Synthesis and Antidepressant Activity Profile of Some Novel Benzothiazole Derivatives
Abstract
:1. Introduction
2. Results and Discussion
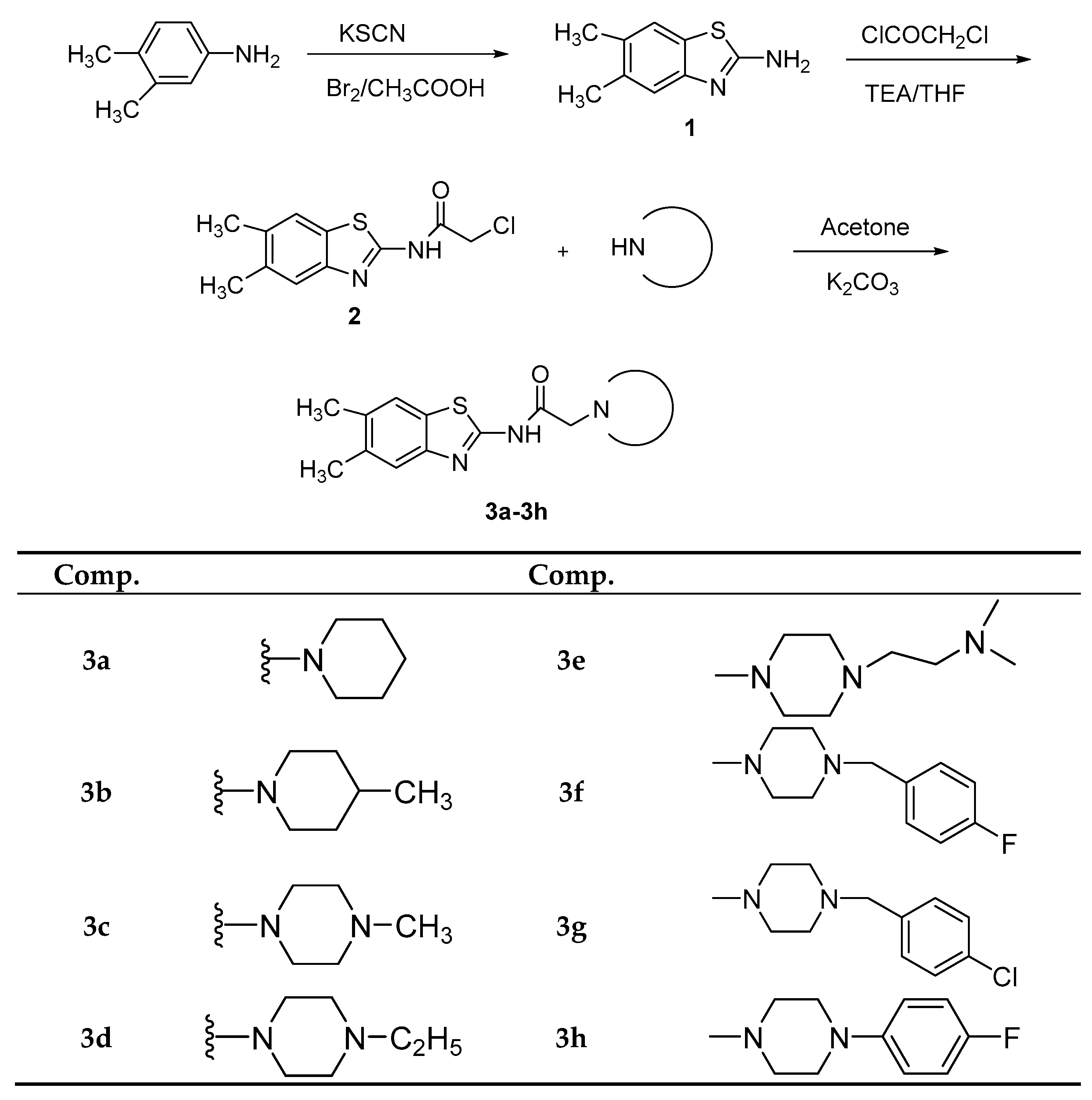
2.1. Chemistry
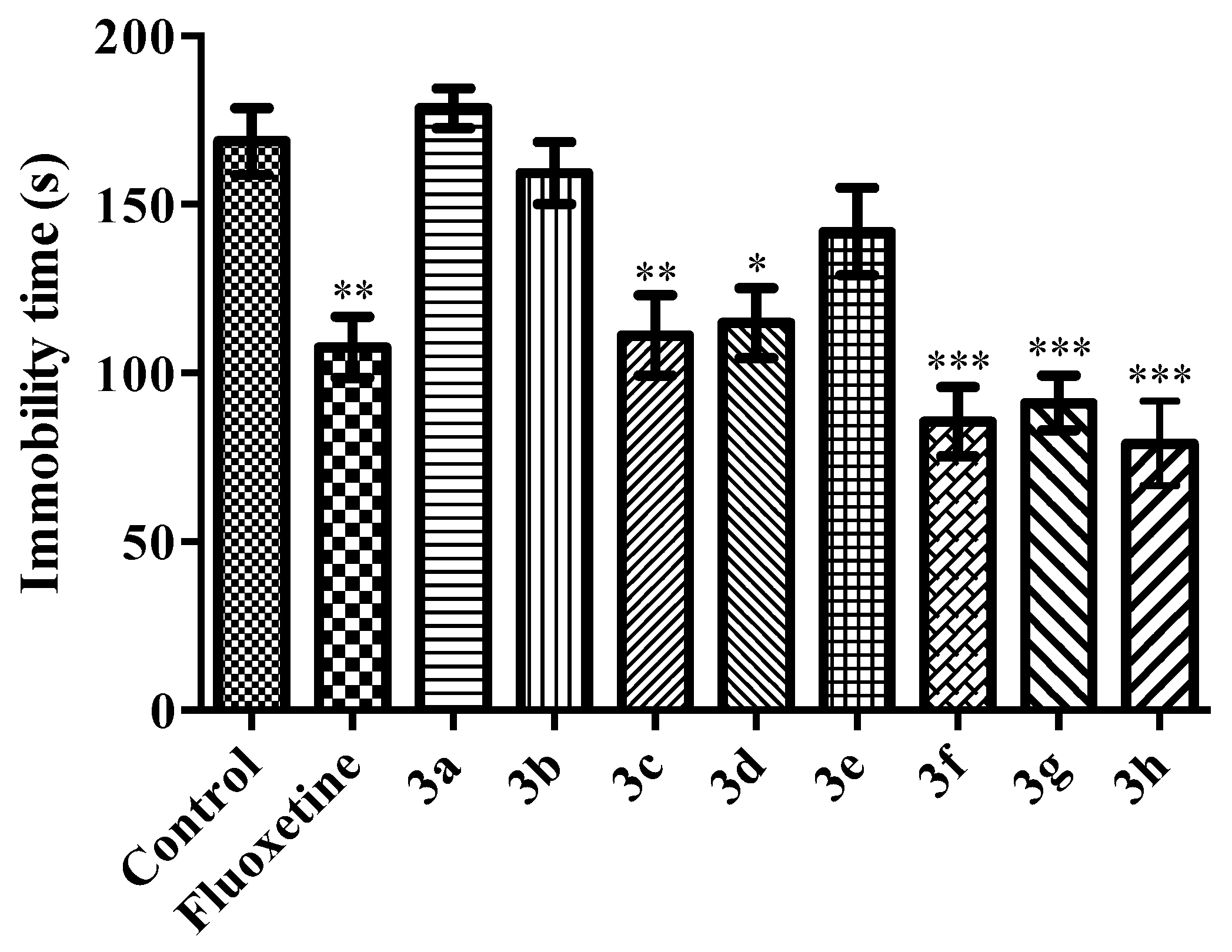
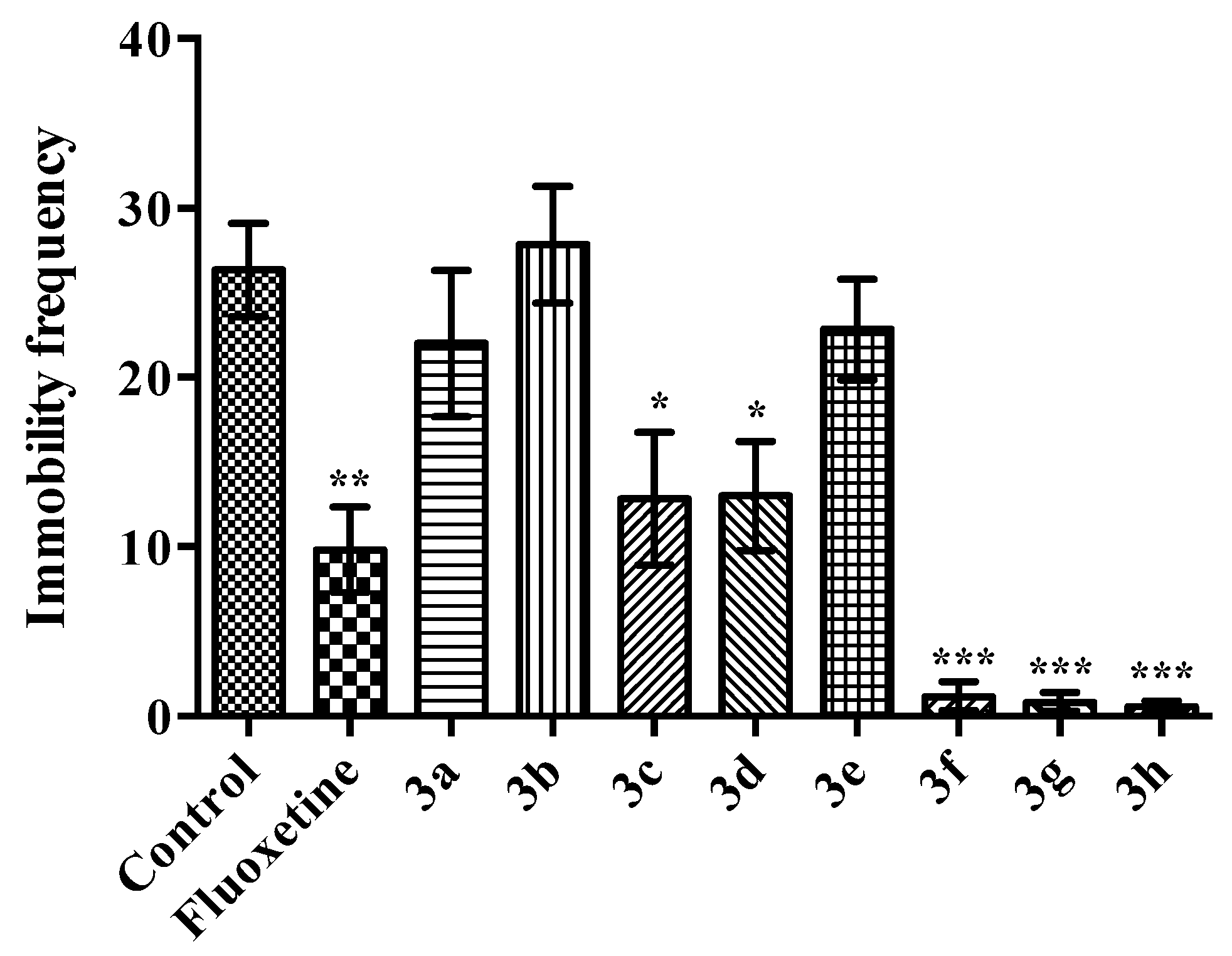
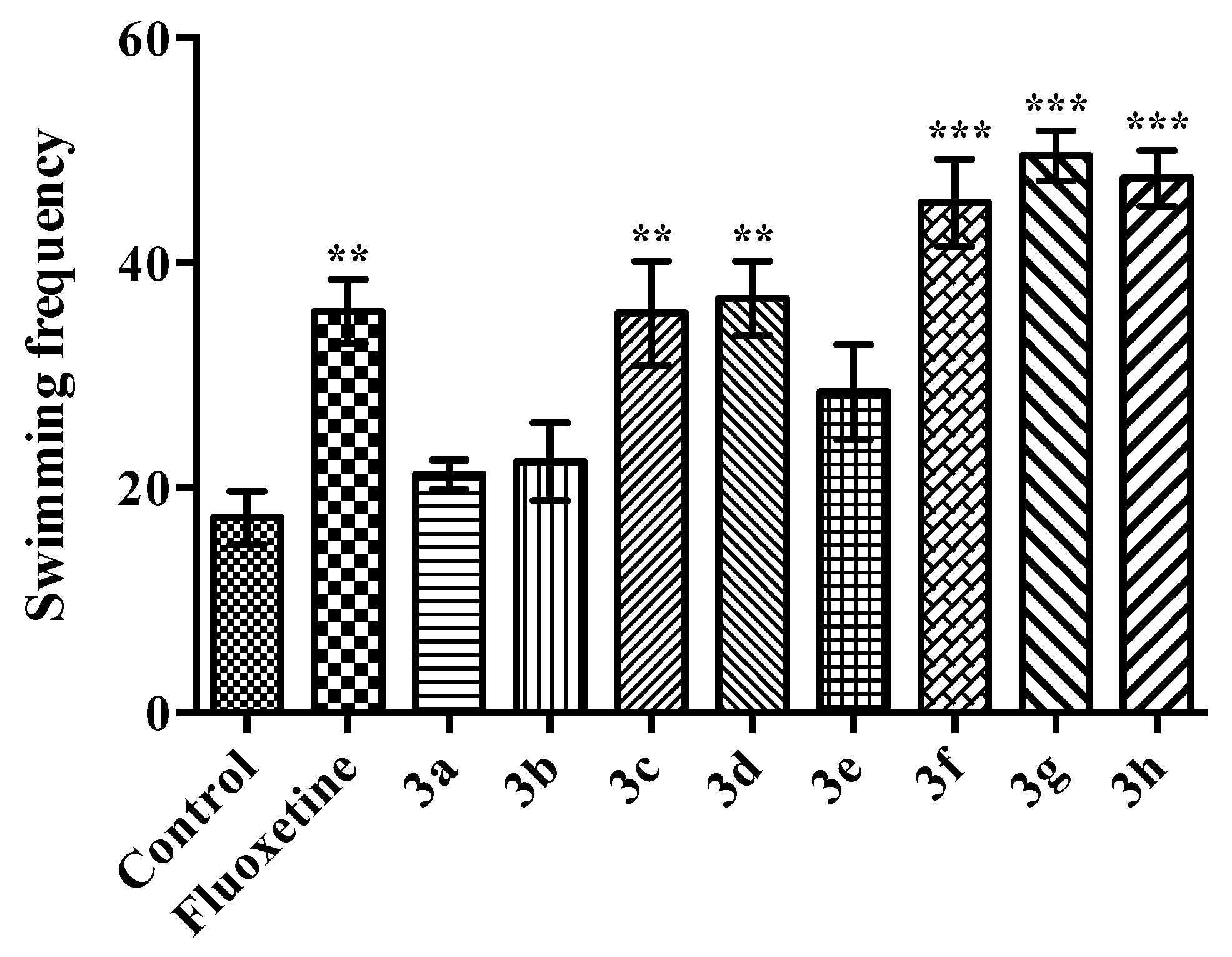
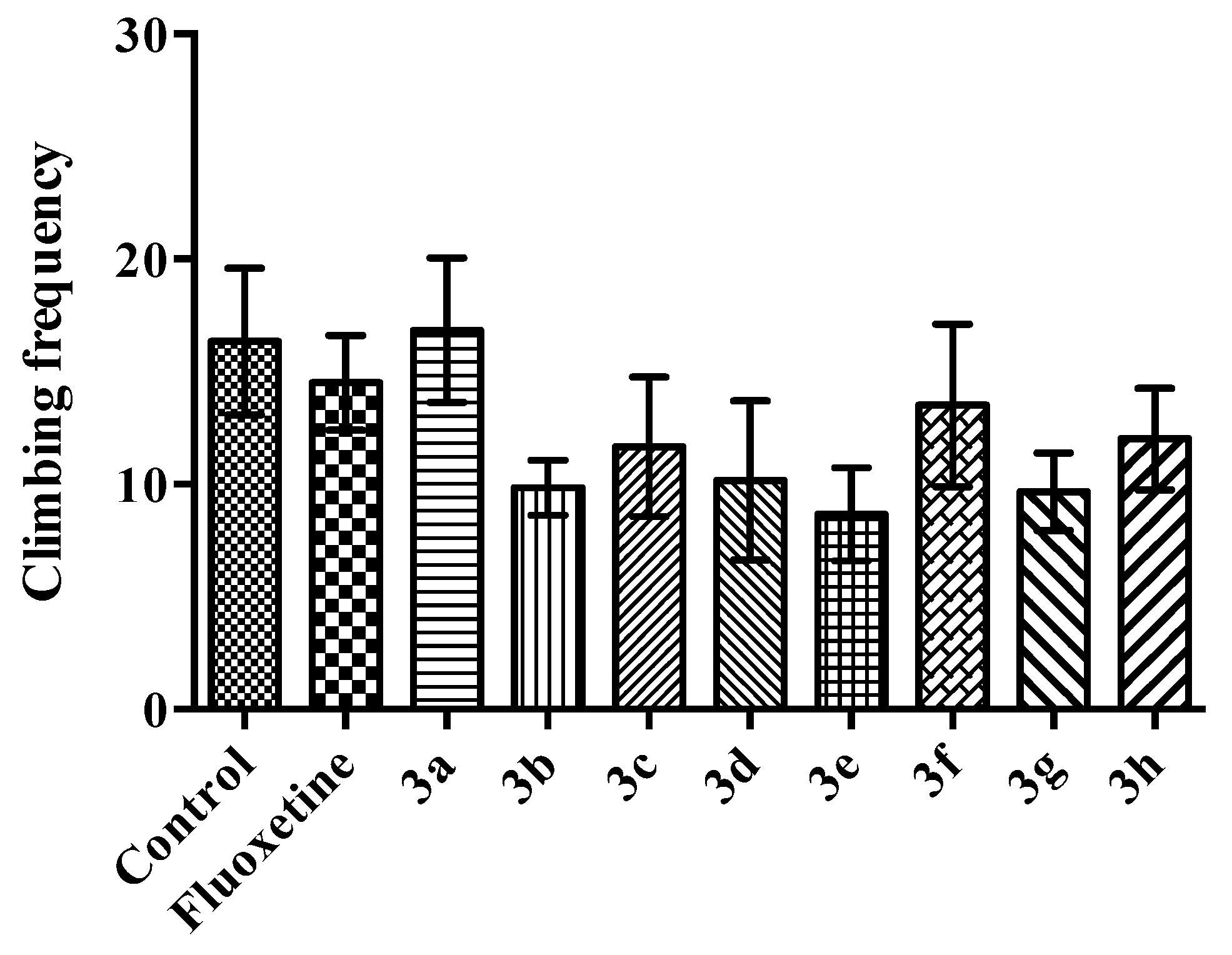
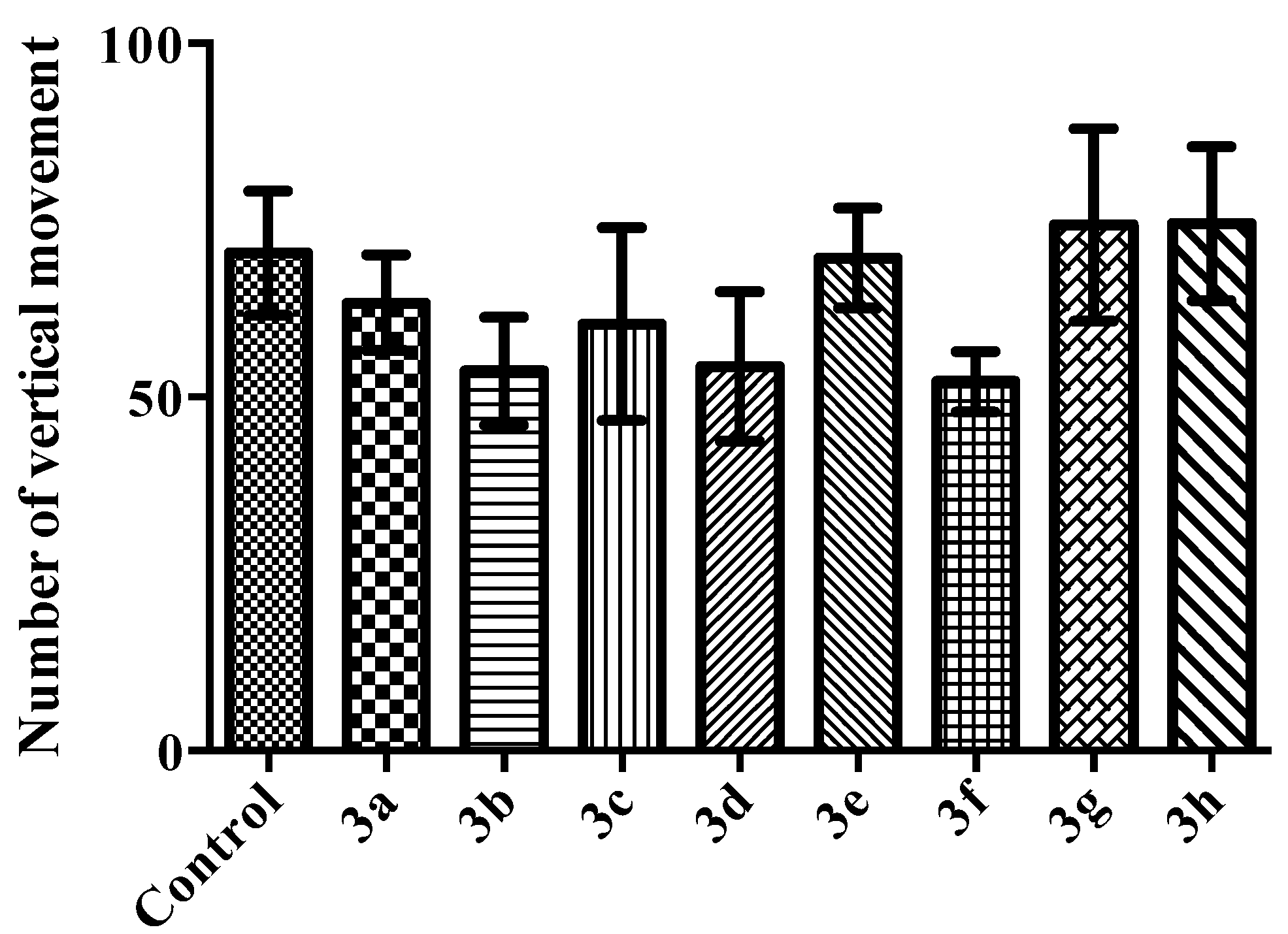
2.2. Pharmacology
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of 5,6-Dimethylbenzo[d]thiazol-2-amine (1)
3.1.2. Synthesis of 2-Chloro-N-(5,6-dimethylbenzo[d]thiazol-2-yl) Acetamide (2)
3.1.3. General Procedure for the Synthesis of N-(5,6-Dimethylbenzo[d]thiazol-2-yl)-2-(4-substituted piperazin/piperidine-1-yl) Acetamides 3a–3h
3.2. Prediction of ADME Parameters
3.3. Pharmacology
3.3.1. Animals
3.3.2. Drugs and Treatments
3.3.3. Behavioral Experiments
Tail Suspension Test
Modified Forced Swimming Test
- Immobility: Mouse was in an upright position on the surface with its front paws together and making only those movements required to keep the head above the water.
- Swimming: Mouse was moving in horizontally throughout the swim chamber and crossing into another quadrant.
- Climbing: Mouse was making vertical movements with its forepaws along the side of the swim chamber.
Activity Cage Test
3.3.4. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pytka, K.; Podkowa, K.; Rapacz, A.; Podkowa, A.; Żmudzka, E.; Olczyk, A.; Sapa, J.; Filipek, B. The role of serotonergic, adrenergic and dopaminergic receptors in antidepressant-like effect. Pharmacol. Rep. 2016, 68, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; He, Z.M.; Zhu, H.Y.; Gao, Y.G.; Zhao, Y.; Yang, H.; Zhang, L.X. Involvement of serotonergic, noradrenergic and dopaminergic systems in the antidepressant-like effect of ginsenoside Rb1, a major active ingredient of Panax ginseng C.A. Meyer. J. Ethnopharmacol. 2017, 204, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, Y.; Ramya, E.M.; Navya, K.; Phani Kumar, G.; Anilakumar, K.R. Antidepressant like effects of hydrolysable tannins of Terminalia catappa leaf extract via modulation of hippocampal plasticity and regulation of monoamine neurotransmitters subjected to chronic mild stress (CMS). Biomed. Pharmacother. 2017, 86, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Guo, F.; Fu, Z.W.; Zhang, B.; Huang, C.G.; Li, Y. Timosaponin derivative YY-23 acts as a non-competitive NMDA receptor antagonist and exerts a rapid antidepressant-like effect in mice. Acta Pharmacol. Sin. 2016, 37, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Hroch, L.; Aitken, L.; Benek, O.; Dolezal, M.; Kuca, K.; Gunn-Moore, F.; Musilek, K. Benzothiazoles-scaffold of interest for CNS targeted drugs. Curr. Med. Chem. 2015, 22, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Yurttaş, L.; Özkay, Y.; Akalın-Çiftçi, G.; Ulusoylar-Yıldırım, Ş. Synthesis and anticancer activity evaluation of N-[4-(2-methylthiazol-4-yl)phenyl]acetamide derivatives containing (benz)azole moiety. J. Enzym. Inhib. Med. Chem. 2014, 29, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Lad, N.P.; Manohar, Y.; Mascarenhas, M.; Pandit, Y.B.; Kulkarni, M.R.; Sharma, R.; Salkar, K.; Suthar, A.; Pandit, S.S. Methylsulfonyl benzothiazoles (MSBT) derivatives: Search for new potential antimicrobial and anticancer agents. Bioorg. Med. Chem. Lett. 2017, 27, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Turan-Zitouni, G.; Özkay, Y.; Özdemir, A.; Kaplancıklı, Z.A.; Altıntop, M.D. Synthesis of some benzothiazole based piperazine-dithiocarbamate derivatives and evaluation of their anticancer activities. Lett. Drug Des. Discov. 2011, 8, 830–837. [Google Scholar] [CrossRef]
- Akhtar, T.; Hameed, S.; Al-Masoudi, N.A.; Loddo, R.; La Colla, P. In vitro antitumor and antiviral activities of new benzothiazole and 1,3,4-oxadiazole-2-thione derivatives. Acta Pharm. 2008, 58, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Netalkar, P.P.; Netalkar, S.P.; Budagumpi, S.; Revankar, V.K. Synthesis, crystal structures and characterization of late first row transition metal complexes derived from benzothiazole core: Anti-tuberculosis activity and special emphasis on DNA binding and cleavage property. Eur. J. Med. Chem. 2014, 79, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Siddiqui, A.A.; Saha, S.J.; De, R.; Mazumder, S.; Banerjee, C.; Iqbal, M.S.; Nag, S.; Adhikari, S.; Bandyopadhyay, U. Antimalarial activity of small-molecule benzothiazole hydrazones. Antimicrob. Agents Chemother. 2016, 60, 4217–4228. [Google Scholar] [CrossRef] [PubMed]
- Yurttaş, L.; Özkay, Y.; Duran, M.; Turan-Zitouni, G.; Özdemir, A.; Canturk, Z.; Kücükoğlu, K.; Kaplancıklı, Z.A. Synthesis and antimicrobial activity evaluation of new dithiocarbamate derivatives bearing thiazole/benzothiazole rings. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1166–1173. [Google Scholar] [CrossRef]
- Amnerkar, N.D.; Bhongade, B.A.; Bhusari, K.P. Synthesis and biological evaluation of some 4-(6-substituted-1,3-benzothiazol-2-yl)amino-1,3-thiazole-2-amines and their Schiff bases. Arab. J. Chem. 2015, 8, 545–552. [Google Scholar] [CrossRef]
- Dar, A.A.; Shadab, M.; Khan, S.; Ali, N.; Khan, A.T. One-pot synthesis and evaluation of antileishmanial activities of functionalized S-alkyl/aryl benzothiazole-2-carbothioate scaffold. J. Org. Chem. 2016, 81, 3149–3160. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, C.; Alam, M.S.; Hamid, H.; Javed, K.; Bano, S.; Dhulap, A.; Ali, Y.; Nazreen, S.; Haider, S. Synthesis and evaluation of pyrazolines bearing benzothiazole as anti-inflammatory agents. Bioorg. Med. Chem. 2014, 22, 5804–5812. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Díaz, H.; Villalobos-Molina, R.; Ortiz-Andrade, R.; Díaz-Coutiño, D.; Medina-Franco, J.L.; Webster, S.P.; Binnie, M.; Estrada-Soto, S.; Ibarra-Barajas, M.; León-Rivera, I.; et al. Antidiabetic activity of N-(6-substituted-1,3-benzothiazol-2-yl)benzenesulfonamides. Bioorg. Med. Chem. Lett. 2008, 18, 2871–2877. [Google Scholar] [CrossRef] [PubMed]
- Karali, N.; Güzel, O.; Ozsoy, N.; Ozbey, S.; Salman, A. Synthesis of new spiroindolinones incorporating a benzothiazole moiety as antioxidant agents. Eur. J. Med. Chem. 2010, 45, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.J.; Yabut, S.C.; Almond, H.R., Jr.; Andrade-Gordon, P.; Corcoran, T.W.; De Garavilla, L.; Kauffman, J.A.; Abraham, W.M.; Recacha, R.; Chattopadhyay, D.; et al. Potent, small-molecule inhibitors of human mast cell tryptase. Antiasthmatic action of a dipeptide-based transition-state analogue containing a benzothiazole ketone. J. Med. Chem. 2003, 46, 3865–3876. [Google Scholar] [PubMed]
- Khan, K.M.; Mesaik, M.A.; Abdalla, O.M.; Rahim, F.; Soomro, S.; Halim, S.A.; Mustafa, G.; Ambreen, N.; Khalid, A.S.; Taha, M.; et al. The immunomodulation potential of the synthetic derivatives of benzothiazoles: Implications in immune system disorders through in vitro and in silico studies. Bioorg. Chem. 2016, 64, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, N.A.; Abdel-Aziz, H.A.; Kamel, G.M.; Fakhr, I.M. Convenient synthesis, anti-inflammatory, analgesic and ulcerogenic activites of some new bis-hydrazones and pyrazole derivatives. Acta Pol. Pharm. 2013, 70, 469–480. [Google Scholar] [PubMed]
- Kaplancikli, Z.A.; Altintop, M.D.; Turan-Zitouni, G.; Ozdemir, A.; Can, O.D. Synthesis and analgesic activity of some acetamide derivatives. J. Enzym. Inhib. Med. Chem. 2012, 27, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Siddiqui, N. New benzo[d]thiazol-2-yl-aminoacetamides as potential anticonvulsants: Synthesis, activity and prediction of molecular properties. Arch. Pharm. (Weinheim) 2015, 348, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.C.; Zhang, H.J.; Jin, C.M.; Quan, Z.S. Synthesis and biological evaluation of novel benzothiazole derivatives as potential anticonvulsant agents. Molecules 2016, 21, 164. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Quintanova, C.; Marques, S.M.; Esteves, A.R.; Cardoso, S.M.; Santos, M.A. Design, synthesis and neuroprotective evaluation of novel tacrine-benzothiazole hybrids as multi-targeted compounds against Alzheimer's disease. Bioorg. Med. Chem. 2013, 21, 4559–4569. [Google Scholar] [CrossRef] [PubMed]
- Özkay, U.D.; Can, O.D.; Özkay, Y.; Öztürk, Y. Effect of benzothiazole/piperazine derivatives on intracerebroventricular streptozotocin-induced cognitive deficits. Pharmacol. Rep. 2012, 64, 834–847. [Google Scholar] [CrossRef]
- Mohsen, U.A.; Kaplancikli, Z.A.; Özkay, Y.; Yurttaş, L. Synthesis and evaluation of anti-acetylcholinesterase activity of some benzothiazole based new piperazine-dithiocarbamate derivatives. Drug Res. 2015, 65, 176–183. [Google Scholar] [CrossRef]
- Demir Özkay, U.; Can, O.D.; Sağlık, B.N.; Çevik, U.A.; Levent, S.; Özkay, Y.; Ilgın, S.; Atlı, Ö. Design, synthesis, and AChE inhibitory activity of new benzothiazole-piperazines. Bioorg. Med. Chem. Lett. 2016, 26, 5387–5394. [Google Scholar] [CrossRef] [PubMed]
- Kaya, B.; Sağlık, B.N.; Levent, S.; Özkay, Y.; Kaplancıklı, Z.A. Synthesis of some novel 2-substituted benzothiazole derivatives containing benzylamine moiety as monoamine oxidase inhibitory agents. J. Enzym. Inhib. Med. Chem. 2016, 31, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Firooznia, F.; Cheung, A.W.; Brinkman, J.; Grimsby, J.; Gubler, M.L.; Hamid, R.; Marcopulos, N.; Ramsey, G.; Tan, J.; Wen, Y.; et al. Discovery of benzothiazole-based adenosine A2B receptor antagonists with improved A2A selectivity. Bioorg. Med. Chem. Lett. 2011, 21, 1933–1936. [Google Scholar] [CrossRef] [PubMed]
- Anzini, M.; Chelini, A.; Mancini, A.; Cappelli, A.; Frosini, M.; Ricci, L.; Valoti, M.; Magistretti, J.; Castelli, L.; Giordani, A.; et al. Synthesis and biological evaluation of amidine, guanidine, and thiourea derivatives of 2-amino(6-trifluoromethoxy)benzothiazole as neuroprotective agents potentially useful in brain diseases. J. Med. Chem. 2010, 53, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Carboni, S.; Hiver, A.; Szyndralewiez, C.; Gaillard, P.; Gotteland, J.P.; Vitte, P.A. AS601245 (1,3-benzothiazol-2-yl(2-[[2-(3-pyridinyl)ethyl]amino]-4-pyrimidinyl)acetonitrile): A c-Jun NH2-terminal protein kinase inhibitor with neuroprotective properties. J. Pharmacol. Exp. Ther. 2004, 310, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Sinhmar, A.; Sharma, A.; Rajak, H.; Pathak, D.P. Medicinal significance of benzothiazole scaffold: An insight view. J. Enzym. Inhib. Med. Chem. 2013, 28, 240–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Y.; Zhao, S.; Xu, X.; Liu, X.; Liu, B.F.; Zhang, G. Synthesis and biological evaluation of a series of benzoxazole/benzothiazole-containing 2,3-dihydrobenzo[b][1,4]dioxine derivatives as potential antidepressants. Bioorg. Med. Chem. Lett. 2014, 24, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Etukala, J.R.; Eyunni, S.V.; Setola, V.; Roth, B.L.; Ablordeppey, S.Y. Benzothiazoles as probes for the 5HT1A receptor and the serotonin transporter (SERT): A search for new dual-acting agents as potential antidepressants. Eur. J. Med. Chem. 2012, 53, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Rana, A.; Khan, S.A.; Ahsan, W.; Alam, M.S.; Ahmed, S. Analgesic and antidepressant activities of benzothiazole-benzamides. Biomed. Pharmacol. J. 2008, 1, 297–300. [Google Scholar]
- Oliveira, C.E.; Sari, M.H.; Zborowski, V.A.; Araujo, P.C.; Nogueira, C.W.; Zeni, G. p,p′-Methoxyl-diphenyl diselenide elicits an antidepressant-like effect in mice without discontinuation anxiety phenotype. Pharmacol. Biochem. Behav. 2017, 154, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Can, Ö.D.; Özkay, Ü.D.; Üçel, U.İ. Anti-depressant-like effect of vitexin in BALB/c mice and evidence for the involvement of monoaminergic mechanisms. Eur. J. Pharmacol. 2013, 699, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berl.) 1985, 85, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Markou, A.; Lucki, I. Assessing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol. Sci. 2002, 23, 238–245. [Google Scholar] [CrossRef]
- Santiago, R.M.; Barbiero, J.; Martynhak, B.J.; Boschen, S.L.; da Silva, L.M.; Werner, M.F.; Da Cunha, C.; Andreatini, R.; Lima, M.M.; Vital, M.A. Antidepressant-like effect of celecoxib piroxicam in rat models of depression. J. Neural Transm. (Vienna) 2014, 121, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Brocardo, P.S.; Budni, J.; Kaster, M.P.; Santos, A.R.; Rodrigues, A.L. Folic acid administration produces an antidepressant-like effect in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Neuropharmacology 2008, 54, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Demir Özkay, Ü.; Yurttaş, L.; Özkay, Y.; Üçel, U.I.; Can, Ö.D.; Öztürk, Y. Synthesis of new 1-phenyl-2-(4-substituted-piperazin-1-yl)-propanol derivatives and evaluation of their antidepressant-like effects. Arch. Pharm. Res. 2013, 36, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Van de Waterbeemd, H.; Gifford, E. ADMET in silico modelling: Towards prediction paradise? Nat. Rev. Drug Discov. 2003, 2, 192–204. [Google Scholar] [CrossRef] [PubMed]
- QikProp, version 4.8; Schrödinger, LLC: New York, NY, USA, 2016.
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Duffy, E.M. Prediction of drug solubility from structure. Adv. Drug Deliv. Rev. 2002, 54, 355–366. [Google Scholar] [CrossRef]
- Demir Özkay, Ü.; Özkay, Y.; Can, Ö.D. Synthesis and analgesic effects of 2-(2-carboxyphenylsulfanyl)-N-(4-substitutedphenyl)acetamide derivatives. Med. Chem. Res. 2011, 20, 152–157. [Google Scholar] [CrossRef]
- Can, O.D.; Demir Özkay, Ü.; Kıyan, H.T.; Demirci, B. Psychopharmacological profile of Chamomile (Matricaria recutita L.) essential oil in mice. Phytomedicine 2012, 19, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Detke, M.J.; Lucki, I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: The effects of water depth. Behav. Brain Res. 1996, 73, 43–46. [Google Scholar] [CrossRef]
- Palotai, M.; Telegdy, G.; Tanaka, M.; Bagosi, Z.; Jászberényi, M. Neuropeptide AF induces anxiety-like and antidepressant-like behavior in mice. Behav. Brain Res. 2014, 274, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Can, O.D.; Ozkay, U.D. Effects of Hypericum montbretti extract on the central nervous system and involvement of GABA (A)/Benzodiazepine receptors in its pharmacological activity. Phytother. Res. 2012, 26, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Votava, M.; Hess, L.; Slíva, J.; Krsiak, M.; Agová, V. Dexmedetomidine selectively suppresses dominant behavior in aggressive and sociable mice. Eur. J. Pharmacol. 2005, 523, 79–85. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 3a–h are available from the authors. |

| Comp | RB | CNS | MW | MV | DHB | AHB | LogP | LogS | PCaco | PM | %HOA | PSA | VRF | VRT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3a | 3 | 1 | 303.42 | 1023 | 1 | 6 | 2.58 | −3.55 | 729.17 | 5 | 93.28 | 49.84 | 0 | 0 |
| 3b | 3 | 1 | 317.45 | 1056 | 1 | 6 | 2.78 | −3.78 | 712.35 | 5 | 94.29 | 49.82 | 0 | 0 |
| 3c | 3 | 2 | 318.44 | 1065 | 1 | 8 | 1.43 | −2.01 | 152.72 | 6 | 74.38 | 57.29 | 0 | 0 |
| 3d | 4 | 2 | 332.46 | 1128 | 1 | 8 | 1.83 | −2.39 | 161.61 | 6 | 77.18 | 58.61 | 0 | 0 |
| 3e | 3 | 1 | 398.50 | 1256 | 1 | 7 | 3.93 | −5.42 | 680.86 | 5 | 100.00 | 56.25 | 0 | 0 |
| 3f | 5 | 2 | 412.52 | 1309 | 1 | 8 | 3.33 | −3.98 | 175.24 | 7 | 86.57 | 55.97 | 0 | 1 |
| 3g | 5 | 2 | 428.98 | 1337 | 1 | 8 | 3.58 | −4.35 | 175.24 | 7 | 88.07 | 55.97 | 0 | 1 |
| 3h | 8 | 1 | 465.66 | 1562 | 1 | 10 | 3.26 | −3.51 | 39.60 | 9 | 74.63 | 61.00 | 0 | 1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demir Özkay, Ü.; Kaya, C.; Acar Çevik, U.; Can, Ö.D. Synthesis and Antidepressant Activity Profile of Some Novel Benzothiazole Derivatives. Molecules 2017, 22, 1490. https://doi.org/10.3390/molecules22091490
Demir Özkay Ü, Kaya C, Acar Çevik U, Can ÖD. Synthesis and Antidepressant Activity Profile of Some Novel Benzothiazole Derivatives. Molecules. 2017; 22(9):1490. https://doi.org/10.3390/molecules22091490
Chicago/Turabian StyleDemir Özkay, Ümide, Ceren Kaya, Ulviye Acar Çevik, and Özgür Devrim Can. 2017. "Synthesis and Antidepressant Activity Profile of Some Novel Benzothiazole Derivatives" Molecules 22, no. 9: 1490. https://doi.org/10.3390/molecules22091490




