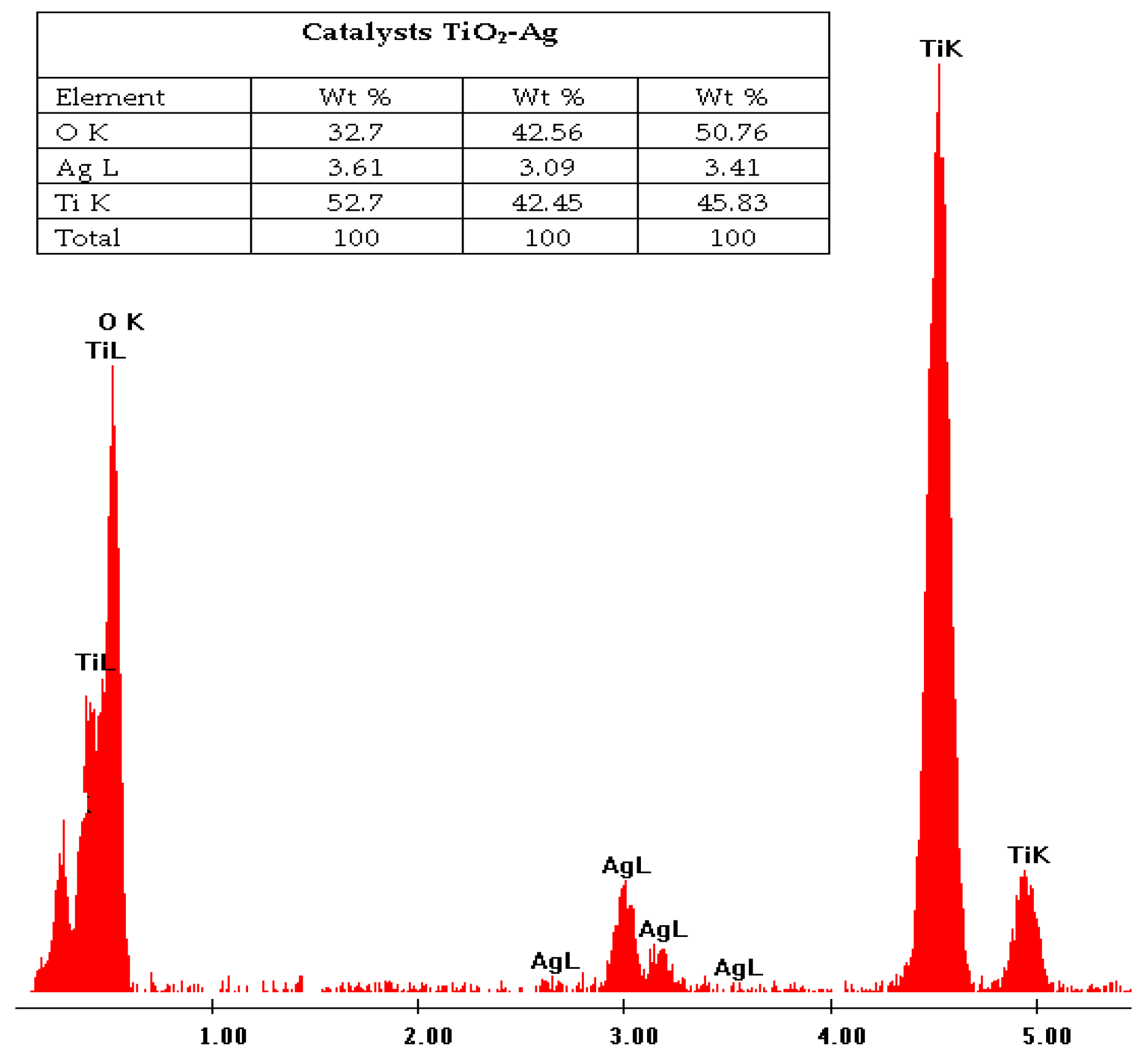
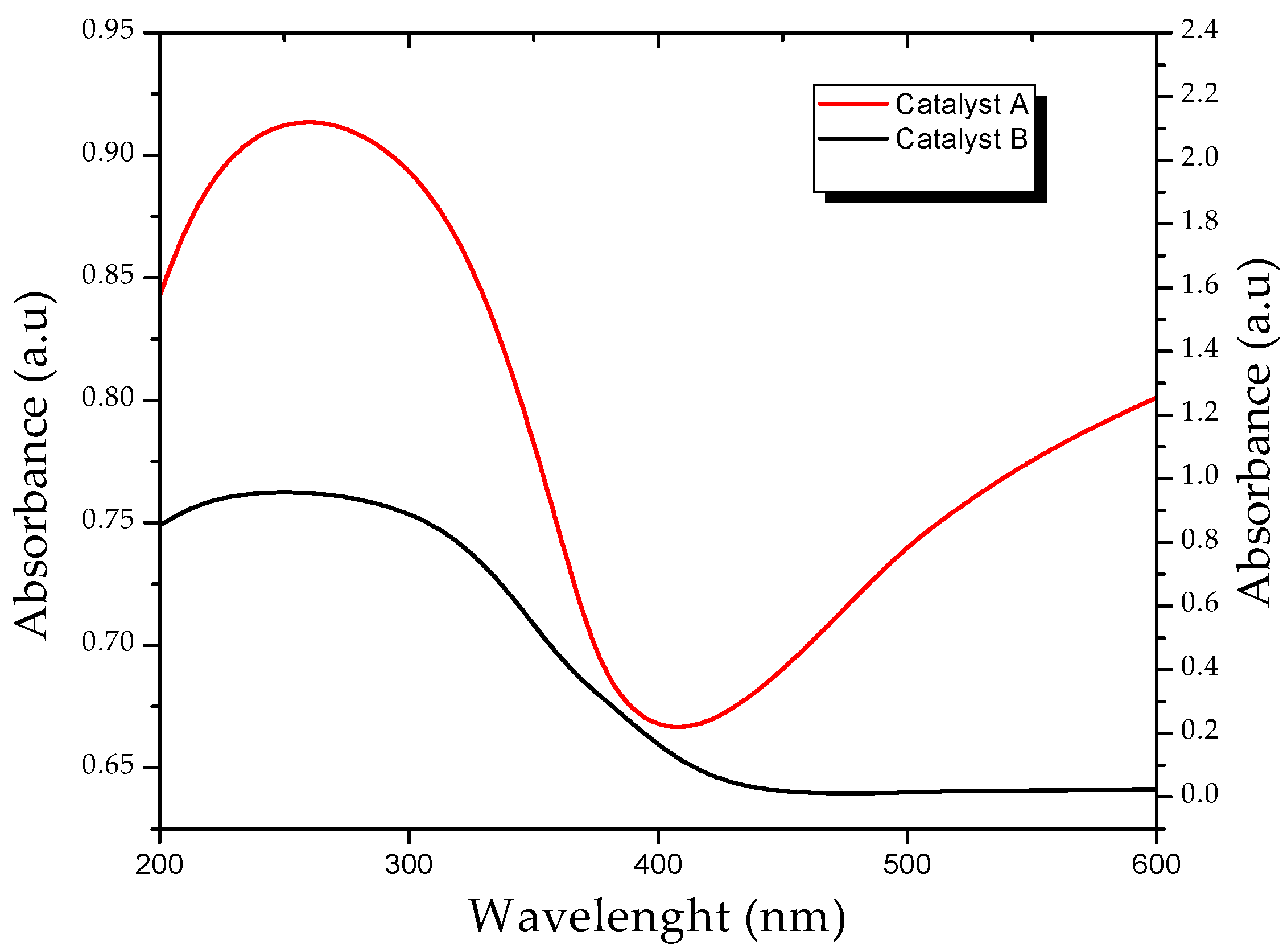
3.1. Characterization of the Catalyst (XRD, EDS and Diffuse Reflectance)
3.2. Kinetics of the Reaction
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lacombe, S.; Keller, N. Photocatalysis: Fundamentals and applications in JEP 2011. Environ. Sci. Pollut. Res. 2012, 19, 3651–3654. [Google Scholar] [CrossRef] [PubMed]
- Kazuya, N.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar]
- Maeda, K. Photocatalytic water splitting using semiconductor particles: History and recent developments. J. Photochem. Photobiol. C 2011, 12, 237–268. [Google Scholar] [CrossRef]
- Hinstho, N.; Petrik, L.; Nechaev, A.; Titinchi, S.; Ndungu, P. Photocatalytic activity of titanium dioxide carbon nanotube composites modified with silver and palladium nanoparticles. Appl. Catal. B Environ. 2014, 156, 273–283. [Google Scholar]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2017, in press. [Google Scholar] [CrossRef]
- Gmurek, M.; Olak-Kucharczyk, M.; Ledakowicz, S. Photochemical decomposition of endocrine disrupting compounds—A review. Chem. Eng. J. 2017, 310, 437–456. [Google Scholar] [CrossRef]
- Cesaro, A.; Belgiorno, V. Removal of endocrine disruptors from urban wastewater by advanced oxidation processes (AOPs): A review. Open Biotechnol. J. 2016, 10. [Google Scholar] [CrossRef]
- Sornalingam, K.; McDonagh, A.; Zhou, J.L. Photodegradation of estrogenic endocrine disrupting steroidal hormones in aqueous systems: Progress and future challenges. Sci. Total Environ. 2016, 550, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of pharmaceuticals and endocrine disrupting compounds from water by zinc oxide-based photocatalytic degradation: A review. Sustain. Cities Soc. 2016, 27, 407–418. [Google Scholar] [CrossRef]
- Yujie, H.; Sutton, N.B.; Rijnaarts, H.H.; Langenhoff, A.M. Degradation of pharmaceuticals in wasterwater using immobilized TiO2 photocatalysis under simulated solar radiation. Appl. Catal. B Environ. 2016, 182, 132–141. [Google Scholar]
- Dionysiou, D.T.; Suidan, M.; Baudin, I.; Lainé, J.M. Oxidation of organic contaminants in a rotating disk photocatalytic reactor: Reaction kinetics in the liquid phase and the role of mass transfer based on the dimensionless Damköhler number. Appl. Catal. B Environ. 2002, 38, 1–16. [Google Scholar] [CrossRef]
- Zhang, A.; Zhou, M.; Han, L.; Zhou, Q. The combinating of rotating disk photocatalytic reactor and TiO2 nanotube arrays for the environmental pollutants removal. J. Hazard. Mater. 2011, 186, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.N.; Chang, C.Y.; Huang, H.J.; Tsai, D.P.; Wu, N.L. Photocatalytic degradation of methyl orange by a multilayer rotating disk reactor. Environ. Sci. Pollut. Res. 2012, 19, 3743–3750. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yi, H.; Diwen, Y.; Ye, Y.; Tiantian, T.; Yaling, W.; Jinping, J. Ahigly efficient dual rotating disk photocatalytic fuel cell with wedged surface TiO2 nanopore anode and hemoglobin cathode. Catalysts 2016, 6, 2–12. [Google Scholar]
- Li, K.; Zhang, H.; He, Y.; Tang, T.; Ying, D.; Wang, Y.; Sun, T.; Jia, J. Novel wedge structured rotating disk photocatalytic reactor for post treatment of actual textile wasterwater. Chem. Eng. J. 2015, 268, 10–20. [Google Scholar] [CrossRef]
- Fang, L.; Wai, S.; Haibao, H.; Jiantao, L.; Leung, Y.C. A photocatalytic rotating disc reactor with TiO2 nanowire arrays deposited for industrial wasterwater treatment. Molecules 2017, 22, 2–13. [Google Scholar]
- Kim, S.; Cho, H.; Joo, H.; Her, N.; Han, J.; Yi, K.; Kim, J.; Yoon, J. Evaluation of performance with small and scale-up rotating and flat-reactors; photocatalytic degradation of bisphenol A, 17β-estradiol, and 17α-ethynyl estradiol under solar irradiation. J. Hazard. Mater. 2017, 336, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, R.D.K.; Mohammad, S.S.; Abdul, A.A.R.; Wan, M.A.W.D. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar]
- Reli, M.; Koci, K.; Matejka, V.; Kovar, P.; Obalova, L. Effect of calcination temperature and calcination time on the kaolinite/TIO2 composite for photocatalytic reduction of CO2. Geosci. Eng. 2012, 58, 10–22. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, B.; Chen, J. Effects of calcination temperature on preparation of boron-doped TiO2 by sol-gel method. Int. J. Photoenergy 2012, 2012. [Google Scholar] [CrossRef]
- Murashkina, A.A.; Murzin, P.D.; Rudakova, A.V.; Ryabchuk, V.K.; Emeline, A.V.; Bahnemann, D.W. Influence of the dopant concentration on the photocatalytic activity: Al-doped TiO2. J. Phys. Chem. C 2015, 119, 24695–24703. [Google Scholar] [CrossRef]
- Cui, X.; Xu, W.; Xie, Z.; Dorman, J.A.; Gutierrez, M.T.; Wang, Y. Effect of dopant concentration on visible light driven photocatalytic activity of Sn1-x AgxS2. Dalton Trans. 2016, 45, 16290–16297. [Google Scholar] [CrossRef] [PubMed]
- Liga, M.V.; Bryant, E.L.; Colvin, V.L.; Li, Q. Virus inactivation by silver doped titanium dioxide nanoparticles for drinking water treatment. Water Res. 2011, 45, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Serry, M.K.; Reenamole, G.; Floris, P.; Pillai, S.C. Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J. Photochem. Photobiol. A Chem. 2007, 189, 258–263. [Google Scholar] [CrossRef]
- Arfaj, E.A. Structure and photocatalysis activity of silver doped titanium oxide nanotubes array for degradation of pollutants. Superlattices Microstruct. 2013, 62, 285–291. [Google Scholar] [CrossRef]
- Manríquez, M.E.; Hernández, J.G. Espectroscopía UV-Vis y su Aplicación en Catálisis en Caracterización de Catalizadores, 1nd ed.; Hernandez, M.L., Cedeño, Eds.; Academia de catálisis de México: Ciudad de Mexico, Mexico, 2015; pp. 315–338. ISBN 9781500289331. [Google Scholar]
- Lin, J.C.; De Luna, M.D.; Aranzamendez, G.L.; Lu, M.C. Degradations of acetaminophen via a K2S2O8-doped TiO2 photocatalyst under visible light irradiation. Chemosphere 2016, 155, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Yang, W.; Chou, C.; Liou, S.Y. Hollow mesoporous TiO2 microspheres for enhanced photocatalytic degradation of acetaminophen in water. Chemosphere 2016, 152, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, C.; Montalvo, C.; Ceron, J.; Moctezuma, E. Photocatalytic degradation of acetaminophen. Int. J. Environ. Res. 2011, 5, 1071–1078. [Google Scholar]
- Moctezuma, E.; Leyva, E.; Aguilar, C.A.; Luna, R.A.; Montalvo, C. Photocatalytic degradation of paracetamol: Intermediates and total reaction mechanism. J. Hazard. Mater. 2012, 243, 130–138. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montalvo-Romero, C.; Aguilar-Ucán, C.; Alcocer-Dela hoz, R.; Ramirez-Elias, M.; Cordova-Quiroz, V. A Semi-Pilot Photocatalytic Rotating Reactor (RFR) with Supported TiO2/Ag Catalysts for Water Treatment. Molecules 2018, 23, 224. https://doi.org/10.3390/molecules23010224
Montalvo-Romero C, Aguilar-Ucán C, Alcocer-Dela hoz R, Ramirez-Elias M, Cordova-Quiroz V. A Semi-Pilot Photocatalytic Rotating Reactor (RFR) with Supported TiO2/Ag Catalysts for Water Treatment. Molecules. 2018; 23(1):224. https://doi.org/10.3390/molecules23010224
Chicago/Turabian StyleMontalvo-Romero, Carlos, Claudia Aguilar-Ucán, Roberto Alcocer-Dela hoz, Miguel Ramirez-Elias, and Victor Cordova-Quiroz. 2018. "A Semi-Pilot Photocatalytic Rotating Reactor (RFR) with Supported TiO2/Ag Catalysts for Water Treatment" Molecules 23, no. 1: 224. https://doi.org/10.3390/molecules23010224





