Characterization of Physico-Chemical Properties and Antioxidant Capacities of Bioactive Honey Produced from Australian Grown Agastache rugosa and its Correlation with Colour and Poly-Phenol Content
Abstract
:1. Introduction
2. Results and Discussion
2.1. Honey Samples
2.2. Physico-Chemical Properties
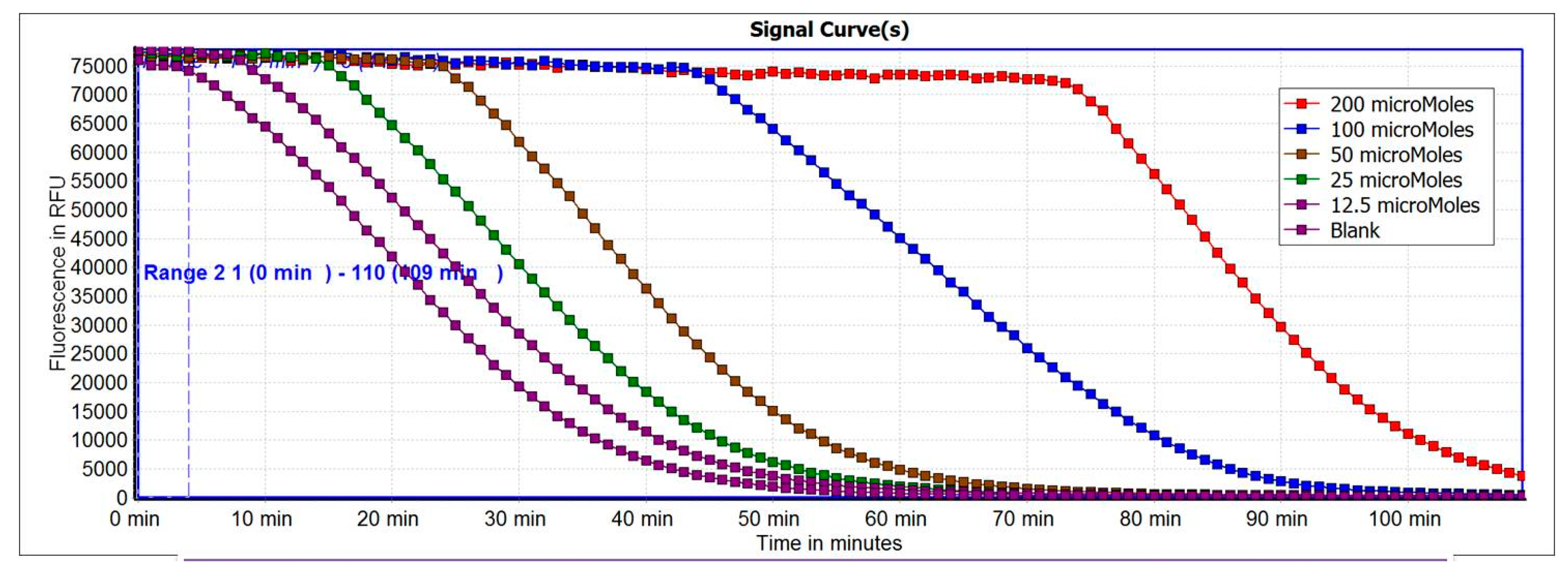
2.3. Antioxidant Capacity
2.4. Correlation between Parameters
3. Materials and Methods
3.1. Mono-floral Agastache Honey Production
3.2. Physico-Chemical Properties
3.2.1. pH Measurement
3.2.2. Moisture Content
3.2.3. Determination of Protein Content
3.2.4. Colour Analysis
3.2.5. Determination of Total Phenolic Content
3.2.6. Determination of Flavonoid Content
3.3. Antioxidant Capacity
3.3.1. Determination of Radical Scavenging Capacity against DPPH• (Antiradical Activity)
3.3.2. Free Radical Scavenging Capacity against ABTS•+ (TEAC Assay)
3.3.3. Oxygen Radical Absorbance Capacity Assay
3.3.4. Ferric Reducing Antioxidant Power Assay
3.4. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Molan, P.C. Potential of Honey in the Treatment of Wounds and Burns. Am. J. Clin. Dermatol. 2001, 2, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Molan, P. Re-introducing honey in the management of wounds and ulcers—Theory and practice. Ostomy Wound Manag. 2002, 48, 28–40. [Google Scholar]
- Bang, L.M.; Buntting, C.; Molan, P. The effect of dilution on the rate of hydrogen peroxide production in honey and its implications for wound healing. J. Altern. Complement. Med. 2003, 9, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Yamani, H.A.; Pang, E. C.; Mantri, N.; Deighton, M.A. Antimicrobial Activity of Tulsi (Ocimum tenuiflorum) Essential Oil and Their Major Constituents against Three Species of Bacteria. Front. Microbiol. 2016, 7, 681. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Introduction. In Oxidative Stress: Oxidants and Antioxidants; Academic Press: London, UK, 1991. [Google Scholar]
- Niu, L.; Mantri, N.; Li, C.G.; Xue, C.; Wohlmuth, H.; Pang, E.C.K. Detection of Panax quinquefolius in Panax ginseng using “Subtracted Diversity Array”. J. Sci. Food Agric. 2011, 91, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Olarte, A.; Mantri, N.; Nugent, G.; Li, C.G.; Xue, C.; Pang, E.C.K. A gDNA microarray for genotyping Salvia species. Mol. Biotechnol. 2013, 54, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Mantri, N.; Hu, Y.; Lu, J.; Jiang, W.; Lu, H. Evaluation of bioactive compounds of black mulberry juice after thermal, microwave, ultrasonic processing and storage at different temperatures. Food Sci. Technol. Int. 2015, 21, 392–399. [Google Scholar]
- Alvarez-Suarez, J.M.; Tulipani, S.; Díaz, D.; Estevez, Y.; Romandini, S.; Giampieri, F.; Damiani, E.; Astolfi, P.; Bompadre, S.; Battino, M. Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem. Toxicol. 2010, 48, 2490–2499. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mehta, A.; Berenbaum, M.; Zangerl, A.R.; Engeseth, N.J. Honeys from Different Floral Sources as Inhibitors of Enzymatic Browning in Fruit and Vegetable Homogenates. J. Agric. Food Chem. 2000, 48, 4997–5000. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Inoue, R.; Kanamori, N.; Suzuki, N.; Nagashima, T. Characterization of honey from different floral sources. Its functional properties and effects of honey species on storage of meat. Food Chem. 2006, 97, 256–262. [Google Scholar] [CrossRef]
- Taormina, P.J.; Niemira, B.A.; Beuchat, L.R. Inhibitory activity of honey against foodborne pathogens as influenced by the presence of hydrogen peroxide and level of antioxidant power. Int. J. Food Microbiol. 2001, 69, 217–225. [Google Scholar] [CrossRef]
- Gheldof, N.; Wang, X.-H.; Engeseth, N.J. Buckwheat Honey Increases Serum Antioxidant Capacity in Humans. J. Agric. Food Chem. 2003, 51, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Buratti, S.; Benedetti, S.; Cosio, M.S. Evaluation of the antioxidant power of honey, propolis and royal jelly by amperometric flow injection analysis. Talanta 2007, 71, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Maffei Facino, R. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal. Chim. Acta 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Gałkowska, D.; Fortuna, T.; Witczak, T. Phenolic profile and antioxidant properties of Polish honeys. Int. J. Food Sci. Technol. 2011, 46, 528–534. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Pontis, J.A.; Costa, L.A.M.A.D.; Silva, S.J.R.D.; Flach, A. Color, phenolic and flavonoid content and antioxidant activity of honey from Roraima, Brazil. Food Sci. Technol. 2014, 34, 69–73. [Google Scholar] [CrossRef]
- Jun, H.J.; Chung, M.J.; Dawson, K.; Rodriguez, R.L.; Houng, S.J.; Cho, S.Y.; Jeun, J.; Kim, J.Y.; Kim, K.H.; Park, K.W.; et al. Nutrigenomic analysis of hypolipidemic effects of Agastache rugosa essential oils in HepG2 cells and C57BL/6 mice. Food Sci. Biotechnol. 2010, 19, 219–227. [Google Scholar] [CrossRef]
- Hong, J.-J.; Choi, J.-H.; Oh, S.-R.; Lee, H.-K.; Park, J.-H.; Lee, K.-Y.; Kim, J.-J.; Jeong, T.-S.; Oh, G.T. Inhibition of cytokine-induced vascular cell adhesion molecule-1 expression; possible mechanism for anti-atherogenic effect of Agastache rugosa. FEBS Lett. 2001, 495, 142–147. [Google Scholar] [CrossRef]
- Oh, H.M.; Kang, Y.J.; Kim, S.H.; Lee, Y.S.; Park, M.K.; Heo, J.M.; Sun, J.; Kim, H.J.; Kang, E.S.; Kim, H.J.; et al. Agastache rugosa leaf extract inhibits the iNOS expression in ROS 17/2.8 cells activated with TNF-α and IL-1β. Arch. Pharm. Res. 2005, 28, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, S.M. Adam, Phytochemistry and bioactivity of aromatic and medicinal plants from the genus Agastache (Lamiaceae). Phytochem. Rev. 2014, 13, 391–416. [Google Scholar] [CrossRef] [PubMed]
- Yamani, H.; Mantri, N.; Morrison, P.D.; Pang, E. Analysis of the volatile organic compounds from leaves, flower spikes, and nectar of Australian grown Agastache rugosa. BMC Complement. Altern. Med. 2014, 14, 495. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.H.; Liu, C.F.; Lai, P.H.; Nie, F.; Shi, J. Study on the essential oil obtained from the calyx of wild Agastache rugosa. Sci. Technol. Food Ind. 2009, 10, 44. [Google Scholar]
- Chandler, B.V. Composition of Australian Honeys; Commonwealth Scientific and Industrial Research Organization: Melbourne, Australia, 1974.
- Alzahrani, H.A.; Alsabehi, R.; Boukraâ, L.; Abdellah, F.; Bellik, Y.; Bakhotmah, B.A. Antibacterial and antioxidant potency of floral honeys from different botanical and geographical origins. Molecules 2012, 17, 10540–10549. [Google Scholar] [CrossRef] [PubMed]
- Gethin, G.T.; Cowman, S.; Conroy, R.M. The impact of Manuka honey dressings on the surface pH of chronic wounds. Int. Wound J. 2008, 5, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius, 2001. In Codex Standard for Honey; FAO: Rome, Italy, 2001.
- Finola, M.S.; Lasagno, M.C.; Marioli, J.M. Microbiological and chemical characterization of honeys from central Argentina. Food Chem. 2007, 100, 1649–1653. [Google Scholar] [CrossRef]
- Anklam, E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Azeredo, L.D.C.; Azeredo, M.A.A.; de Souza, S.R.; Dutra, V.M.L. Protein contents and physicochemical properties in honey samples of Apis mellifera of different floral origins. Food Chem. 2003, 80, 249–254. [Google Scholar] [CrossRef]
- Adams, C.J.; Boult, C.H.; Deadman, B.J.; Farr, J.M.; Grainger, M.N.C.; Manley-Harris, M.; Snow, M.J. Isolation by HPLC and characterisation of the bioactive fraction of New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 2008, 343, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Sulaiman, S.; Khalil, M.; Gan, S. Evaluation of physicochemical and antioxidant properties of sourwood and other Malaysian honeys: A comparison with manuka honey. Chem. Cent. J. 2013, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Yaoa, L.; Jiang, Y.; Singanusong, R.; Datta, N.; Raymont, K. Phenolic acids in Australian Melaleuca, Guioa, Lophostemon, Banksia and Helianthus honeys and their potential for floral authentication. Food Res. Int. 2005, 38, 651–658. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Yao, L.; Jiang, Y.; Singanusong, R.; D’Arcy, B.; Datta, N.; Caffin, N.; Raymont, K. Flavonoids in Australian Melaleuca, Guioa, Lophostemon, Banksia and Helianthus honeys and their potential for floral authentication. Food Res. Int. 2004, 37, 166–174. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Gheldof, N.; Engeseth, N.J. Antioxidant Capacity of Honeys from Various Floral Sources Based on the Determination of Oxygen Radical Absorbance Capacity and Inhibition of in Vitro Lipoprotein Oxidation in Human Serum Samples. J. Agric. Food Chem. 2002, 50, 3050–3055. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant Capacity of Tea and Common Vegetables. J. Agric. Food Chem. 1996, 44, 3426–3431. [Google Scholar] [CrossRef]
- Wang, H.; Cao, G.; Prior, R.L. Total Antioxidant Capacity of Fruits. J. Agric. Food Chem. 1996, 44, 701–705. [Google Scholar] [CrossRef]
- Gorjanović, S.Ž.; Alvarez-Suarez, J.M.; Novaković, M.M.; Pastor, F.T.; Pezo, L.; Battino, M.; Sužnjević, D.Ž. Comparative analysis of antioxidant activity of honey of different floral sources using recently developed polarographic and various spectrophotometric assays. J. Food Compos. Anal. 2013, 30, 13–18. [Google Scholar] [CrossRef]
- Brudzynski, K.; Miotto, D. The relationship between the content of Maillard reaction-like products and bioactivity of Canadian honeys. Food Chem. 2011, 124, 869–874. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Al, M.L.; Daniel, D.; Moise, A.; Bobis, O.; Laslo, L.; Bogdanov, S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Khalil, M.I.; Alam, N.; Moniruzzaman, M.; Sulaiman, S.A.; Gan, S.H. Phenolic Acid Composition and Antioxidant Properties of Malaysian Honeys. J. Food Sci. 2011, 76, C921–C928. [Google Scholar] [CrossRef] [PubMed]
- Frankel, S.; Robinson, G.E.; Berenbaum, M.R. Antioxidant capacity and correlated characteristics of 14 unifloral honeys. J. Apic. Res. 1998, 37, 27–31. [Google Scholar] [CrossRef]
- Saxena, S.; Gautam, S.; Sharma, A. Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem. 2010, 118, 391–397. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W.; Latimer, G.W., Jr. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1990. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Barros, L.; Calhelha, R.C.; Vaz, J.A.; Ferreira, I.C.; Baptista, P.; Estevinho, L.M. Antimicrobial activity and bioactive compounds of Portuguese wild edible mushrooms methanolic extracts. Eur. Food Res. Technol. 2007, 225, 151–156. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Baltrušaitytė, V.; Venskutonis, P.R.; Čeksterytė, V. Radical scavenging activity of different floral origin honey and beebread phenolic extracts. Food Chem. 2007, 101, 502–514. [Google Scholar] [CrossRef]
- Brangoulo, H.L.; Molan, P.C. Assay of the antioxidant capacity of foods using an iron(II)-catalysed lipid peroxidation model for greater nutritional relevance. Food Chem. 2011, 125, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Gillespie, K.M.; Chae, J.M.; Ainsworth, E.A. Rapid measurement of total antioxidant capacity in plants. Nat. Protoc. 2007, 2, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Honey Samples | pH | Moisture (%) | Protein (µg/g) | Colour (A450, mAU) |
|---|---|---|---|---|
| Agastache | 4.10 ± 0.1 | 17.0 ± 0.5 | 1428 ± 83.4 | 461 ± 8.8 |
| Manuka | 4.03 ± 0.2 | 19.5 ± 0.3 | 903 ± 107 | 1007 ± 4.0 |
| Tea tree | 4.10 ± 0.1 | 20.0 ± 0.15 | 1319 ±18.4 | 507 ± 15.4 |
| Jelly bush | 3.84 ± 0.23 | 14.5 ± 0.5 | 1384 ± 64.2 | 1135 ± 3.1 |
| Super manuka | 3.83 ± 0.12 | 17.0 ± 0.35 | 1016 ± 143.1 | 726 ± 3.7 |
| Jarrah | 4.30 ± 0.12 | 21.0 ± 0.5 | 2178 ± 100 | 518 ± 2.6 |
| Honey Samples | TPC (GAE µg/g) | TFC (CE µg/g) | DPPH• (µmol TE/g) | TEAC (µmol TE/g) | ORAC (µmol TE/g) | FRAP (µmol TE/g) |
|---|---|---|---|---|---|---|
| Agastache | 853.6 ± 5.0 | 26.67 ± 5.6 | 9.85 ± 1.98 | 26.88 ± 0.32 | 19.78 ± 1.1 | 3.61 ± 0.02 |
| Manuka | 1288.0 ± 102.8 | 37.64 ± 7.2 | 18.69 ± 0.9 | 30.72 ± 0.27 | 24.82 ± 0.5 | 3.68 ± 0.04 |
| Tea-tree | 1263.5 ± 143.1 | 20.08 ± 4.3 | 17.25 ± 1.7 | 13.60 ± 0.35 | 14.16 ± 0.2 | 2.72 ± 0.16 |
| Jelly bush | 1415.6 ± 126 | 53.91 ± 10.9 | 17.25 ± 0.8 | 23.84 ± 0.29 | 26.95 ± 0.9 | 3.36 ± 0.15 |
| Super manuka | 974.4 ± 26.9 | 24.90 ± 4.3 | 11.34 ± 0.69 | 21.28 ± 0.14 | 12.40 ± 0.3 | 3.28 ± 0.02 |
| Jarrah | 1028.7 ± 27.4 | 39.3 ± 8.9 | 6.87 ± 0.77 | 20.96 ± 0.33 | 12.44 ± 0.5 | 3.34 ± 0.03 |
| COLOUR | TPC | TFC | DPPH• | |
|---|---|---|---|---|
| DPPH• | 0.925 | 0.826 | 0.484 | |
| TFC | 0.685 | 0.866 | ||
| TPC | 0.944 | |||
| COLOUR |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anand, S.; Pang, E.; Livanos, G.; Mantri, N. Characterization of Physico-Chemical Properties and Antioxidant Capacities of Bioactive Honey Produced from Australian Grown Agastache rugosa and its Correlation with Colour and Poly-Phenol Content. Molecules 2018, 23, 108. https://doi.org/10.3390/molecules23010108
Anand S, Pang E, Livanos G, Mantri N. Characterization of Physico-Chemical Properties and Antioxidant Capacities of Bioactive Honey Produced from Australian Grown Agastache rugosa and its Correlation with Colour and Poly-Phenol Content. Molecules. 2018; 23(1):108. https://doi.org/10.3390/molecules23010108
Chicago/Turabian StyleAnand, Sushil, Edwin Pang, George Livanos, and Nitin Mantri. 2018. "Characterization of Physico-Chemical Properties and Antioxidant Capacities of Bioactive Honey Produced from Australian Grown Agastache rugosa and its Correlation with Colour and Poly-Phenol Content" Molecules 23, no. 1: 108. https://doi.org/10.3390/molecules23010108




