Synthesis and DFT Calculations of Novel Vanillin-Chalcones and Their 3-Aryl-5-(4-(2-(dimethylamino)-ethoxy)-3-methoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbaldehyde Derivatives as Antifungal Agents
Abstract
:1. Introduction
2. Results
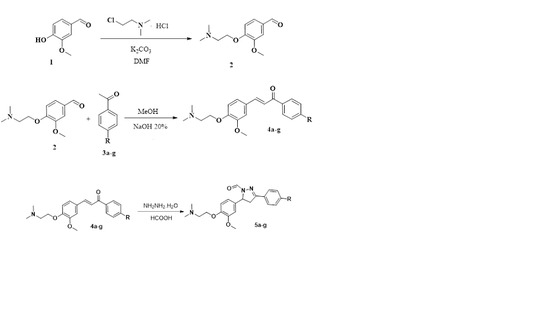
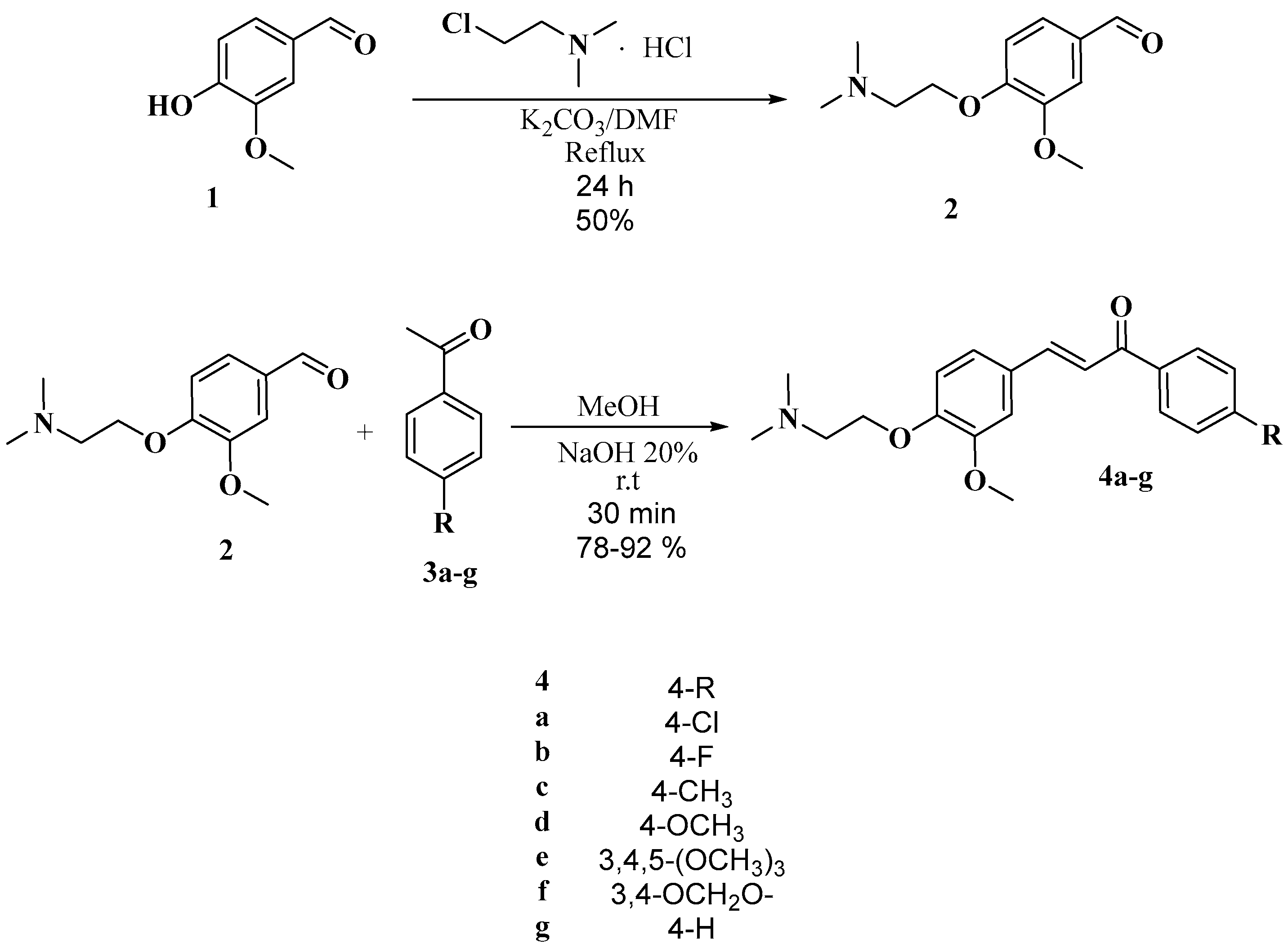
2.1. Chemistry
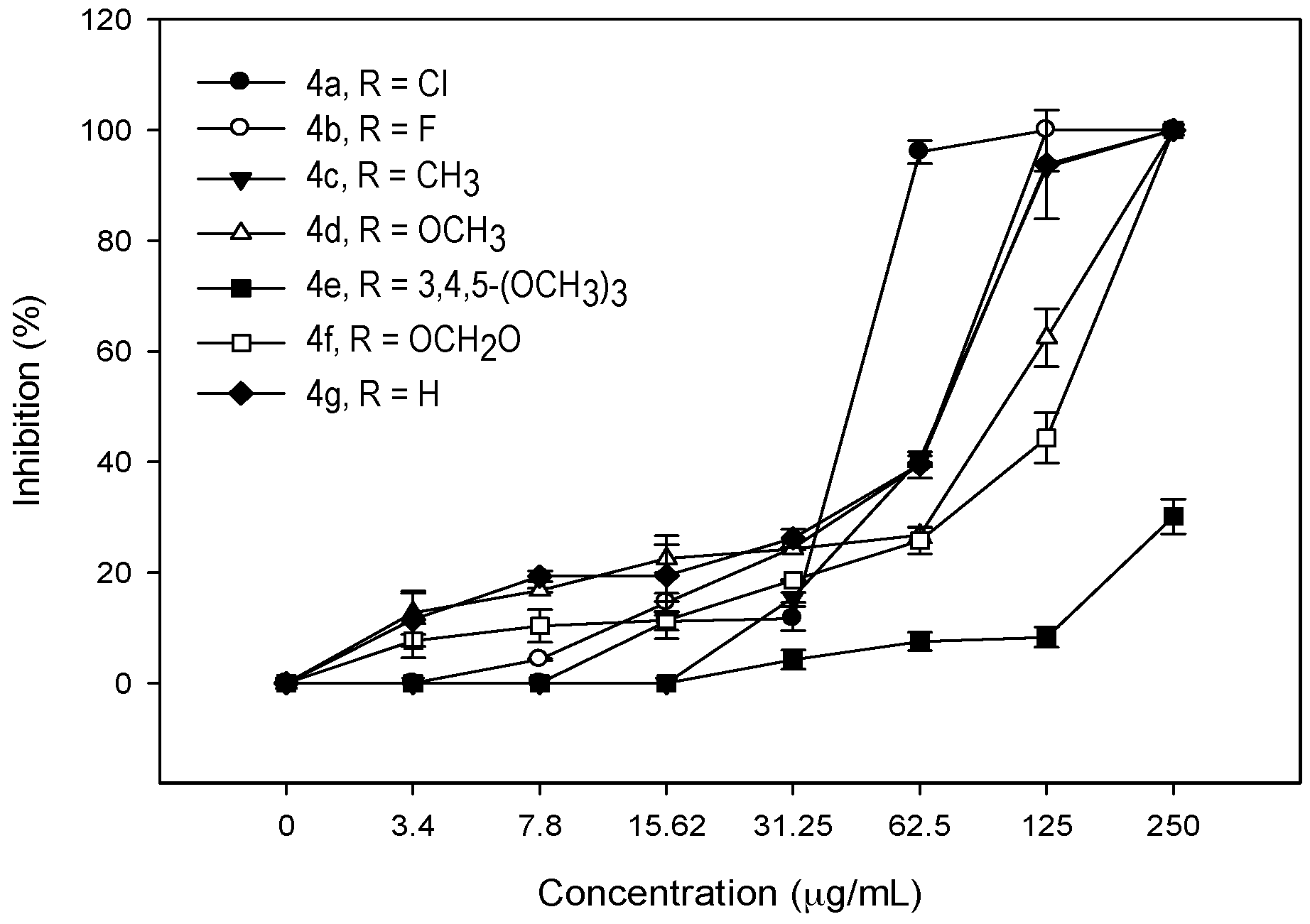
2.2. Antifungal Activity
2.3. Second Order Studies of Compounds 4 against C. neoformans
2.4. DFT Calculations
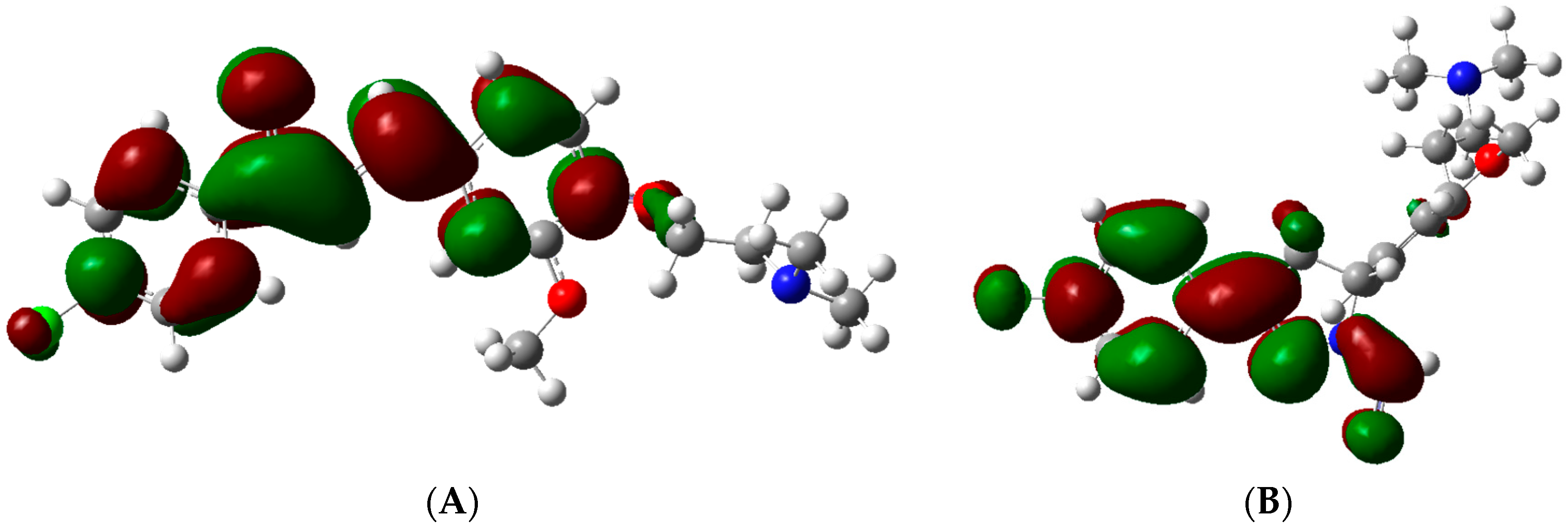
2.4.1. Chalcones vs. Pyrazolines: A Chemical Reactivity Analysis
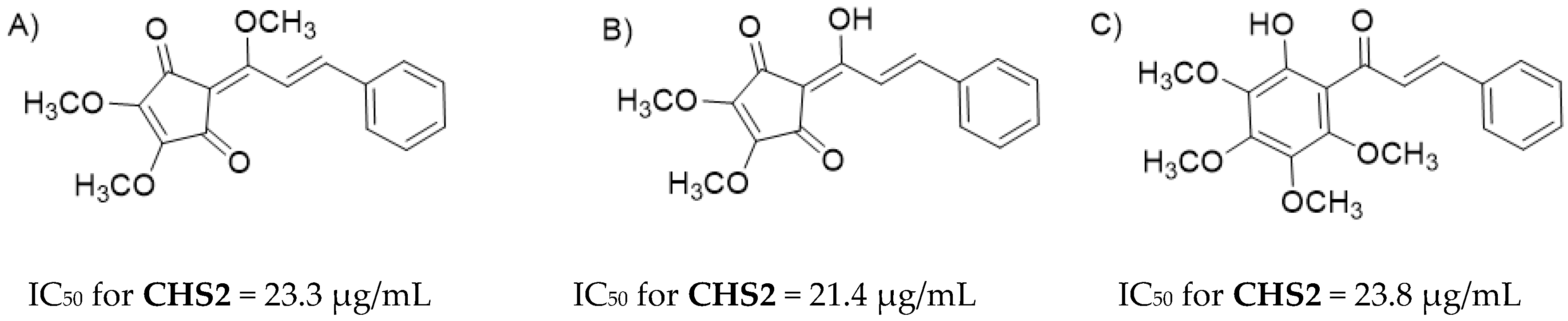
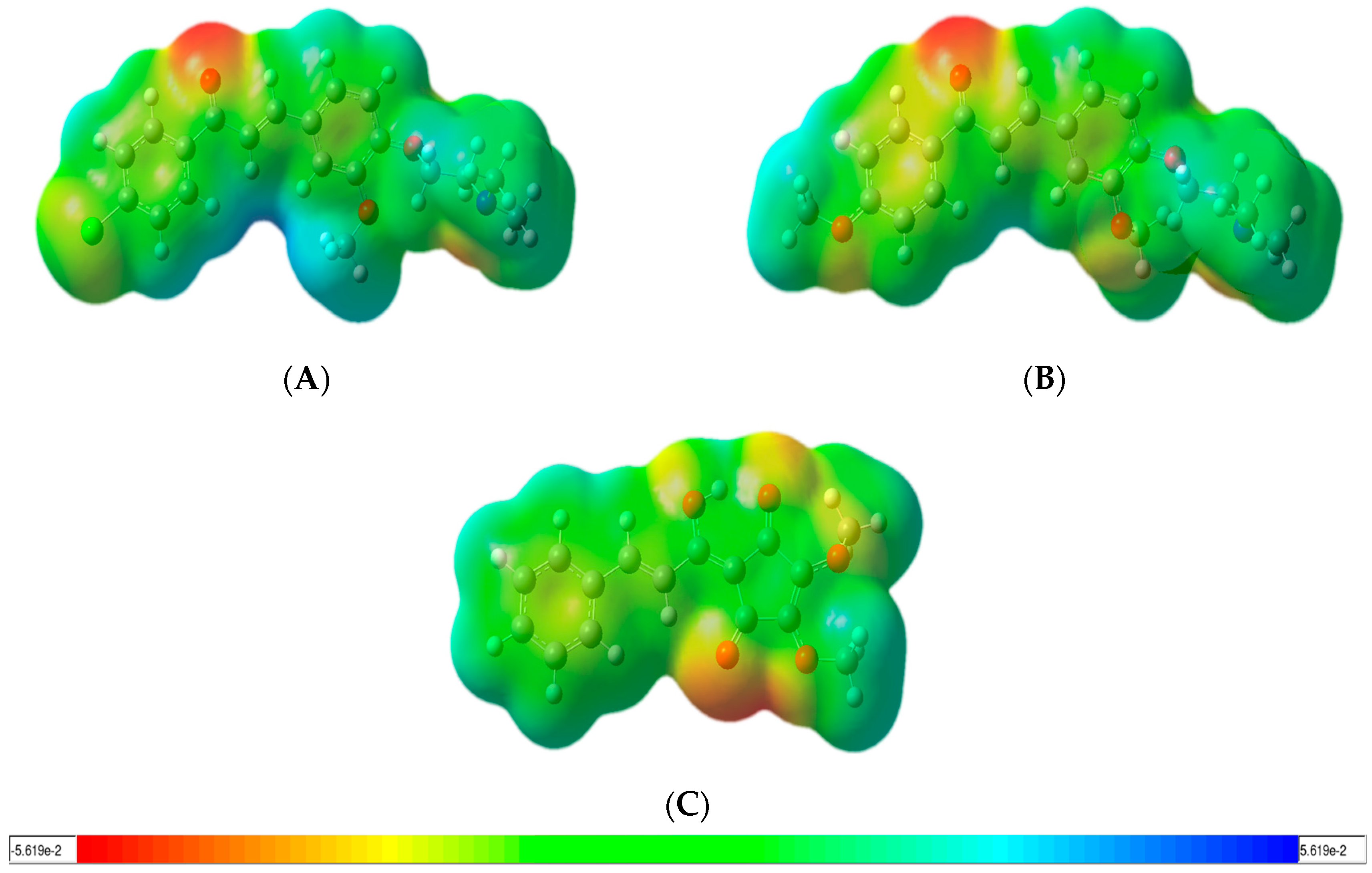
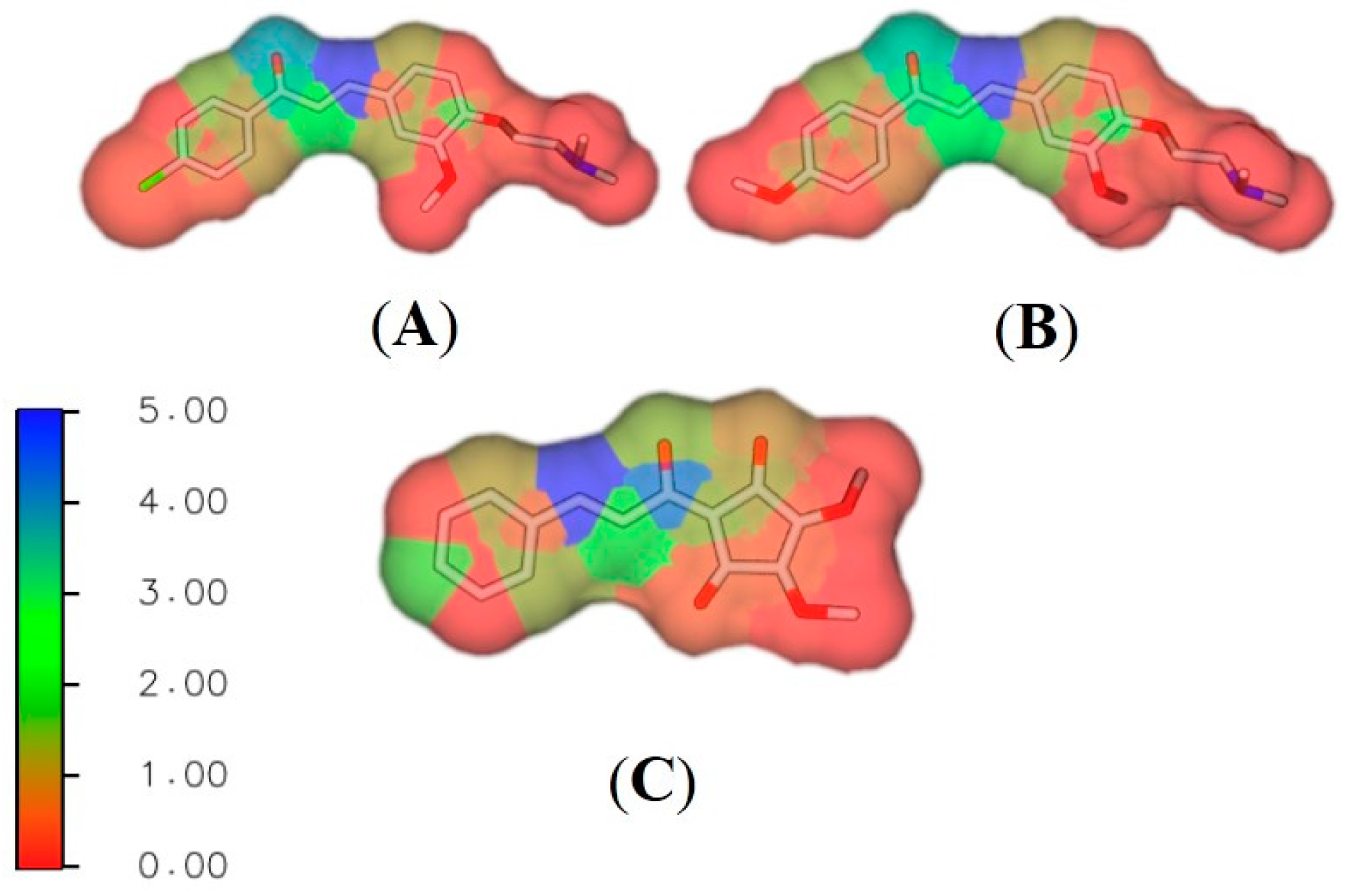
2.4.2. Reactivity Analysis on Chalcone Series
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.2.1. Synthesis of the Precursor 4-(2-(Dmethylamino)ethoxy)-3-methoxybenzaldehyde (2)
3.2.2. General Process for the Synthesis of Compounds 4a–g
3.2.3. General Process for the Synthesis of Compounds 5a–g
3.3. Antifungal Activity
3.3.1. Microorganisms and Media
3.3.2. Antifungal Susceptibility Testing
3.3.3. Fungal Growth Inhibition Percentage Determination
3.4. Computational Details
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Odds, F.C. Antifungal agents: Their diversity and increasing sophistication. Mycologist 2003, 17, 51–55. [Google Scholar] [CrossRef]
- Denning, D.W.; Bromley, M.J. How to bolster the antifungal pipeline. Science 2015, 347, 1414–1416. [Google Scholar] [CrossRef] [PubMed]
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008, 51, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, I.; Summerbell, R.C. The dermatophytes. Clin. Microbiol. Rev. 1995, 8, 240–259. [Google Scholar] [CrossRef]
- Sabatelli, F.; Patel, R.; Mann, P.; Mendrick, C.; Norris, C.; Hare, R.; Loebenberg, D.; Black, T.; McNicholas, P. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob. Agents Chemother. 2006, 50, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Bastert, J.; Schaller, M.; Korting, H.; Evans, E. Current and future approaches to antimycotic treatment in the era of resistant fungi and immunocompromised hosts. Int. J. Antimicrob. Agents 2001, 17, 81–91. [Google Scholar] [CrossRef]
- Damodar, K.; Kim, J.K.; Jun, J.G. Synthesis and pharmacological properties of naturally occurring prenylated and pyranochalcones as potent anti-inflammatory agents. Chin. Chem. Lett. 2016, 27, 698–702. [Google Scholar] [CrossRef]
- Vembu, S.; Pazhamalai, S.; Gopalakrishnan, M. Synthesis, spectral characterization, and effective antifungal evaluation of 1H-tetrazole containing 1,3,5-triazine dendrimers. Med. Chem. Res. 2016, 25, 1916–1924. [Google Scholar] [CrossRef]
- Sivacumar, P.M.; Kumar, T.M.; Doble, M. Antifungal activity, mechanism and QSAR studies on chalcones. Chem. Biol. Drug Des. 2009, 74, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Muškinja, J.; Burmudžija, A.; Ratković, Z.; Ranković, B.; Kosanić, M.; Bogdanović, G.A.; Novaković, S.B. Ferrocenyl chalcones with O-alkylated vanillins: Synthesis, spectral characterization, microbiological evaluation, and single-crystal X-ray analysis. Med. Chem. Res. 2016, 25, 1744–1753. [Google Scholar] [CrossRef]
- Mazzone, G.; Galano, A.; Alvarez-Idaboy, J.R.; Russo, N. Coumarin-chalcone hybrids as peroxyl radical scavengers: Kinetics and mechanisms. J. Chem. Inf. Model. 2016, 56, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Tadigoppula, N.; Korthikunta, V.; Gupta, S.; Kancharla, P.; Khaliq, T.; Soni, A.; Srivastava, R.K.; Srivastava, K.; Puri, S.K.; Raju, K.S.R. Synthesis and insight into the structure-activity relationships of chalcones as antimalarial agents. J. Med. Chem. 2012, 56, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Mohanakrishnan, D.; Sharma, U.K.; Kumar, R.; Sinha, A.K.; Sahal, D. Desing, economical synthesis and antiplasmodial evaluation of vanillin derived allylated chalcones and their marked synergism with artemisinin againts chloroquine resistant strains of plasmodium falciparum. Eur. J. Med. Chem. 2014, 79, 350–368. [Google Scholar] [CrossRef] [PubMed]
- Pingaew, R.; Saekee, A.; Mandi, P.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis, biological evaluation and molecular docking of novel chalcone-coumarin hybrids as anticancer and antimalarial agents. Eur. J. Med. Chem. 2014, 85, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.; Simoens, M.; Falchi, G.; Lavaggi, L.; Piro, O.E.; Castellano, E.E.; Vidal, A.; Azquete, A.; Monge, A.; López, A.; et al. Synthetic chalcones, flavonones, and flavones as antitutumoral agents: Biological and structure-activity relationships. Bioorg. Med. Chem. 2007, 15, 3356–3367. [Google Scholar] [CrossRef] [PubMed]
- López, S.N.; Castelli, M.V.; Zacchino, S.A.; Dominguez, J.N.; Lobo, G.; Charris-Charris, J.; Cortés, J.C.G.; Ribas, J.C.; Devia, C.; Rodríguez, A.M.; et al. In vitro antifungal evaluation and structure-activity relationships of a new series of chalcones derivatives and synthetic analogues, with inhibitory properties againts polymers of the fungal cell wall. Bioorg. Med. Chem. 2001, 9, 1999–2013. [Google Scholar] [CrossRef]
- Boeck, P.; Leal, P.C.; Yunes, R.A.; Filho, V.C.; López, S.; Sortino, M.; Escalante, A.; Furlán, R.L.E.; Zacchino, S. Antifungal activity and studies on mode of action of novel xanthoxyline-derived chalcones. Arch. Pharm. 2005, 338, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lahtchev, K.L.; Batovska, D.I.; Parushev, S.P.; Ubiyvovk, V.M.; Sibirny, A.A. Antifungal, activity of chalcones: A Mechanistic study using various yeast strain. Eur. J. Med. Chem. 2008, 43, 2220–2238. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, Z. A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem. 2007, 42, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, B.F.; Abdel-Aziz, H.A.; Ahmed, E.M. Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3-arylbutan-1-ones and 3-(benzofuran-2-yl)-4,5-dihydro-5-aryl-1-[4-(aryl)-1,3-thiazol-2-yl]-1H-pyrazoles. Eur. J. Med. Chem. 2009, 44, 2632–2635. [Google Scholar] [CrossRef] [PubMed]
- Montoya, A.; Quiroga, J.; Abonia, R.; Derita, M.; Sortino, M.; Ornelas, A.; Zacchino, S.; Insuasty, B. Hybrid molecules containing a 7-chloro-4-aminoquinoline nucleus and a substituted 2-pyrazoline with antiproliferative and antifungal activity. Molecules 2016, 21, 969. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sayed, M.A.; Bayomi, S.M.; El-Sherbeny, M.A.; Abdel-Aziz, N.I.; ElTahir, K.E.H.; Shehatou, G.S.; Alaa, A.-M. Synthesis, anti-inflammatory, analgesic, COX-1/2 inhibition activities and molecular docking study of pyrazoline derivatives. Bioorg. Med. Chem. 2016, 24, 2032–2042. [Google Scholar] [CrossRef] [PubMed]
- Insuasty, B.; Montoya, A.; Becerra, D.; Quiroga, J.; Abonia, R.; Robledo, S.; Vélez, I.D.; Upegui, Y.; Nogueras, M.; Cobo, J. Synthesis of novel analogs of 2-pyrazoline obtained from [(7-chloroquinolin-4-yl) amino] chalcones and hydrazine as potential antitumor and antimalarial agents. Eur. J. Med. Chem. 2013, 67, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Insuasty, B.; Tigreros, A.; Orozco, F.; Quiroga, J.; Abonía, R.; Nogueras, M.; Sanchez, A.; Cobo, J. Synthesis of novel pyrazolic analogues of chalcones and their 3-aryl-4-(3-aryl-4,5-dihydro-1H-pyrazol-5-yl)-1-phenyl-1H-pyrazole derivatives as potential antitumor agents. Bioorg. Med. Chem. 2010, 18, 4965–4974. [Google Scholar] [CrossRef] [PubMed]
- Burmudžija, A.; Ratković, Z.; Muškinja, J.; Janković, N.; Ranković, B.; Kosanić, M.; Đorđević, S. Ferrocenyl based pyrazoline derivatives with vanillic core: Synthesis and investigation of their biological properties. RSC Adv. 2016, 6, 91420–91430. [Google Scholar] [CrossRef]
- Hernández-Vázquez, E.; Castaneda-Arriaga, R.; Ramírez-Espinosa, J.J.; Medina-Campos, O.N.; Hernández-Luis, F.; Chaverri, J.P.; Estrada-Soto, S. 1,5-Diarylpyrazole and vanillin hybrids: Synthesis, biological activity and DFT studies. Eur. J. Med. Chem. 2015, 100, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, A.; Yang, J. One-pot three-component mild synthesis of 2-aryl-3-(9-alkylcarbazol-3-yl) thiazolidin-4-ones. J. Heterocycl. Chem. 2012, 49, 1458–1461. [Google Scholar] [CrossRef]
- Chakraborty, A.; Pan, S.; Chattaraj, P.K. Biological activity and toxicity: A conceptual DFT approach. In Applications of Density Functional Theory to Biological and Bioinorganic Chemistry; Springer: Berlin, Germany, 2013; Volume 150, pp. 143–179. [Google Scholar]
- Sarkar, A.; Middya, T.R.; Jana, A.D. A QSAR study of radical scavenging antioxidant activity of a series of flavonoids using DFT based quantum chemical descriptors—The importance of group frontier electron density. J. Mol. Model. 2012, 18, 2621–2631. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, T.P.J.; Ibarra, R.G.; Acosta, J.J.M. 2D, 3D-QSAR and molecular docking of 4(1H)-quinolones analogues with antimalarial activities. J. Mol. Graph. Model. 2013, 46, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Khan, S.I.; Kandi, S.K.; Raj, K.; Sun, G.; Yang, X.; Molina, A.D.C.; Ni, N.; Wang, B.; Rawat, D.S. Synthesis, antimalarial activity and cytotoxic potential of new monocarbonyl analogues of curcumin. Bioorg. Med. Chem. Lett. 2013, 23, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). CLSI Document M27-A3: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Volume 14, pp. 1–25. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). CLSI Document M38-A2: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Volume 16, pp. 1–35. [Google Scholar]
- Patel, P.A.; Bhadani, V.N.; Bhatt, P.V.; Purohit, D.M. Synthesis and biological evaluation of novel chalcone and pyrazoline derivatives bearing substituted vanillin nucleus. J. Heterocycl. Chem. 2015, 52, 1119–1125. [Google Scholar] [CrossRef]
- Butts, A.; Krysan, D.J. Antifungal drug discovery: Something old and something new. PLoS Pathog. 2012, 8, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Wannemuehler, K.A.; Marston, B.J.; Govender, N.; Pappas, P.G.; Chiller, T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009, 23, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Trpković, A.; Pekmezović, M.; Barać, A.; Radović, L.C.; Arsenijević, V.A. In vitro antifungal activities of amphotericin B, 5-fluorocytosine, fluconazole and itraconazole against Cryptococcus neoformans isolated from cerebrospinal fluid and blood from patients in Serbia. J. Mycol. Méd. J. Med. Mycol. 2012, 22, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E.J.; Roling, E.E.; Petzold, C.R.; Keele, D.J.; Klepser, M.E. In vitro activity of micafungin (FK-463) against Candida spp.: Microdilution, time-kill, and postantifungal-effect studies. Antimicrob. Agents Chemother. 2002, 46, 3846–3853. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, T. Über die zuordnung von wellenfunktionen und eigenwerten zu den einzelnen elektronen eines Atoms. Physica 1934, 1, 104–113. [Google Scholar] [CrossRef]
- Pearson, R.G. The principle of maximum hardness. Acc. Chem. Res. 1993, 26, 250–255. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Chalcone scaffolds as anti-infective agents: Structural and molecular target perspectives. Eur. J. Med. Chem. 2015, 101, 496–524. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.M.; Tupe, S.G.; Deshpande, M.V. Chitin synthase inhibitors as antifungal agents. Mini Rev. Med. Chem. 2013, 13, 222–236. [Google Scholar] [PubMed]
- Hwang, E.I.; Lee, Y.M.; Lee, S.M.; Yeo, W.H.; Moon, J.S.; Kang, T.H.; Park, K.D.; Kim, S.U. Inhibition of chitin synthase 2 and antifungal activity of lignans from the stem bark of Lindera erythrocarpa. Planta Medica 2007, 73, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Dorfmueller, H.C.; Ferenbach, A.T.; Borodkin, V.S.; van Aalten, D.M. A structural and biochemical model of processive chitin synthesis. J. Biol. Chem. 2014, 289, 23020–23028. [Google Scholar] [CrossRef] [PubMed]
- Saxena, I.M.; Brown, R.M., Jr.; Fevre, M.; Geremia, R.A.; Henrissat, B. Multidomain architecture of beta-glycosyl transferases: Implications for mechanism of action. J. Bacteriol. 1995, 177, 1419. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.K.; Tho Nguyen, M. Fukui function and local softness as reactivity descriptors. In Chemical Reactivity Theory: A Density Functional View; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Sure, R.; Grimme, S. Corrected small basis set Hartree-Fock method for large systems. J. Comput. Chem. 2013, 34, 1672–1685. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Sánchez-Márquez, J.; Zorrilla, D.; Sánchez-Coronilla, A.; Desireé, M.; Navas, J.; Fernández-Lorenzo, C.; Alcántara, R.; Martín-Calleja, J. Introducing “UCA-FUKUI” software: Reactivity-index calculations. J. Mol. Model. 2014, 20, 2492. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Compound | Structure | Fungal Species | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C.a. | C.n. | A.fu. | A.fl. | A.n. | M.g. | T.r. | T.m. | ||
| 4a |  | 125/250 | 62.5/125 | i | i | i | 31.25/31.25 | 31.25/31.25 | 31.25/31.25 |
| 4b |  | 250/>250 | 125/125 | i | i | i | 62.5/125 | 62.5/125 | 62.5/125 |
| 4c |  | 250/nf | 125/125 | i | i | i | 62.5/62.5 | 62.5/62.5 | 31.25/31.25 |
| 4d |  | i | 250/250 | i | i | i | 62.5/250 | 62.5/250 | 62.5/125 |
| 4e |  | i | i | i | i | i | i | i | i |
| 4f |  | i | 250/nf | i | i | i | 250/250 | 250/250 | 125/250 |
| 4g |  | i | 125/250 | i | i | i | 62.5/250 | 62.5/125 | 62.5/125 |
| 5a |  | i | 125/125 | i | i | i | 125/250 | 125/250 | 125/250 |
| 5b |  | i | 250/250 | i | i | i | i | i | i |
| 5c |  | i | 250/nf | i | i | i | 250/nf | 250/nf | 250/nf |
| 5d |  | i | i | i | i | i | i | i | i |
| 5e |  | i | i | i | i | i | i | i | i |
| 5f |  | i | i | i | i | i | i | i | i |
| 5g |  | i | i | i | i | i | i | i | i |
| Amphotericin B | - | 0.78 | 0.25 | 0.50 | 0.50 | 0.50 | 0.12 | 0.07 | 0.07 |
| Terbinafine | - | 0.50 | 0.25 | 0.12 | 0.50 | 0.25 | 0.05 | 0.02 | 0.02 |

| Concentrations in µg/mL | ||||||||
|---|---|---|---|---|---|---|---|---|
| R | Compound | 250 | 125 | 62.5 | 31.25 | 15.62 | 7.81 | 3.9 |
| 4-Cl | 4a | 100 | 100 | 96.0 ± 2.0 | 11.7 ± 2.2 | 11.2 ± 1.6 | 0 | 0 |
| 4-F | 4b | 100 | 100 | 39.4 ± 2.4 | 24.5 ± 0.1 | 14.6 ± 1.7 | 4.3 ± 0.1 | 0 |
| 4-CH3 | 4c | 100 | 93.3 ± 0.7 | 40.5 ± 0.6 | 15.5 ± 0.9 | 0 | 0 | 0 |
| 4-OCH3 | 4d | 100 | 62.4 ± 5.2 | 26.8 ± 1.4 | 24.3 ± 0.2 | 22.6 ± 2.5 | 16.8 ± 0.4 | 12.7 ± 3.9 |
| 3,4,5-(OCH3)3 | 4e | 30.1 ± 3.1 | 8.35 ± 1.8 | 7.6 ± 1.6 | 4.3 ± 1.7 | 0 | 0 | 0 |
| OCH2O | 4f | 100 | 44.3 ± 4.5 | 25.8 ± 2.4 | 18.7 ± 0.1 | 11.4 ± 3.4 | 10.4 ± 2.9 | 7.7 ± 3.1 |
| H | 4g | 100 | 93.8 ± 9.8 | 39.6 ± 0.4 | 26.2 ± 1.7 | 19.5 ± 7.1 | 19.3 ± 0.9 | 11.5 ± 4.8 |
| Compound | Clinical Strains of C. neoformans | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C.n. ATCC 32264 | C.n. IM 983040 | C.n. IM 972724 | C.n. IM 042074 | C.n. IM 983036 | C.n. IM 00319 | |||||||||||||
| MIC100 | MIC80 | MIC50 | MIC100 | MIC80 | MIC50 | MIC100 | MIC80 | MIC50 | MIC100 | MIC80 | MIC50 | MIC100 | MIC80 | MIC50 | MIC100 | MIC80 | MIC50 | |
| 4a | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 |
| 4b | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 |
| 4c | 125 | 125 | 62.5 | 125 | 125 | 62.5 | 125 | 125 | 62.5 | 125 | 125 | 62.5 | 125 | 125 | 62.5 | 125 | 125 | 62.5 |
| 4g | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 |
| AmpB | 0.5 | 0.25 | 0.25 | 0.12 | 0.25 | 0.5 | ||||||||||||
| Compound | a EHOMO | a ELUMO | a η | b S [×10−4] | a χ | a ω |
|---|---|---|---|---|---|---|
| 4a | −181.43 | −9.81 | 171.62 | 58.27 | 95.62 | 26.64 |
| 4b | −184.91 | −6.80 | 178.12 | 56.14 | 95.86 | 25.79 |
| 4c | −182.83 | −4.06 | 178.77 | 55.94 | 93.45 | 24.42 |
| 4d | −181.33 | −2.22 | 179.12 | 55.83 | 91.77 | 23.51 |
| 4e | −177.92 | −4.74 | 173.18 | 57.74 | 91.33 | 24.08 |
| 4f | −177.39 | −3.93 | 173.46 | 57.65 | 90.66 | 23.69 |
| 4g | −183.73 | −5.16 | 178.56 | 56.00 | 94.45 | 24.98 |
| 5a | −179.82 | 5.72 | 185.54 | 53.90 | 87.05 | 20.42 |
| 5b | −177.46 | 11.23 | 188.69 | 53.00 | 83.11 | 18.30 |
| 5c | −175.37 | 13.29 | 188.66 | 53.01 | 81.04 | 17.41 |
| 5d | −170.17 | 16.60 | 186.77 | 53.54 | 76.79 | 15.78 |
| 5e | −172.30 | 14.28 | 186.58 | 53.60 | 79.01 | 16.73 |
| 5f | −170.49 | 13.86 | 184.35 | 54.24 | 78.31 | 16.63 |
| 5g | −173.32 | 12.88 | 186.20 | 53.71 | 80.22 | 17.28 |
| Methyllinderone | −171.65 | −2.95 | 168.70 | 59.28 | 87.30 | 22.59 |
| Kanakugiol | −175.60 | −8.42 | 167.18 | 59.82 | 92.01 | 25.32 |
| Linderone | −177.74 | −11.09 | 166.65 | 60.01 | 94.42 | 26.75 |
| EHOMO | ELUMO | η | S | χ | ω | pMIC-C.a. | pMIC-C.n. | pMIC-M.g. | pMIC-T.r. | pMIC-T.m. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EHOMO | 1.00 | ||||||||||
| ELUMO | 0.26 | 1.00 | |||||||||
| η | −0.68 | 0.53 | 1.00 | ||||||||
| S | 0.68 | −0.53 | −1.00 | 1.00 | |||||||
| χ | −0.83 | −0.76 | 0.15 | −0.15 | 1.00 | ||||||
| ω | −0.54 | −0.96 | −0.26 | 0.26 | 0.92 | 1.00 | |||||
| pMIC-C.a. | −0.01 | −0.82 | −0.62 | 0.62 | 0.49 | 0.73 | 1.00 | ||||
| pMIC-C.n. | −0.59 | −0.85 | −0.13 | 0.14 | 0.89 | 0.92 | 0.75 | 1.00 | |||
| pMIC-M.g. | −0.77 | −0.49 | 0.31 | −0.30 | 0.81 | 0.68 | 0.56 | 0.81 | 1.00 | ||
| pMIC-T.r. | −0.77 | −0.49 | 0.31 | −0.30 | 0.81 | 0.68 | 0.56 | 0.81 | 1.00 | 1.00 | |
| pMIC-T.m. | −0.68 | −0.36 | 0.32 | −0.32 | 0.67 | 0.53 | 0.47 | 0.77 | 0.90 | 0.90 | 1.00 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illicachi, L.A.; Montalvo-Acosta, J.J.; Insuasty, A.; Quiroga, J.; Abonia, R.; Sortino, M.; Zacchino, S.; Insuasty, B. Synthesis and DFT Calculations of Novel Vanillin-Chalcones and Their 3-Aryl-5-(4-(2-(dimethylamino)-ethoxy)-3-methoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbaldehyde Derivatives as Antifungal Agents. Molecules 2017, 22, 1476. https://doi.org/10.3390/molecules22091476
Illicachi LA, Montalvo-Acosta JJ, Insuasty A, Quiroga J, Abonia R, Sortino M, Zacchino S, Insuasty B. Synthesis and DFT Calculations of Novel Vanillin-Chalcones and Their 3-Aryl-5-(4-(2-(dimethylamino)-ethoxy)-3-methoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbaldehyde Derivatives as Antifungal Agents. Molecules. 2017; 22(9):1476. https://doi.org/10.3390/molecules22091476
Chicago/Turabian StyleIllicachi, Luis Alberto, Joel José Montalvo-Acosta, Alberto Insuasty, Jairo Quiroga, Rodrigo Abonia, Maximiliano Sortino, Susana Zacchino, and Braulio Insuasty. 2017. "Synthesis and DFT Calculations of Novel Vanillin-Chalcones and Their 3-Aryl-5-(4-(2-(dimethylamino)-ethoxy)-3-methoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbaldehyde Derivatives as Antifungal Agents" Molecules 22, no. 9: 1476. https://doi.org/10.3390/molecules22091476