Protective Mechanism of the Antioxidant Baicalein toward Hydroxyl Radical-Treated Bone Marrow-Derived Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Animals
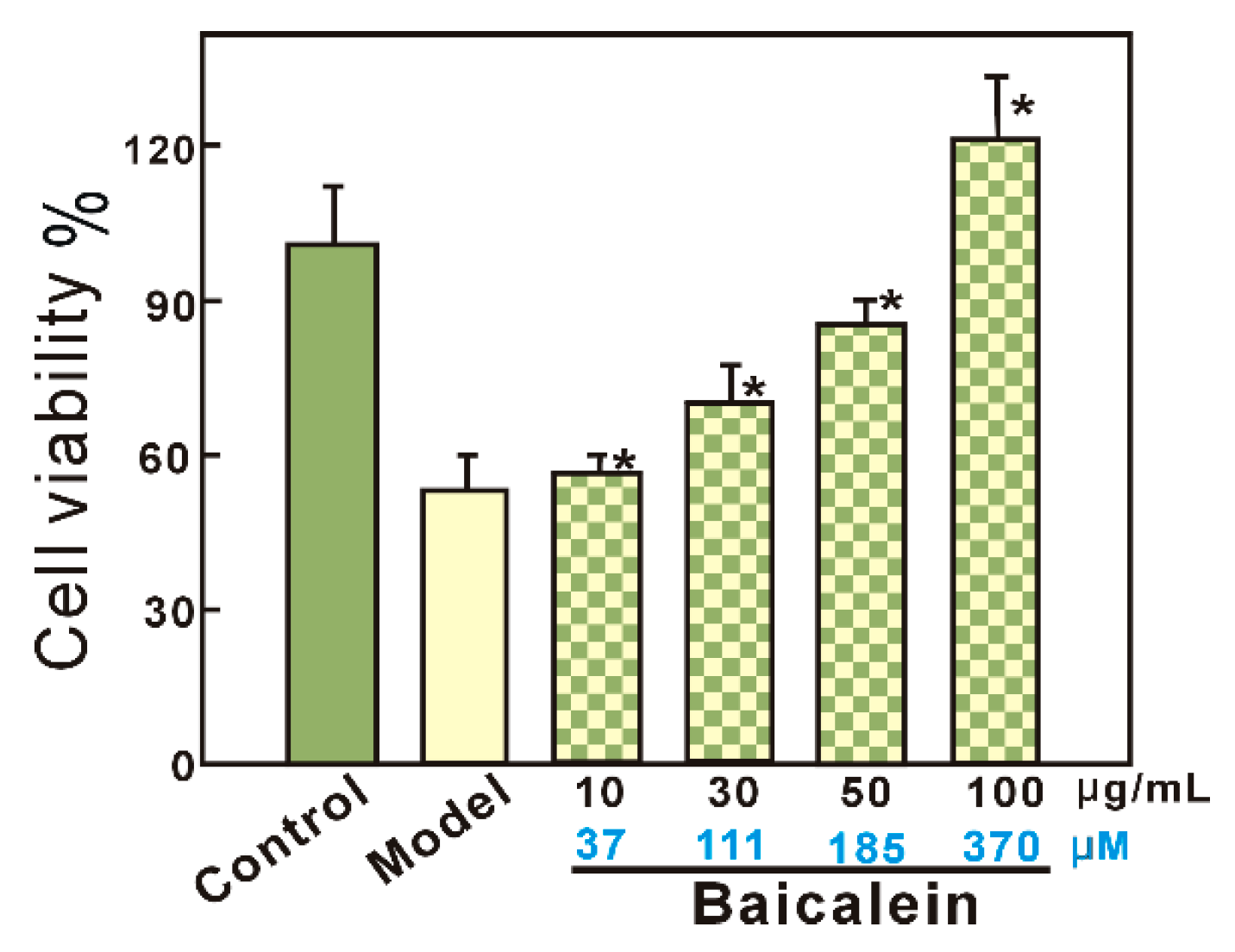
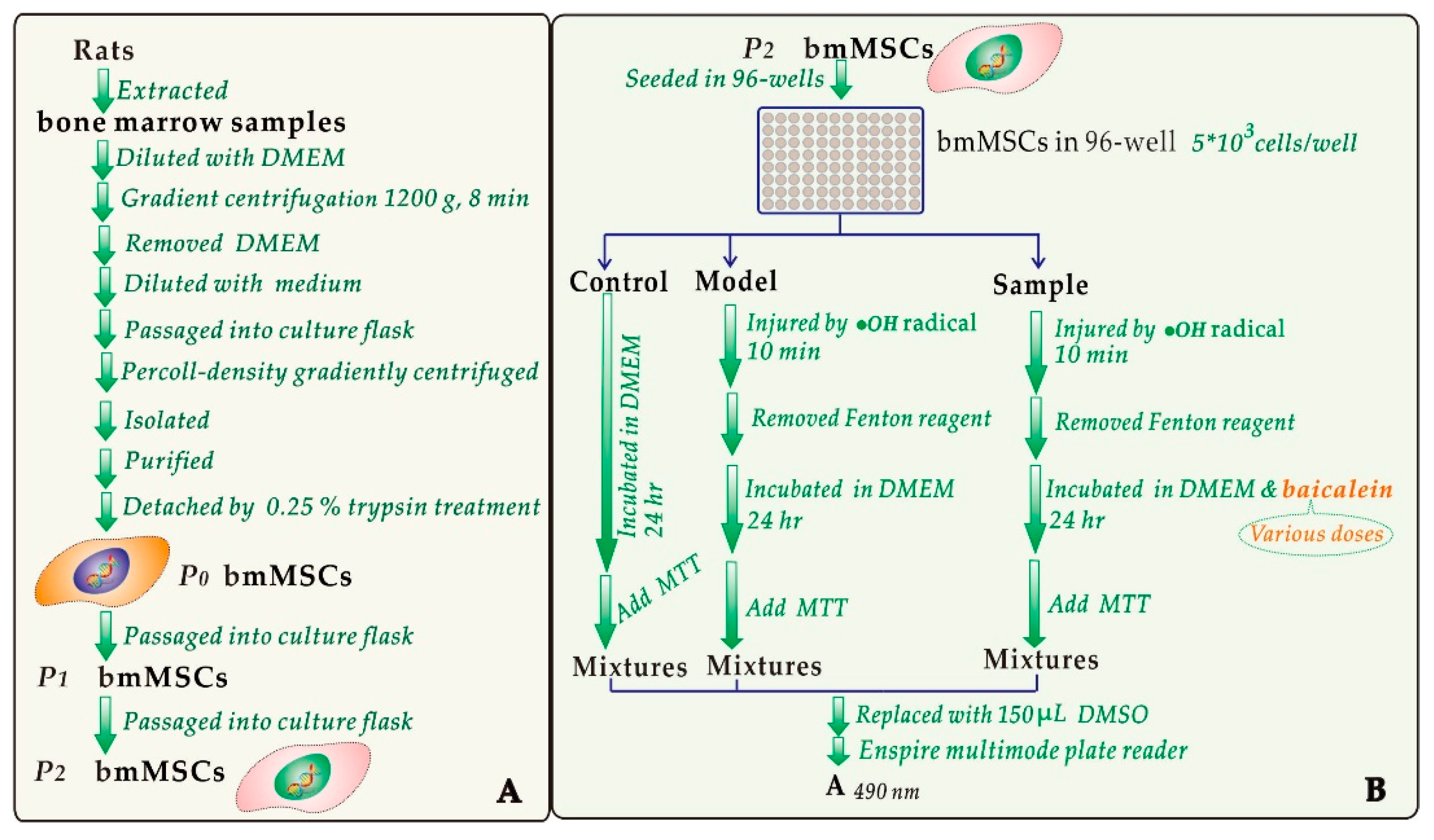
3.2. Protective Effect against •OH-Induced Damage to bmMSCs (MTT Assay)
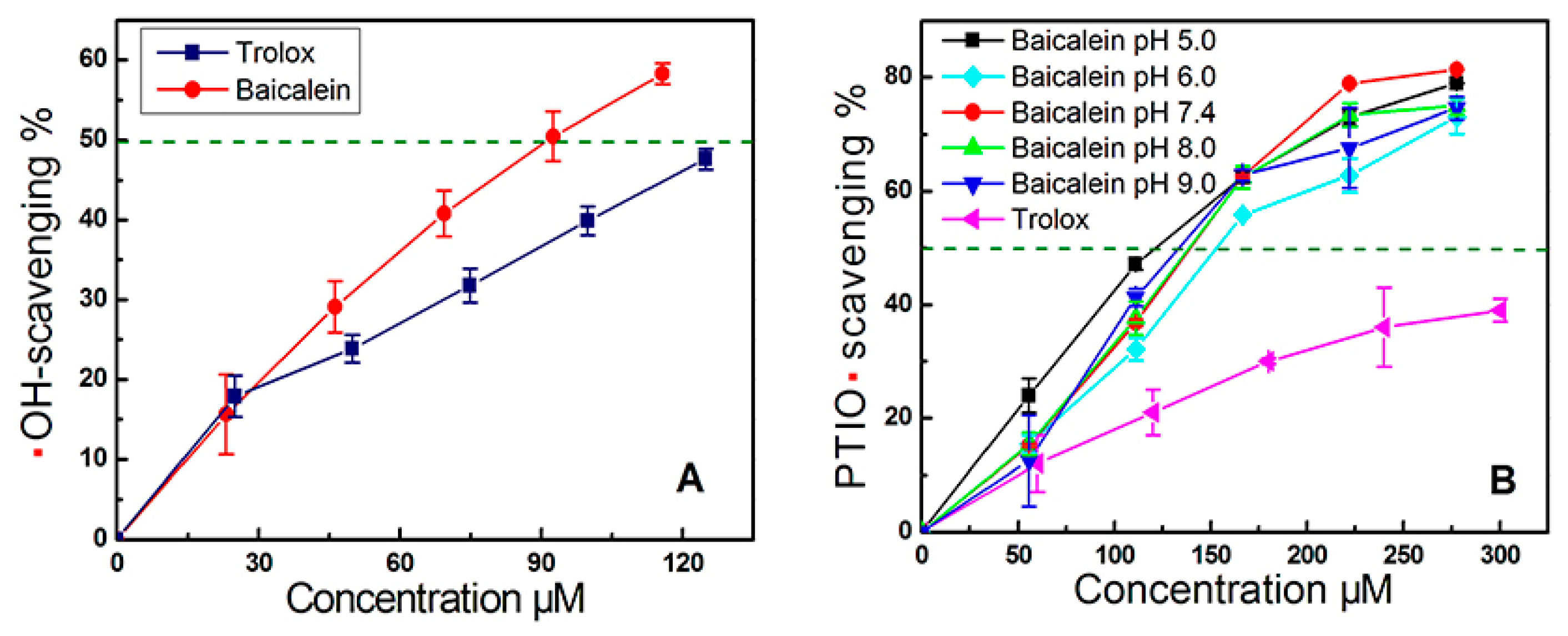
3.3. Hydroxyl Radical (•OH) Scavenging Assay
3.4. PTIO• Scavenging Assay
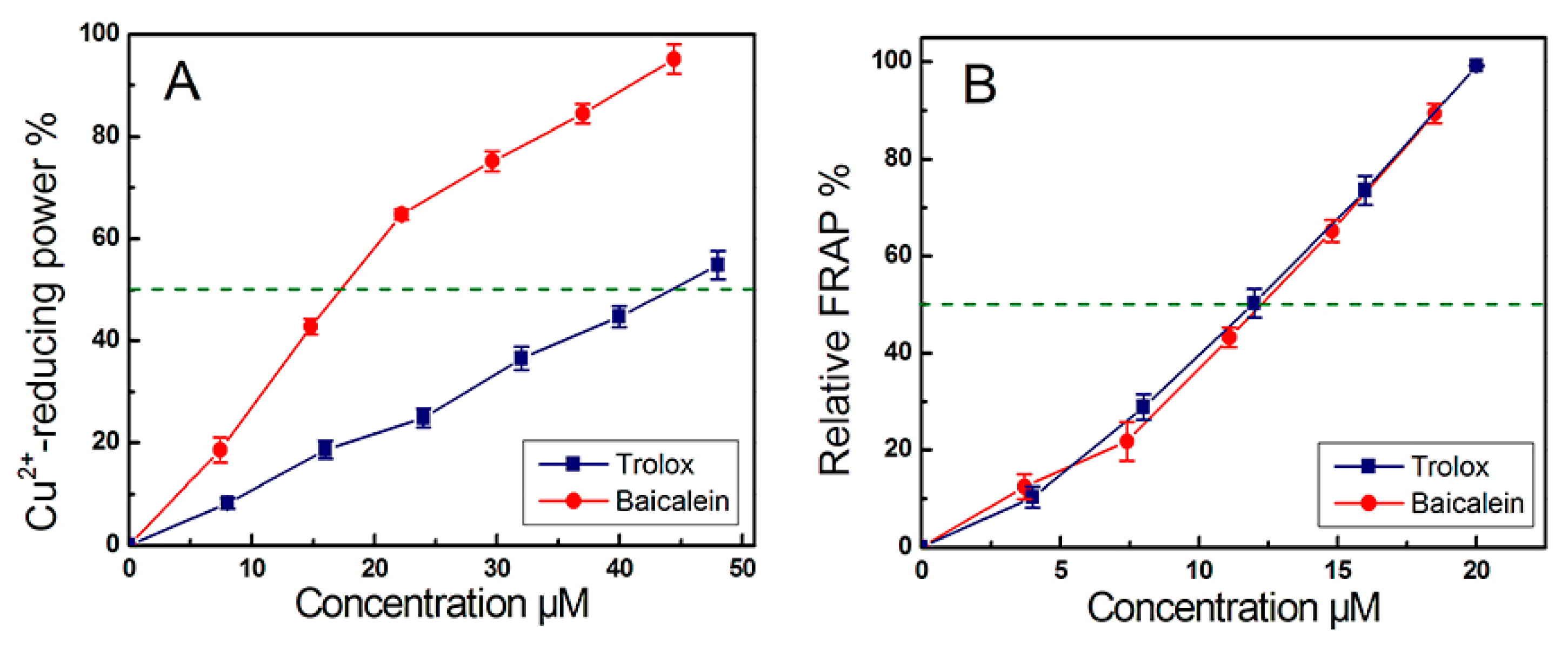
3.5. Cu2+-Reducing Power Assay
3.6. Ferric (Fe3+) Reducing Antioxidant Power (FRAP) Assay
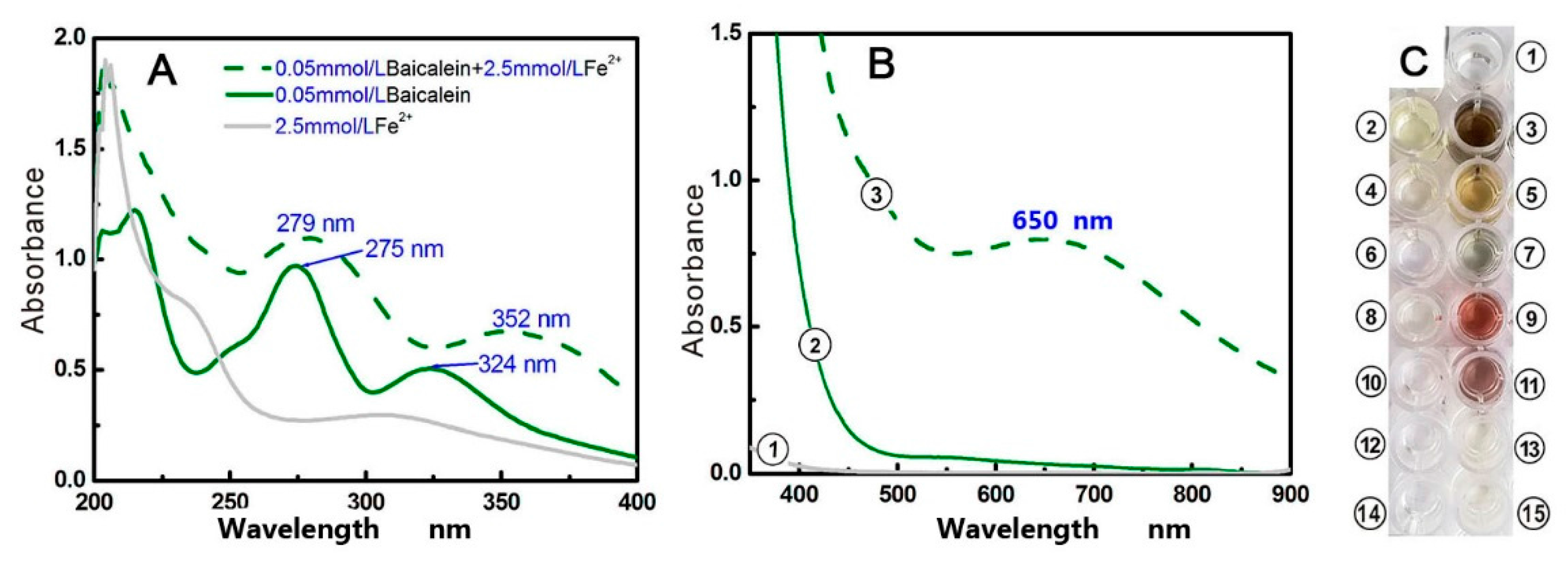
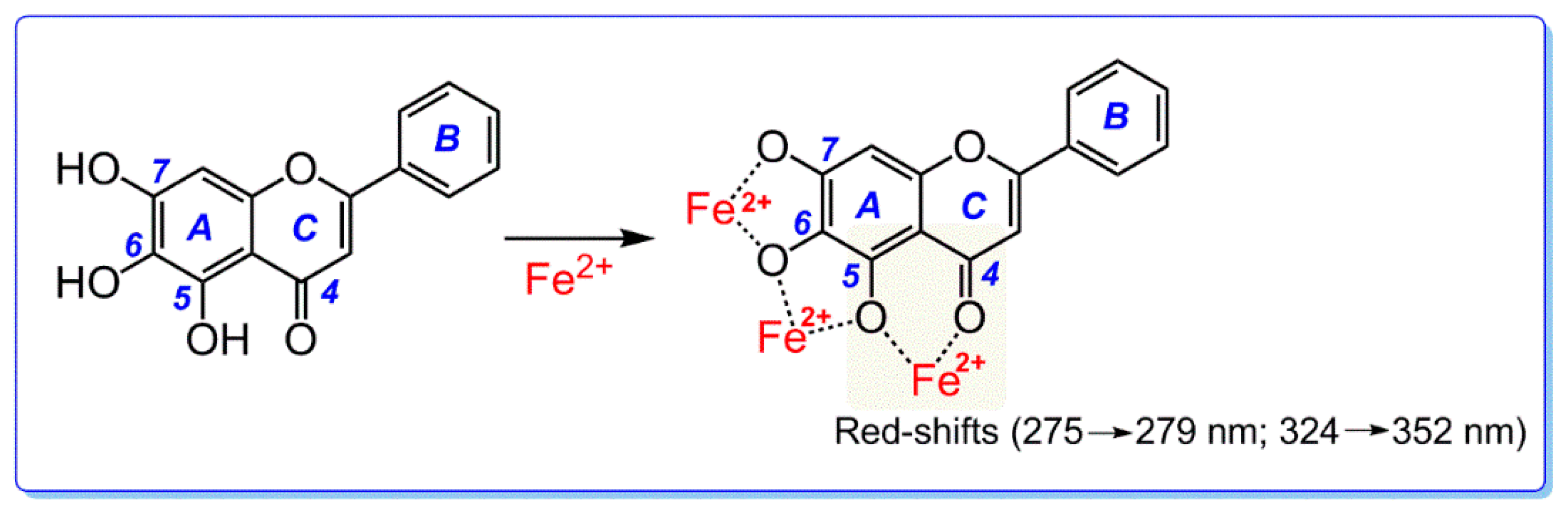
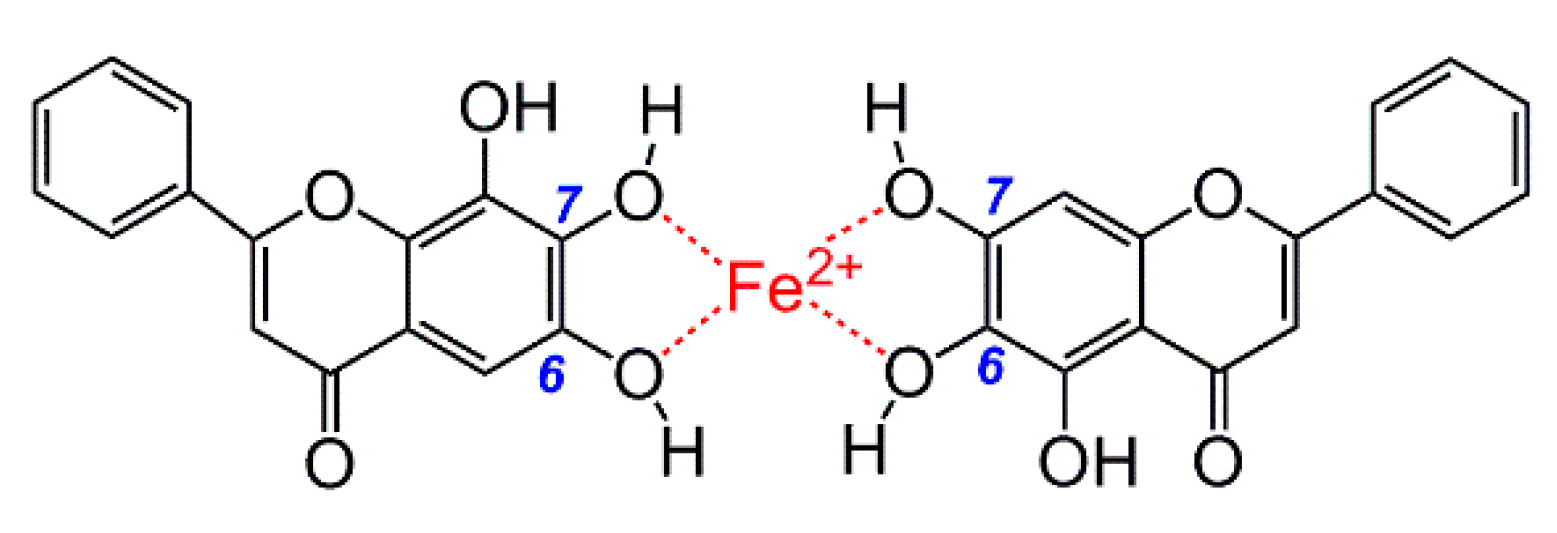
3.7. Ultraviolet-Visible (UV-Vis) Spectra Determination of Fe2+ Binding
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis (3-ethylbenzo-thiazoline-6-sulfonic acid) |
| bmMSCs | bone marrow-derived mesenchymal stem cells |
| DMEM | Dulbecco’s modified Eagle’s medium |
| ET | Electron transfer |
| FBS | Fetal bovine serum |
| FRAP | ferric ion reducing antioxidant power |
| MTT | methyl thiazolyl tetrazolium |
| Neocuproine | 2,9-dimethyl-1,10-phenanthroline |
| PTIO• | 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide radical |
| ROS | reactive oxygen species |
| Trolox | (±)-6-hydroxyl-2,5,7,8-tetramethlychromane-2-carboxylic acid |
| TPTZ | 2,4,6-tripyridyl triazine |
References
- Waite, G.N.; Waite, L.R.; Hughes, E.F.; Balcavage, W.X. Biophotonic hydrogen peroxide production by antibodies, T cells, and T-cell membranes. Biochem. Biophys. Res. Commun. 2005, 338, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.R.; Phillips, D.H. Oxidative DNA damage mediated by copper(II), iron(II) and nickel(II) fenton reactions: Evidence for site-specific mechanisms in the formation of double-strand breaks, 8-hydroxydeoxyguanosine and putative intrastrand cross-links. Mutat. Res. 1999, 424, 23–36. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Li, F.; Liang, A.; Xu, Z.; Bai, Y.; Mai, W.; Han, L.; Chen, D. Maclurin protects against hydroxyl radical-induced damages to mesenchymal stem cells: Antioxidant evaluation and mechanistic insight. Chem. Biol. Interact. 2014, 219, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, G.; Wang, X.; Liu, D.H.; Deng, R.D.; Li, H.; Zhou, J.H.; Li, Y.W.; Zeng, H.P.; Chen, D.F. Targeting of the Shh pathway by atractylenolides promotes chondrogenic differentiation of mesenchymal stem cells. Biol. Pharm. Bull. 2012, 35, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Denu, R.A.; Hematti, P. Effects of Oxidative Stress on Mesenchymal Stem Cell Biology. Oxid. Med. Cell. Longev. 2016, 2016, 2989076. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hu, Y.; Lv, C.; Tu, G. Effects of Reactive Oxygen Species on Differentiation of Bone Marrow Mesenchymal Stem Cells. Ann. Transpl. 2016, 21, 695–700. [Google Scholar] [CrossRef]
- Tsay, J.; Yang, Z.; Ross, F.P.; Cunningham-Rundles, S.; Lin, H.; Coleman, R.; Mayer-Kuckuk, P.; Doty, S.B.; Grady, R.W.; Giardina, P.J.; et al. Bone loss caused by iron overload in a murine model: Importance of oxidative stress. Blood 2010, 116, 2582–2589. [Google Scholar] [CrossRef] [PubMed]
- Datta, I.; Bhonde, R. Can mesenchymal stem cells reduce vulnerability of dopaminergic neurons in the substantia nigra to oxidative insult in individuals at risk to Parkinson’s disease? Cell Biol. Int. 2012, 36, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, L.; Li, Y.; Zhang, J.; Chen, J.; Lu, W.; Zhao, X.; Lai, Y.; Chen, D.; Wei, G. Protective effect of sinapine against hydroxyl radical-induced damage to mesenchymal stem cells and possible mechanisms. Chem. Pharm. Bull. 2016, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Meng, H.; Wang, X.; Zhao, C.; Peng, J.; Wang, Y. Differentiation of Bone Marrow Mesenchymal Stem Cells in Osteoblasts and Adipocytes and its Role in Treatment of Osteoporosis. Med. Sci. Monit. 2016, 22, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, D.; Mai, Y.; Wen, B.; Wang, X. Concordance between antioxidant activities in vitro and chemical components of Radix Astragali (Huangqi). Nat. Prod. Res. 2012, 26, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Zeng, H. Synthesis, antioxidation activity of (E)-9-p-Tolyl-3-[2-(8-hydroxy-quinol-2-yl)vinyl]-carbazole and (E)-9-(p-Anisyl)-3-[2-(8-hydroxy-quinol-2-yl)vinyl]-carbazole and their induction proliferation of mesenchymal stem cells. Acta Chim. Sin. 2009, 67, 974–982. [Google Scholar]
- Wang, T.; Zeng, G.; Li, X.; Zeng, H. In vitro studies on the antioxidant and protective effect of 2-substituted-8-hydroxyquinoline derivatives against H2O2-induced oxidative stress in BMSCs. Chem. Biol. Drug Des. 2010, 75, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, Y.; Wong, N.K.; Xiao, J.; So, K.F. Oxidative Stress in Stem Cell Aging. Cell Transpl. 2017, 26, 1483–1495. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Yoshihara, H.; Fujimori, K. Suppression of Very Early Stage Of Adipogenesis by Baicalein, a Plant-Derived Flavonoid through Reduced Akt-C/EBP alpha-GLUT4 Signaling-Mediated Glucose Uptake in 3T3-L1 Adipocytes. PLoS ONE 2016, 11, e0163640. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.J.; Choi, H.S.; Jeon, H.J.; Woo, M.S.; Lee, B.Y. Baicalein inhibits lipid accumulation by regulating early adipogenesis and m-TOR signaling. Food Chem. Toxicol. 2014, 67, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Tang, J.J.; Chen, J.; Zhang, P.; Wang, T.; Chen, T.Y.; Yan, B.; Huang, B.; Wang, L.; Huang, M.J.; et al. Regulation of bone formation by baicalein via the mTORC1 pathway. Drug Des. Dev. Ther. 2015, 9, 5169–5183. [Google Scholar]
- Gao, Z.; Huang, K.; Xu, H. Protective effects of flavonoids in the roots of Scutellaria baicalensis Georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells. Pharmacol. Res. 2001, 43, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; He, G.; Du, G. Neuroprotective effect of baicalein in patients with Parkinson’s disease. Zhongguo Zhong Yao Za Zhi 2012, 37, 421–425. [Google Scholar] [PubMed]
- Yoshino, M.; Murakami, K. Interaction of iron with polyphenolic compounds: Application to antioxidant characterization. Anal. Biochem. 1998, 257, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Shieh, D.E.; Liu, L.T.; Lin, C.C. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000, 20, 2861–2865. [Google Scholar] [PubMed]
- Ren, J.; Meng, S.; Lekka, C.E.; Kaxiras, E. Complexation of flavonoids with iron: Structure and optical signatures. J. Phys. Chem. B 2008, 112, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.A.; Wei, Y.; Guo, M. Iron-binding and anti-Fenton properties of baicalein and baicalin. J. Inorg. Biochem. 2009, 103, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.L.; Cho, E.J.; Jang, M.H. Cytoprotective effect of baicalein against peroxynitrite-induced toxicity in LLC-PK(1) cells. Food Chem. Toxicol. 2008, 46, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mai, W.; Wang, L.; Han, W. A hydroxyl-scavenging assay based on DNA damage in vitro. Anal. Biochem. 2013, 438, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, Q.; Jiang, S.; Li, F.; Lin, J.; Han, L.; Hong, Y.; Lu, W.; Gao, Y.; Chen, D. Flos Chrysanthemi Indici protects against hydroxyl-induced damages to DNA and MSCs via antioxidant mechanism. J. Saudi Chem. Soc. 2015, 19, 454–460. [Google Scholar] [CrossRef]
- Li, X.C.; Liu, J.J.; Lin, J.; Wang, T.T.; Huang, J.Y.; Lin, Y.Q.; Chen, D.F. Protective effects of dihydromyricetin against •OH-Induced mesenchymal stem cells damage and mechanistic chemistry. Molecules 2016, 21, 604. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, X.; Chen, L.; Lu, W.; Chen, X.; Han, L.; Chen, D. Protective effect against hydroxyl radical-induced DNA damage and antioxidant mechanism of [6]-gingerol: A Chemical Study. Bull. Korean Chem. Soc. 2014, 35, 1633–1638. [Google Scholar] [CrossRef]
- Fang, Y.Z.; Zheng, R.L. Reactive oxygen species in theory and application of free radical biology. In Theory and Application of Free Radical Biology, 2nd ed.; Science Press: Beijing, China, 2002; pp. 98–99. [Google Scholar]
- Goldstein, S.; Russo, A.; Samuni, A. Reactions of PTIO and carboxy-PTIO with •NO, •NO2, and •O2−. J. Biol. Chem. 2003, 278, 50949–50955. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Marino, T.; Galano, A.; Russo, N. Radical scavenging ability of gallic acid toward OH and OOH radicals. Reaction mechanism and rate constants from the density functional theory. J. Phys. Chem. B 2014, 118, 10380–10389. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, J.; Gao, Y.; Tian, R.; Chen, D. Nitric Oxide (NO) as Antioxidant Protects HT22 Cells and Biomolecules against Fenton’s Reagent-Induced Damages via Multiple Pathways. Chemistryselect 2016, 1, 585–589. [Google Scholar] [CrossRef]
- Macakova, K.; Mladenka, P.; Filipsky, T.; Riha, M.; Jahodar, L.; Trejtnar, F.; Bovicelli, P.; Proietti Silvestri, I.; Hrdina, R.; Saso, L. Iron reduction potentiates hydroxyl radical formation only in flavonols. Food Chem. 2012, 135, 2584–2592. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.K.; Chang, T.C.; Sheu, J.R.; Wen, K.H.; Chou, D.S. Comparison of free radical formation induced by baicalein and pentamethyl-hydroxychromane in human promyelocytic leukemia cells using electron spin resonance. J. Food Drug Anal. 2014, 22, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Laughton, M.J.; Halliwell, B.; Evans, P.J.; Hoult, J.R. Antioxidant and pro-oxidant actions of the plant phenolics quercetin, gossypol and myricetin. Effects on lipid peroxidation, hydroxyl radical generation and bleomycin-dependent damage to DNA. Biochem. Pharmacol. 1989, 38, 2859–2865. [Google Scholar] [CrossRef]
- Mladěnka, P.; Macáková, K.; Filipský, T.; Zatloukalová, L.; Jahodář, L.; Bovicelli, P.; Silvestri, I.P.; Hrdina, R.; Saso, L. In vitro analysis of iron binding activity of flavonoids. J. Inorg. Biochem. 2011, 105, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Vlachodimitropoulou, E.; Sharp, P.A.; Naftalin, R.J. Quercetin-iron chelates are transported via glucose transporters. Free Radic. Biol. Med. 2011, 50, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Riha, M.; Karlickova, J.; Filipsky, T.; Macakova, K.; Rocha, L.; Bovicelli, P.; Silvestri, I.P.; Saso, L.; Jahodar, L.; Hrdina, R.; et al. In vitro evaluation of copper-chelating properties of flavonoids. RSC Adv. 2014, 4, 32628–32638. [Google Scholar] [CrossRef]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florencio, M.H.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Rajendran, M.; Devapiriam, D. Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem. 2014, 146, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.E.; Novak, E.M.; Maria, D.A.; Velosa, A.S.; Pereira, R.M. Synthesis, characterization and biological evaluation of Rutin-zinc(II) flavonoid -metal complex. Chem. Biol. Interact. 2015, 239, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.H. Chemistry of Chinese Materia Medica, 3rd ed.; Shanghai Scientific & Technical Publishers: Shanghai, China, 1997; pp. 289–299. [Google Scholar]
- Schär, M.Y.; Curtis, P.J.; Hazim, S.; Ostertag, L.M.; Kay, C.D.; Potter, J.F.; Cassidy, A. Orange juice-derived flavanone and phenolic metabolites do not acutely affect cardiovascular risk biomarkers: A randomized, placebo-controlled, crossover trial in men at moderate risk of cardiovascular disease. Am. J. Clin. Nutr. 2015, 101, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Van der Velpen, V.; Hollman, P.C.; van Nielen, M.; Schouten, E.G.; Mensink, M.; van’t Veer, P.; Geelen, A. Large inter-individual variation in isoflavone plasma concentration limits use of isoflavone intake data for risk assessment. Eur. J. Clin. Nutr. 2014, 68, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.P.; Zhang, J.M.; Wang, Q. Analysis of iron and copper contents in plasma and hair for patients with coronary heart disease. Med. Sci. 2003, 38, 89–90. [Google Scholar]
- Chen, D.F.; Li, X.C.; Xu, Z.W.; Liu, X.B.; Du, S.H.; Li, H.; Zhou, J.H.; Zeng, H.P.; Hua, Z.C. Hexadecanoic Acid from Buzhong Yiqi Decoction Induced Proliferation of Bone Marrow Mesenchymal Stem Cells. J. Med. Food 2010, 13, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Solvent effects and improvements in the deoxyribose degradation assay for hydroxyl radical-scavenging. Food Chem. 2013, 141, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Li, X. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-Oxide (PTIO▪) Radical Scavenging: A New and Simple Antioxidant Assay In Vitro. J. Agric. Food Chem. 2017, 65, 6288–6297. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, W.; Mai, W.; Wang, L. Antioxidant activity and mechanism of Tetrahydroamentoflavone in vitro. Nat. Prod. Commun. 2013, 8, 787–789. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, Q.; Wang, T.; Liu, J.; Chen, D. Comparison of the Antioxidant Effects of Quercitrin and Isoquercitrin: Understanding the Role of the 6′’-OH Group. Molecules 2016, 21, 1246. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mai, W.; Chen, D. Chemical study on protective effect against hydroxyl-induced DNA damage and antioxidant mechanism of myricitrin. J. Chin. Chem. Soc. 2014, 61, 383–391. [Google Scholar] [CrossRef]
Sample Availability: Sample of the compound baicalein is available from the authors. |
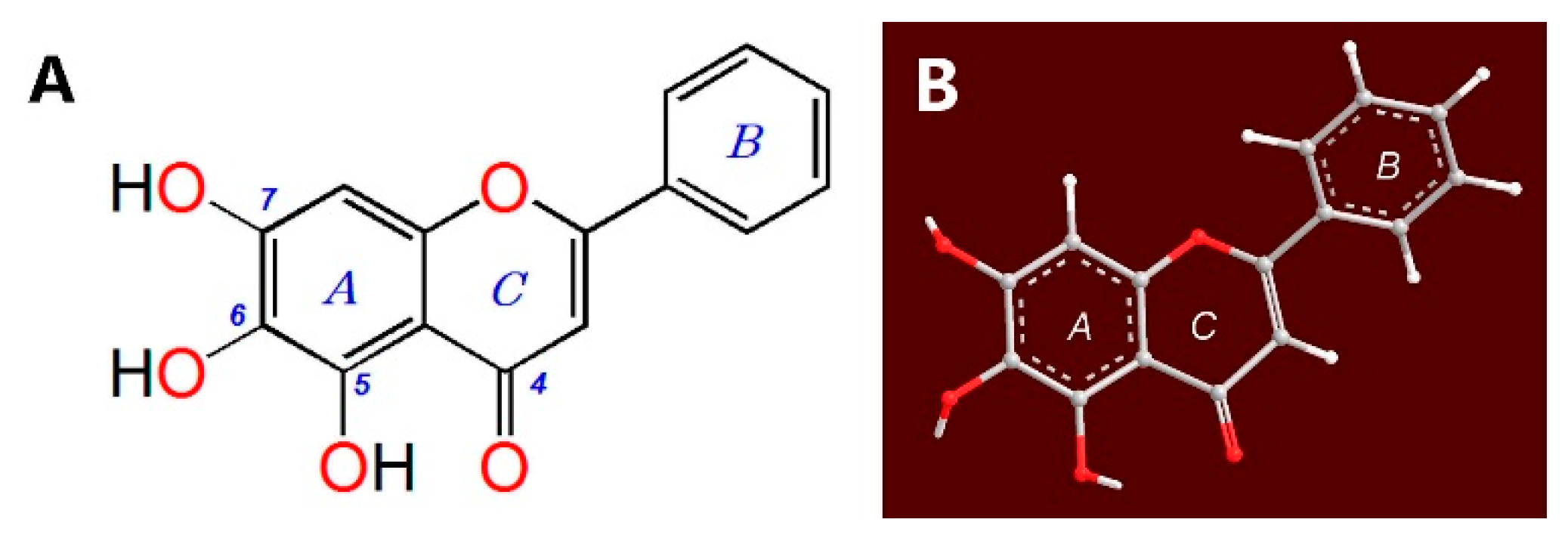
| Assays | Baicalein μM | Trolox μM | Ratio value IC50, Trolox/IC50, Baicalein |
|---|---|---|---|
| •OH-scavenging | 93.7 ± 1.6 a | 137.6 ± 3.6 b | 1.46 |
| PTIO• scavenging * | 188.7 ± 13.1 a | 384.2 ± 23.3 b | 2.03 |
| Cu2+ reducing | 19.0 ± 0.1 a | 44.6 ± 1.5 b | 2.34 |
| FRAP | 11.1 ± 0.0 a | 12.0 ± 0.0 a | 0.96 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Li, X.; Xie, H.; Wang, X.; Xie, Y.; Chen, C.; Chen, D. Protective Mechanism of the Antioxidant Baicalein toward Hydroxyl Radical-Treated Bone Marrow-Derived Mesenchymal Stem Cells. Molecules 2018, 23, 223. https://doi.org/10.3390/molecules23010223
Tian Y, Li X, Xie H, Wang X, Xie Y, Chen C, Chen D. Protective Mechanism of the Antioxidant Baicalein toward Hydroxyl Radical-Treated Bone Marrow-Derived Mesenchymal Stem Cells. Molecules. 2018; 23(1):223. https://doi.org/10.3390/molecules23010223
Chicago/Turabian StyleTian, Yage, Xican Li, Hong Xie, Xiaozhen Wang, Yulu Xie, Chuanbing Chen, and Dongfeng Chen. 2018. "Protective Mechanism of the Antioxidant Baicalein toward Hydroxyl Radical-Treated Bone Marrow-Derived Mesenchymal Stem Cells" Molecules 23, no. 1: 223. https://doi.org/10.3390/molecules23010223





