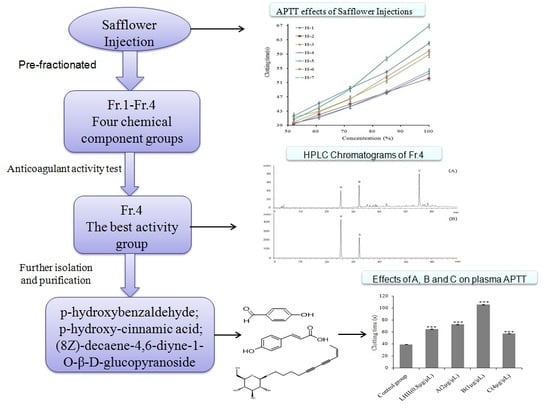
In Vitro Anticoagulant Activity and Active Components of Safflower Injection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Safflower Injection
2.2. Chemicals
2.3. Anticoagulant Activity Screening of Various Safflower Injections
2.3.1. APTT Assay of Safflower Injections
2.3.2. PT Assay of Safflower Injections
2.4. Preparation of Chemical Fractions in Safflower Injections
2.5. APTT Assay of Four Chemical Component Groups
2.6. Analysis, Separation, and Identification of the Active Ingredient Groups
2.7. APTT Assay of Chemical Components
2.8. Statistical Analysis
3. Results and Discussion
3.1. Anticoagulant Activity of Safflower Injection
Effects of Safflower Injection on Plasma APTT and PT
3.2. Effects of Various Chemical Fractions on Plasma APTT
3.3. Structural Analysis of the Main Chemical Components in Fr.4
3.4. Effects of the Main Chemical Components on Plasma APTT
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Han, S.Y.; Li, H.X.; Ma, X.; Zhang, K.; Ma, Z.Z.; Tu, P.F. Protective effects of purified Safflower extract on myocardial ischemia in vivo and in vitro. Phytomedicine 2009, 16, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Pu, R.; Zhao, H.Y.; Liu, X.; Ma, C.; Wang, B.R.; Guo, D.A. Stability and degradation of hydroxysafflor yellow A and anhydrosafflor yellow B in the Safflower injection studied by HPLCDADESIMSn. J. Chin. Pharm. Sci. 2011, 20, 47–56. [Google Scholar] [CrossRef]
- Li, L.; Zhou, L.; Xiong, W.; Zhu, L.; Yang, Y. The Study of Honghua injection’s therapeutic effect on thrombosed illness. World Sci. Technol. 2006, 8, 49–52. [Google Scholar] [CrossRef]
- Liang, X.G.; Liu, L.L.; Zhang, R.Y. Research progress on quality control of Honghua injection. Chin. J. Pharmacovigil. 2013, 10, 468–472. [Google Scholar] [CrossRef]
- Shi, N.N.; Cheng, C.S.; Zha, Z.Q. Clinical study of Safflower injection in treating and preventing the vascular crisis after free flap transplantation. Chin. J. Integr. Tradit. West. Med. 2011, 31, 1322–1327. [Google Scholar]
- Yang, T.L.; Zhang, X.F.; Pan, S.J.; Chen, K.; Cong, J. Safflower Injection combined with alprostadil and sildenafil treats the chronic pulmonary heart disease complicated with pulmonary hypertension. Chin. Tradit. Pat. Med. 2017, 39, 40–46. [Google Scholar] [CrossRef]
- Jiang, W.; Li, G.L. Clinical study on Safflower Injection combined with cilostazol in treatment of acute ischemic cerebral infarction. Drugs Clin. 2017, 32, 1674–5515. [Google Scholar] [CrossRef]
- Li, B. Clinical study of safflower injection combined with compound salvia miltiorrhiza dripping pills in the treatment of unstable angina pectoris. Asia-Pac. Tradit. Med. 2017, 13, 133–134. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Q.; Zhang, Y.; Qin, Y.Y.; He, Q.; Zhang, H.; Gao, H. Screening of bioactivity assay methods of Safflower injection. Chin. J. Pharm. Anal. 2013, 33, 12–17. [Google Scholar] [CrossRef]
- Dong, W.B.; Ye, X.D.; Cheng, M.; Wang, J.Q.; Zheng, G.L. Protective effect of hydroxy safflower yellow A on myocardial ischemia. Chin. J. Clin. Pharmacol. Ther. 2014, 19, 1001–1005. [Google Scholar]
- He, H.B.; Yang, X.Z.; Shi, M.Q.; Zeng, X.W.; Yang, J.; Wu, L.M.; Li, L.D. Protective effects of hydroxysafflor yellow A on acute and chronic congestive cardiac failure mediated by reducing ET-1, NOS and oxidative stress in rats. J. Pharm. Pharmacol. 2008, 60, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, J.R.; Piao, Y.Z.; Dong, N.N.; Jin, M. Inhibitory effect of hydroxysafflor yellow A against rat myocardial mitochondrial injury. Chin. Pharm. J. 2006, 41, 1225–1227. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, C.; Dai, Z.; Zhang, Y.; He, Q.; Hu, X.R.; Gao, H. Active ingredient identification and dose response relationship comparison for the safflower injection from different manufactures. Chin. J. Pharm. Anal. 2012, 32, 1158–1161. [Google Scholar]
- Zhao, J.F.; Liu, J.; Guo, Y.; Liu, Q.; Dai, Z.; Ma, S.C.; Lin, R.C. Chemical constituents from Safflower injection and their bioactivity. China J. Chin. Mater. 2014, 39, 3102–3106. [Google Scholar] [CrossRef]
- Wang, B.S. Study on the Quality Control of Traditional Chinese Medicine Injection for Promoting Blood Circulation to Remove Blood Stasis by Bioassay Assay. Master’s Thesis, Bei Jing University of Chinese Medicine, Beijing, China, 2010. [Google Scholar]
- Fan, H.Y.; Fu, F.H.; Yang, M.Y.; Xu, H.; Zhang, A.H.; Liu, K. Antiplatelet and antithrombotic activities of salvianolic acid A. Thromb. Res. 2010, 126, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.S.; Li, N.; Cao, C.R.; Xiao, Y.; Guo, Y.D.; Zuo, Z.P.; Wang, Z.B. Assay on differences of heparin on various plasma. Chin. J. Pharm. Anal. 2010, 30, 691–693. [Google Scholar] [CrossRef]
- Wang, R.J.; Yang, B.; Fu, M.H. Quality evaluation of Flos Carthami. China J. Chin. Mater. 2008, 33, 2642–2646. [Google Scholar] [CrossRef]
- Li, T.K.; Faridah, A.; Janna, O.A.; Eusni, R.M.T.; Muhajir, H. Anticoagulant activity of polyphenolic-polysaccharides isolated from Melastoma malabathricum L. Evid.-Based Complement. Altern. 2014, 8. [Google Scholar] [CrossRef]
- Li, W.Z.; Wang, J.; Long, R.; Su, G.H.; Bukhory, D.K.; Dai, J.; Jin, N.; Huang, S.Y.; Jia, P.; Li, T.; et al. A possible mechanism underlying the fgl2 coagulation pathway and the inhibition of fgl2 prothrombinase activity. PLoS ONE 2014. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, B.Z.; Du, D.; Guo, X.R.; Xin, G.; Xing, Z.H.; Liang, Y.; Chen, Y.N.; Chen, Q.M.; He, Y.; et al. Anti-thrombosis effect of diosgenyl saponins in vitro and in vivo. Steroids 2013, 78, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |



| Concentration (μg/μL) | APTT (s) | ||||
|---|---|---|---|---|---|
| LHII | Fr.1 | Fr.2 | Fr.3 | Fr.4 | |
| Control Group | 34.0 ± 0.4 | 34.0 ± 0.4 | 34.0 ± 0.4 | 34.0 ± 0.4 | 39.1 ± 0.4 |
| 0.8 | 64.6 ± 0.7 *** | - | - | - | - |
| 0.5 | - | 35.9 ± 0.8 | 35.7 ± 0.5 | 35.3 ± 0.2 | 39.6 ± 0.4 |
| 1.0 | - | 36.6 ± 0.5 | 36.0 ± 0.5 | 39.2 ± 0.8 ** | 41.1 ± 0.5 * |
| 2.0 | - | 37.5 ± 1.0 | 38.0 ± 0.7 ** | 47.6 ± 0.5 *** | 51.4 ± 0.5 *** |
| 4.0 | - | 37.7 ± 0.1 * | 40.0 ± 0.5 ** | 71.5 ± 0.9 *** | 95.4 ± 1.4 ** |
| Sample | Concentration (μg/μL) | APTT (s) |
|---|---|---|
| Control group | - | 39.1 ± 0.4 |
| LHII | 0.8 | 64.6 ± 0.7 *** |
| A | 0.5 | 46.9 ± 0.8 * |
| 1.0 | 53.8 ± 1.0 ** | |
| 2.0 | 72.6 ± 0.9 *** | |
| 4.0 | >200.0 *** | |
| B | 0.5 | 53.8 ± 0.8 ** |
| 1.0 | 105.8 ± 0.8 *** | |
| 1.2 | 158.3 ± 4.7 *** | |
| C | 2.0 | 40.7 ± 0.2 |
| 4.0 | 57.3 ± 0.7 *** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.-H.; Li, S.-F.; Zhao, Y.; Li, H.-X.; Zhang, L.-W. In Vitro Anticoagulant Activity and Active Components of Safflower Injection. Molecules 2018, 23, 170. https://doi.org/10.3390/molecules23010170
Wang K-H, Li S-F, Zhao Y, Li H-X, Zhang L-W. In Vitro Anticoagulant Activity and Active Components of Safflower Injection. Molecules. 2018; 23(1):170. https://doi.org/10.3390/molecules23010170
Chicago/Turabian StyleWang, Kai-Hong, Shi-Fei Li, Yi Zhao, Hong-Xia Li, and Li-Wei Zhang. 2018. "In Vitro Anticoagulant Activity and Active Components of Safflower Injection" Molecules 23, no. 1: 170. https://doi.org/10.3390/molecules23010170