Origin and Formation Mechanism Investigation of Compound Precipitation from the Traditional Chinese Prescription Huang-Lian-Jie-Du-Tang by Isothermal Titration Calorimetry
Abstract
:1. Introduction
2. Results
2.1. The Source of HLJDT CFP
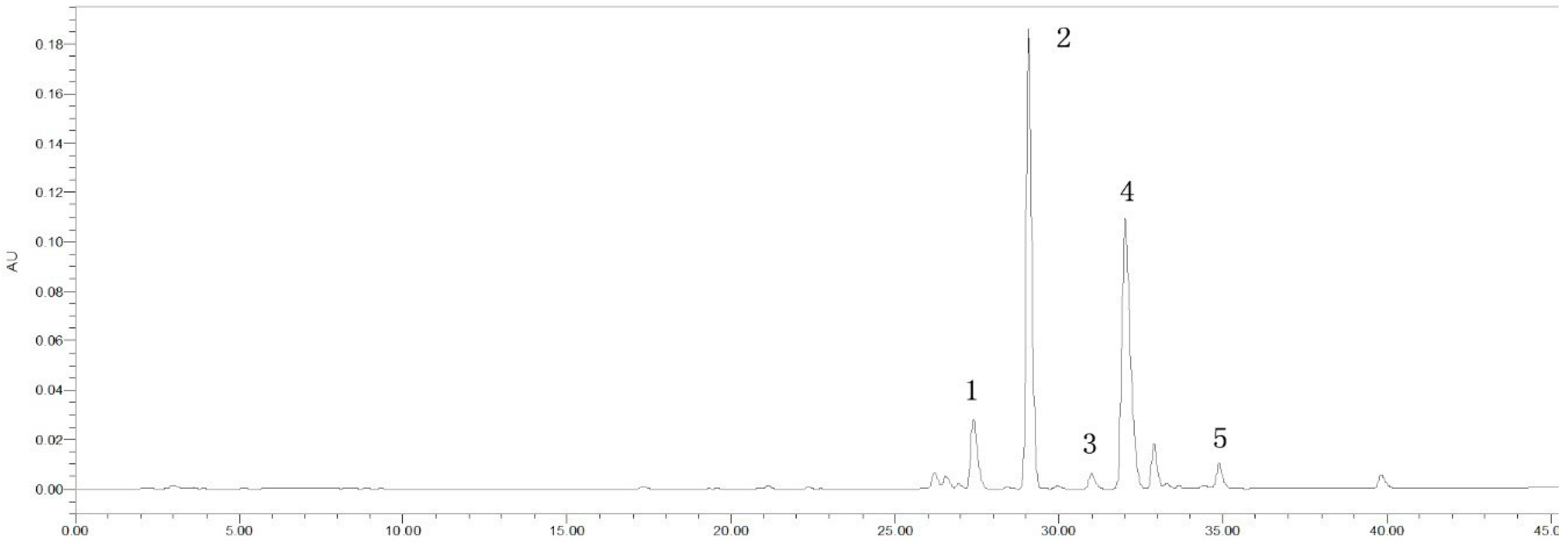
2.2. HPLC-MSn Analysis of the Constituents from HLJDT CFP and SB-CC CFP
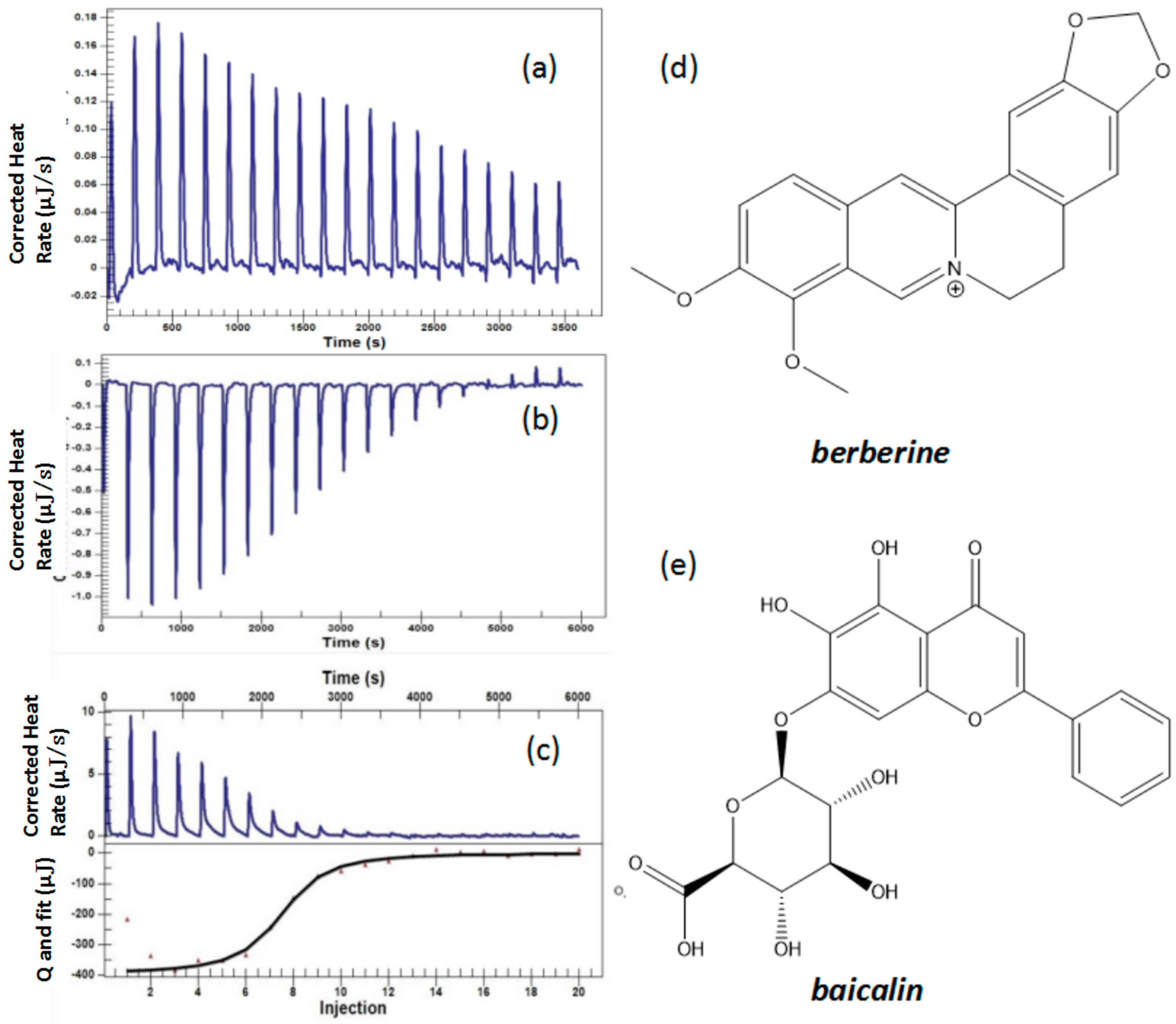
2.3. Formation Mechanism Test Based on ITC
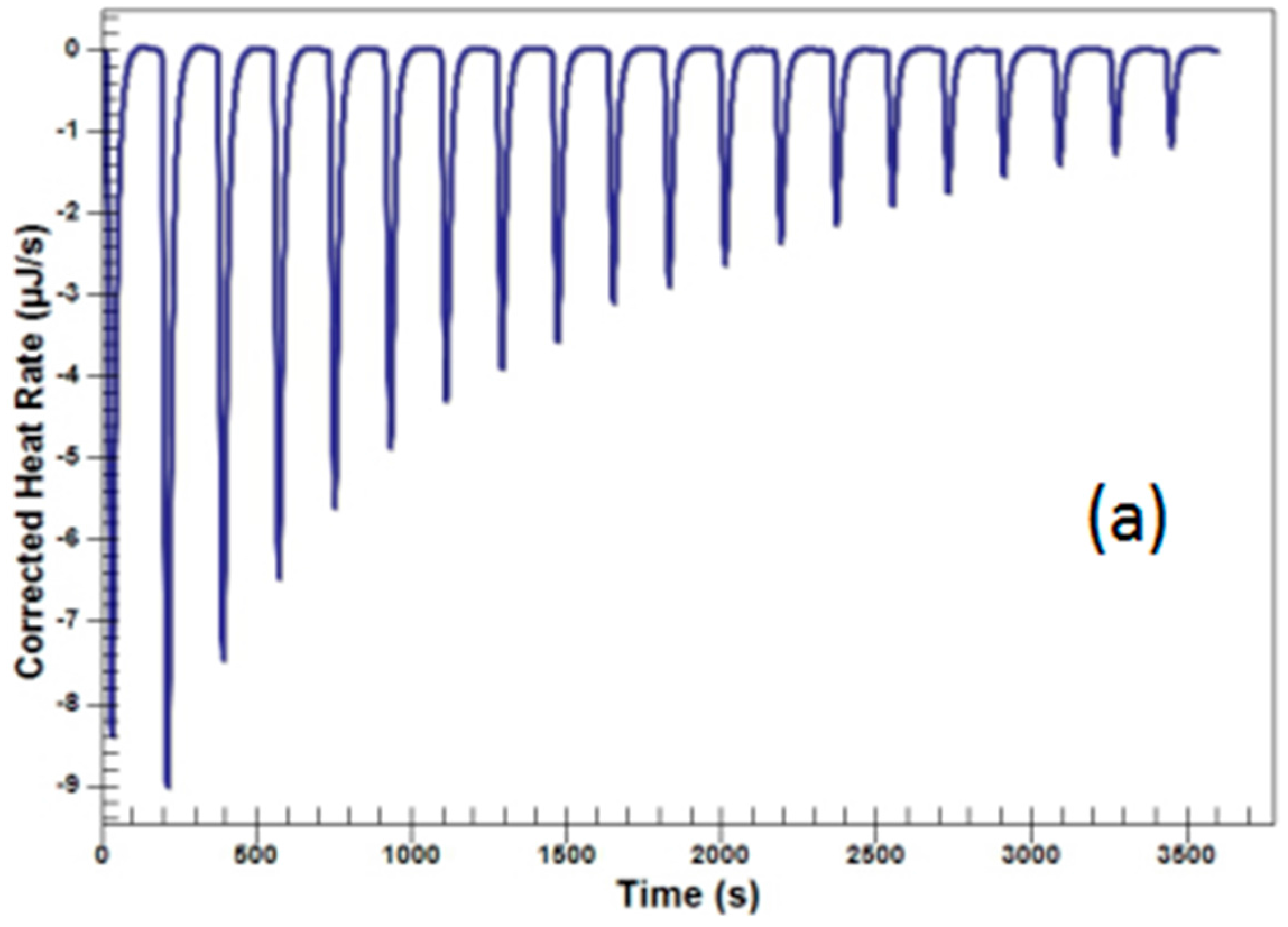
2.3.1. Titration of Decotions of Scutellaria baicalensis and Coptis chinensis
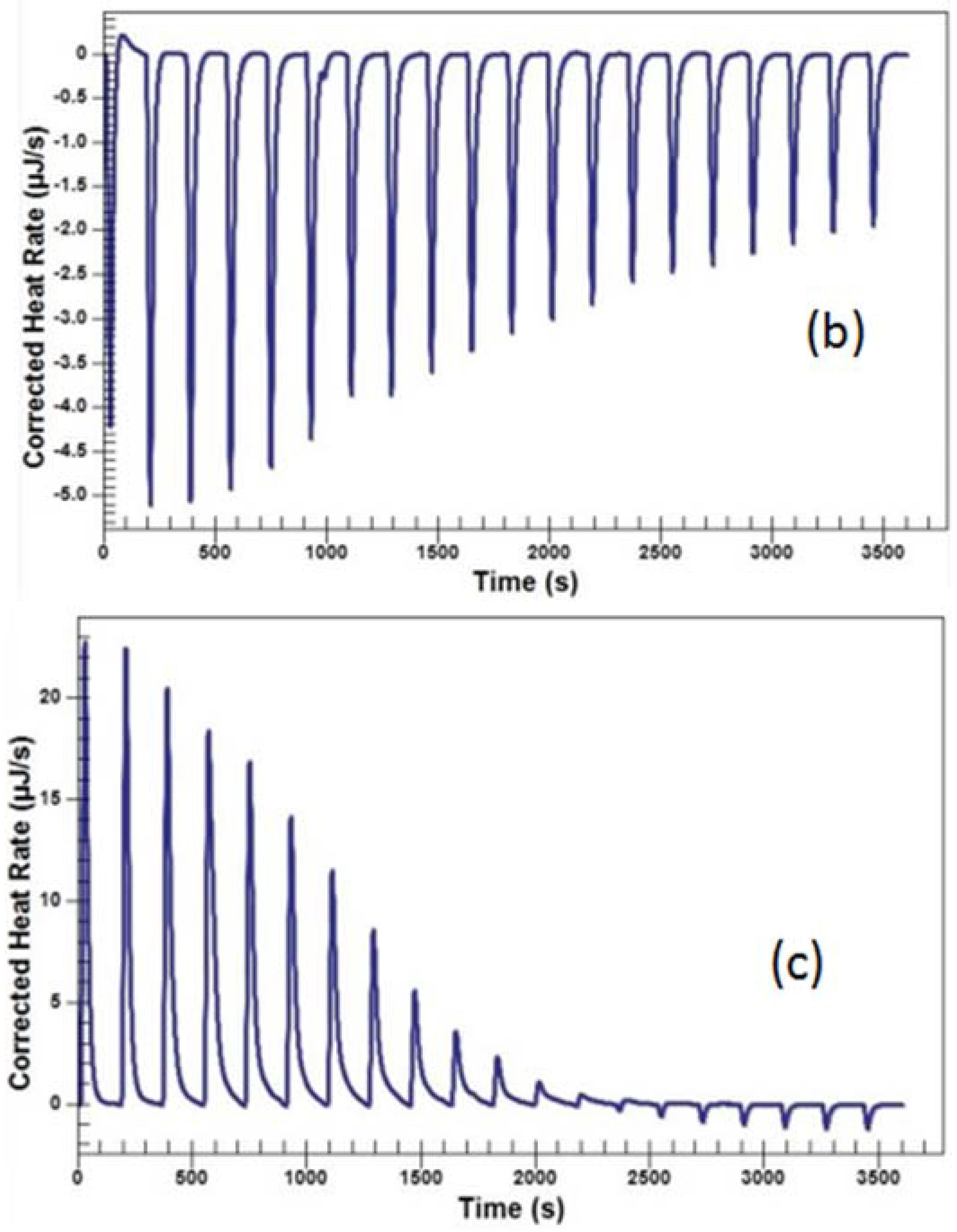
2.3.2. Titration of the Solutions of Baicalin and Berberine
2.4. Protective Effect of the HLJDT CFP and SB-CC CFP on PC12 Cells Injured by CoCl2
3. Discussion
4. Materials and Methods
4.1. Source of HLJDT CFP
4.2. HPLC-MSn Analysis of the Constituents of HLJDT CFP and Scutellaria baicalensis–Coptis chinensis CFP
4.3. Formation Mechanism Test Based on ITC
4.4. Protective Effect of the HLJDT CFP and Scutellaria baicalensis–Coptis chinensis CFP on Injured PC12 Cells
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pei, M.; Duan, X.; Pei, X. Compatability chemistry of acid-alkaline pair medicine of fuzi and gancao in sini decoction. China J. Chin. Mater. Med. 2009, 34, 2047–2050. [Google Scholar]
- Tan, X.; Yan, Y.; Luo, J.; Zhang, W. The fingerprint study of isoflavones in Ge-Gen–Qin-Lian-Tang clear liquid with precipitation. Lishizhen Med. Mater. Med. Res. 2007, 18, 321–323. [Google Scholar]
- Li, J.; Zhang, G.; Yan, M.; Song, H. The study on the pharmacodynamics of the sediment in the Xie-Xin-Tang. Chin. J. Exp. Formulas Chin. Med. 2004, 05, 27–30. [Google Scholar]
- Pei, M.; Duan, X.; Pei, X. Compatibility chemistry of acid-alkaline pair medicine of dahuang and huangbaiin dahuang xiaoshi decoction. China J. Chin. Mater. Med. 2009, 34, 2312–2315. [Google Scholar]
- Qiao, L.; Peng, J.; Pu, X.; Wei, L. studies on precipitate of huanglian-huangqin couples in decoction. China J. Chin. Mater. Med. 1999, 24, 352–353, 382. [Google Scholar]
- Ye, Y.; Huang, C.; Jiang, L.; Shen, X.; Zhu, S.; Rao, Y.; Wang, J.; Zhang, Q. Huanglian-jie-du-tang extract protects against chronic brain injury after focal cerebral ischemia via hypoxia-inducible-factor-1α-regulated vascular endothelial growth factor signaling in mice. Biol. Pharm. Bull. 2012, 35, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Pei, M.; Pei, X. Compatability chemistry of acid-alkaline pair medicine of dahuang and fuzi indahuang fuzi decoction. China J. Chin. Mater. Med. 2009, 34, 2167–2171. [Google Scholar]
- Li, P.; Liao, S.T.; Wang, J.S.; Zhang, Q.; Xu, D.Q.; Lv, Y.; Yang, M.H.; Kong, L.Y. Protection by huang-lian-jie-du decoction and its constituent herbs of lipopolysaccharide-induced acute kidney injury. FEBS Openbio 2017, 7, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.T.; Yang, X.W.; Zhang, Y.; Liu, J.X. Pharmacochemistry and integrated pharmacokinetics of six alkaloids after oral administration of huang-lian-jie-du-tang decoction. J. Asian Nat. Prod. Res. 2014, 16, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Zhao, L.; Hu, Y.; Jiang, P.; Wang, S.; Xiang, L.; Liu, W.; Shan, L.; Zhang, W.; Liu, R. Metabolomic study of collage vn-induced arthritis in rats and the interventional effects of huang-lian-jie-du-tang, a traditional chinese medicine. Evid.-Based Complement. Altern. Med. 2013, 2013, 439690. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Wu, S.J.; Lin, C.C. The anticancer properties and apoptosis-inducing mechanisms of cinnamaldehyde and the herbal prescription huang-lian-jie-du-tang (huáng lián jiě dú tang) in human hepatoma cells. J. Tradit. Complement. Med. 2013, 3, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Li, C.B.; Li, X.X.; Chen, Y.G.; Gao, H.Q.; Bu, P.L.; Zhang, Y.; Ji, X.P. Huang-lian-jie-du-tang protects rats from cardiac damages induced by metabolic disorder by improving inflammation-mediated insulin resistance. PLoS ONE 2013, 8, e67530. [Google Scholar] [CrossRef] [PubMed]
- Zheltovsky, N.V.; Samiylenko, S.P.; Kolomiets, I.N.; Kondratyuk, I.V.; Stepanyugin, A.V. The investigation of interactions of hypoxanthine, xanthine and their methyl and glycosyl derivatives with amino acid carboxylic group by spectroscopic methods. Biopolym. Cell 1993, 9, 17–22. [Google Scholar] [CrossRef]
- Kwok, K.Y.; Xu, J.; Ho, H.M.; Chen, H.B.; Li, M.; Lang, Y.; Han, Q.B. Quality evaluation of commercial huang-lian-jie-du-tang based on simultaneous determination of fourteen major chemical constituents using high performance liquid chromatography. J. Pharm. Biomed. Anal. 2013, 85, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, R.; Yan, W.; Wang, H.; Jia, M.; Zhu, N.; Zhu, Y.; Zhang, Y.; Wang, P.; Lei, H. Compositions, formation mechanism, and neuroprotective effect of compound precipitation from the traditional chinese prescription huang-lian-jie-du-tang. Molecules 2016, 21, 1094. [Google Scholar] [CrossRef] [PubMed]
- Dębowski, D.; Wyrzykowski, D.; Lubos, M.; Rolka, K. Interactions between trypsin and its peptidic inhibitors studied by isothermal titration calorimetry (itc). J. Therm. Anal. Calorim. 2016, 123, 807–812. [Google Scholar] [CrossRef]
- Ràfols, C.; Bosch, E.; Barbas, R.; Prohens, R. The ca(2+)-edta chelation as standard reaction to validate isothermal titration calorimeter measurements (itc). Talanta 2016, 154, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Sokkalingam, P.; Gibb, B.C. Itc and nmr analysis of the encapsulation of fatty acids within a water-soluble cavitand and its dimeric capsule. Supramol. Chem. 2015, 28, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Linkuvienė, V.; Krainer, G.; Chen, W.Y.; Matulis, D. Isothermal titration calorimetry for drug design: Precision of the enthalpy and binding constant measurements and comparison of the instruments. Anal. Biochem. 2016, 515, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, S.; Freire, E. Direct measurement of protein binding energetics by isothermal titration calorimetry. Curr. Opin. Struct. Biol. 2001, 11, 560–566. [Google Scholar] [CrossRef]
- Freyer, M.W.; Lewis, E.A. Isothermal titration calorimetry: Experimental design, data analysis, and probing macromolecule/ligand binding and kinetic interactions. Methods Cell Biol. 2008, 84, 79–113. [Google Scholar] [PubMed]
- Salim, N.N.; Feig, A.L. Isothermal titration calorimetry of RNA. Methods 2009, 47, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Kordás, K.; Bali, K.; Leppävuori, S.; Uusimäki, A.; Nánai, L. Laser direct writing of palladium on polyimide surfaces from solution. Appl. Surf. Sci. 1999, 152, 149–155. [Google Scholar] [CrossRef]
- Ohtaka, H.; Velázquezcampoy, A.; Xie, D.; Freire, E. Overcoming drug resistance in HIV-1 chemotherapy: The binding thermodynamics of Amprenavir and TMC-126 to wild-type and drug-resistant mutants of the HIV-1 protease. Protein Sci. 2002, 11, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, H.; Chu, F.; Xu, X.; Lin, J.; Chen, C.; Li, G.; Cheng, Y.; Wang, L.; Li, Q.; et al. Synthesis and protective effect of new ligustrazine-benzoic acid derivatives against cocl2-induced neurotoxicity in differentiated pc12 cells. Molecules 2013, 18, 13027–13042. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; She, G.; Yang, Y.; Li, Q.; Zhang, H.; Liu, J.; Cao, Y.; Xu, X.; Lei, H. Synthesis and biological evaluation of new ligustrazine derivatives as anti-tumor agents. Molecules 2012, 17, 4972–4985. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Fan, C.; Zhang, Z.; Chen, Y.; Lin, Y.; Jing, W. Design, synthesis and evaluation of the multidrug resistance-reversing activity of pyridine acid esters of podophyllotoxin in human leukemia cells. Bioorg. Med. Chem. Lett. 2016, 26, 4466–4471. [Google Scholar]
- Staff, T.P.O. Correction: Effects of huanglian-jie-du-tang and its modified formula on the modulation of amyloid-β precursor protein processing in alzheimer’s disease models. PLoS ONE 2014, 9, e92954. [Google Scholar] [CrossRef]
- Lapchak, P.A.; Maher, P.; Schubert, D.; Zivin, J.A. Baicalein, an antioxidant 12/15-lipoxygenase inhibitor improves clinical rating scores following multiple infarct embolic strokes. Neuroscience 2007, 150, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Liu, W. Dipole–dipole and H-bonding interactions significantly enhance the multifaceted mechanical properties of thermoresponsive shape memory hydrogels. Adv. Funct. Mater. 2015, 25, 471–480. [Google Scholar] [CrossRef]
- Smigol, V.; Svec, F.; Frechet, J.M. High-performance liquid chromatography of complex mixtures using monodisperse dual-chemistry polymer beads prepared by a pore-size-specific functionalization process. A single column combination of hydrophobic interaction and reversed-phase chromatography. Anal. Chem. 1994, 66, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds HLJDT CFP and SB-CC CFP are available from the authors. |
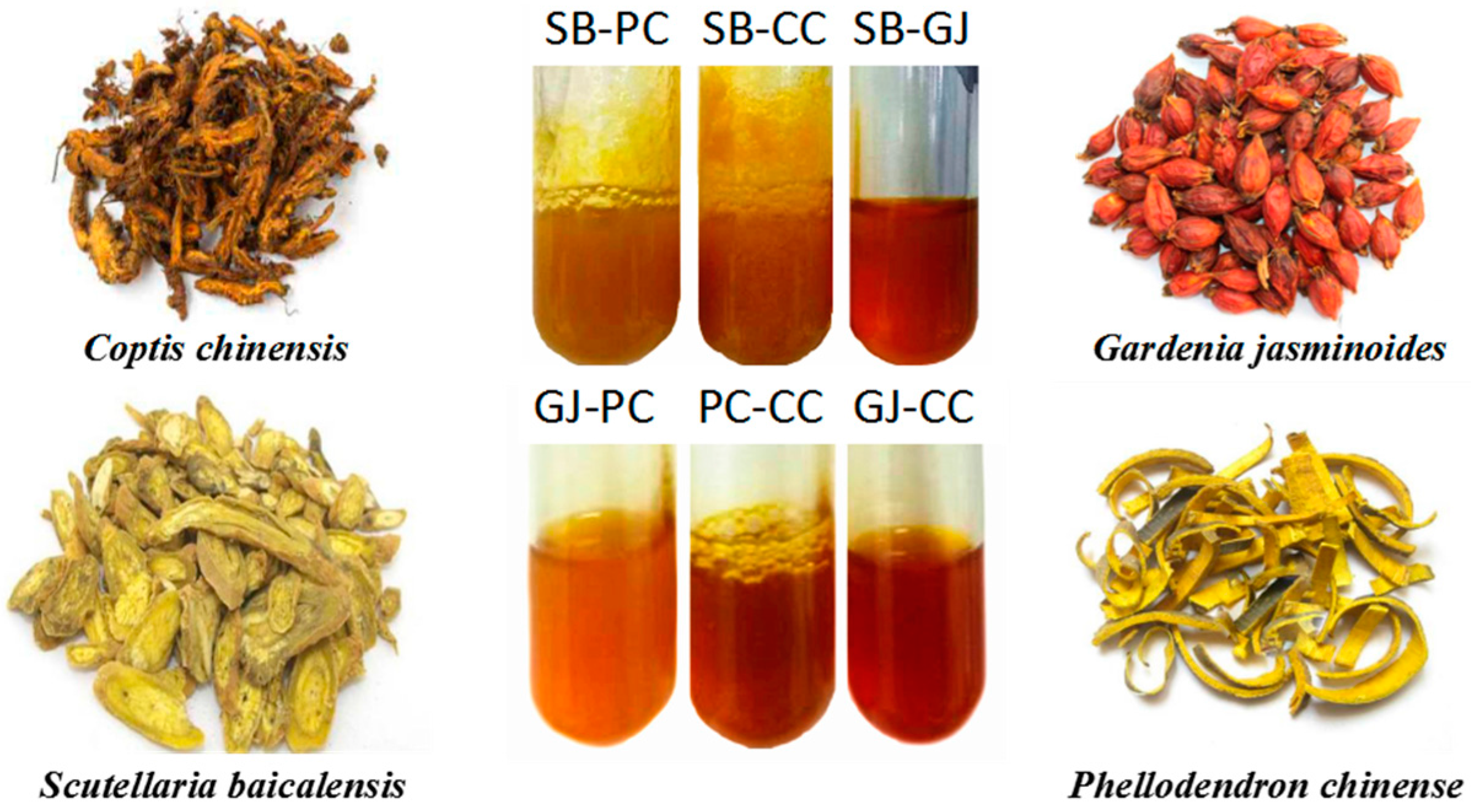
| No. | Batch | Coptis chinensis | Scutellaria baicalensis | Phellodendron chinense | Gardenia jasminoides | Total Weight | Precipitate Weight | Precipitation Rate | Separated Precipitate CFP among HLJDT CFP |
|---|---|---|---|---|---|---|---|---|---|
| HLJDT | 1 | 9.01 g | 6.00 g | 5.98 g | 9.00 g | 29.99 g | 0.78 g | 2.60% | |
| 2 | 8.97 g | 5.99 g | 5.99 g | 9.01 g | 29.98 g | 0.71 g | 2.37% | ||
| 3 | 8.99 g | 6.01 g | 5.98 g | 9.02 g | 30.00 g | 0.75 g | 2.50% | ||
| average | 29.99 ± 0.01 g | 0.75 ± 0.04 g | 2.49 ± 0.12% | ||||||
| SB-CC | 1 | 9.00 g | 6.01 g | 15.01 g | 0.62 g | 4.13% | |||
| 2 | 8.99 g | 5.99 g | 14.98 g | 0.58 g | 3.87% | ||||
| 3 | 9.01 g | 5.98 g | 14.99 g | 0.63 g | 4.20% | ||||
| average | 14.99 ± 0.02 g | 0.61 ± 0.03 g | 4.07 ± 0.20% | 81.33% | |||||
| SB-PC | 1 | 5.99 g | 5.98 g | 11.97 g | 0.06 g | 0.51% | |||
| 2 | 6.01 g | 6.00 g | 12.01 g | 0.05 g | 0.42% | ||||
| 3 | 6.03 g | 5.99 g | 12.02 g | 0.06 g | 0.50% | ||||
| average | 12.00 ± 0.03 g | 0.06 ± 0.01 g | 0.48 ± 0.06% | 8.00% | |||||
| GJ-CC | 1 | 9.02 g | 9.03 g | 18.05 g | — a | — | |||
| 2 | 9.00 g | 9.00 g | 18.00 g | — | — | ||||
| 3 | 9.02 g | 8.99 g | 18.01 g | — | — | ||||
| average | 18.02 ± 0.03 g | — | — | ||||||
| GJ-PC | 1 | 6.00 g | 8.98 g | 14.98 g | — | — | |||
| 2 | 6.01 g | 8.99 g | 15.00 g | — | — | ||||
| 3 | 5.98 g | 9.00 g | 14.98 g | — | — | ||||
| average | 14.99 ± 0.01 g | — | — | ||||||
| SB-PC | 1 | 6.03 g | 8.99 g | 15.02 g | — | — | |||
| 2 | 5.98 g | 9.01 g | 14.98 g | — | — | ||||
| 3 | 6.01 g | 9.03 g | 15.04 g | — | — | ||||
| average | 15.01± 0.04 g | — | — | ||||||
| PC-CC | 1 | 8.98 g | 6.01 g | 14.99 g | — | — | |||
| 2 | 9.01 g | 6.02 g | 15.03 g | — | — | ||||
| 3 | 9.00 g | 5.97 g | 14.97 g | — | — | ||||
| average | 15.00± 0.01 g | — | — |
| Peak | Tr (min) | Compounds | Ms (m/z) | Ms2 (m/z) |
|---|---|---|---|---|
| 1 | 27.401 | Coptisine | 319.81 [M]+ | 291.87 [M − C2H4]+ |
| 2 | 29.001 | Baicalin | 446.97 [M + H]+ | 270.75 [M + H − GLU]+ |
| 3 | 31.008 | Palmatine | 351.92 [M]+ | 335.86 [M − CH4]+ |
| 307.85 [M − CH4 − C2H4]+ | ||||
| 4 | 32.034 | Berberine | 335.85 [M]+ | 319.81 [M − CH4]+ |
| 291.82 [M − CH4 − C2H4]+ | ||||
| 5 | 34.899 | Wogonoside | 460.97 [M + H]+ | 284.80 [M + H − Glu]+ |
| No. | Coptis chinensis into Scutellaria baicalensis (μJ) | Scutellaria baicalensis into Water (μJ) | Coptis chinensis into Water (μJ) | Berberine into Baicalin (μJ) | Berberine into Water (μJ) | Baicalin into Water (μJ) |
|---|---|---|---|---|---|---|
| 1 | — | — | — | — | — | — |
| 2 | 594.11 | −228.2 | −122.09 | 313.21 | −22.00 | −3.35 |
| 3 | 629.88 | −189.2 | −125.01 | 358.41 | −23.77 | −4.36 |
| 4 | 720.57 | −161.6 | −123.93 | 325.81 | −24.89 | −3.85 |
| 5 | 554.46 | −139.1 | −119.18 | 323.80 | −24.49 | −3.55 |
| 6 | 483.12 | −119.7 | −115.41 | 313.13 | −22.15 | −3.50 |
| 7 | 411.30 | −104.6 | −100.42 | 219.95 | −19.87 | −3.20 |
| 8 | 315.20 | −94.20 | −99.69 | 126.31 | −18.40 | −3.13 |
| 9 | 215.62 | −86.06 | −93.10 | 61.22 | −14.93 | −2.81 |
| 10 | 147.73 | −74.72 | −87.18 | 44.42 | −12.87 | −2.58 |
| 11 | 92.88 | −69.86 | −81.03 | 27.56 | −9.98 | −2.86 |
| 12 | 55.09 | −64.10 | −76.37 | 17.60 | −8.68 | −2.60 |
| 13 | 26.51 | −57.33 | −73.13 | 0.78 | −7.15 | −2.34 |
| 14 | 6.84 | −51.45 | −66.79 | −15.98 | −4.01 | −2.14 |
| 15 | −6.99 | −45.71 | −63.61 | −4.83 | −2.49 | −2.00 |
| 16 | −18.57 | −41.89 | −62.47 | −7.92 | 0.06 | −2.01 |
| 17 | −25.25 | −36.65 | −59.78 | 5.23 | −0.80 | −2.07 |
| 18 | −29.76 | −33.33 | −56.14 | 0.85 | 0.36 | −1.75 |
| 19 | −31.58 | −30.35 | −52.34 | 3.31 | 1.80 | −1.16 |
| 20 | −33.05 | −27.86 | −50.15 | −10.56 | 2.11 | −1.70 |
| No. | ΔH (kJ/mol) | −TΔS (kJ/mol) | ΔG (kJ/mol) | n | Ka (1/M) | Kd (M) |
|---|---|---|---|---|---|---|
| Berberine to baicalin | −371.6 | 336.027 | −35.573 | 1.002 | 1.228 × 106 | 8.143 × 10−7 |
| Samples | Proliferation Rate (%) | EC50 (µg/mL) | ||||
|---|---|---|---|---|---|---|
| 3.75 µg/mL | 7.5 µg/mL | 15 µg/mL | 30 µg/mL | 60 µg/mL | ||
| HLJDT supernatant | 2.36 ± 0.30 | 11.46 ± 3.67 | 46.67 ± 6.06 | 60.28 ± 3.83 | 45.24 ± 5.01 | 28.41 ± 2.61 |
| HLJDT CFP | 39.49 ± 7.06 | 53.28 ± 5.57 | 56.46 ± 5.69 | 83.18 ± 4.69 | 19.83 ± 4.20 | 14.71 ± 0.91 |
| SB-CC supernatant | 11.43 ± 3.26 | 14.79 ± 4.01 | 14.19 ± 0.69 | 24.16 ± 5.95 | 10.60 ± 2.93 | 56.47 ± 2.63 |
| SB-CC CFP | 32.58 ± 4.44 | 58.04 ± 5.87 | 66.28 ± 2.19 | 123.91 ± 9.41 | 84.64 ± 4.69 | 6.11 ± 0.12 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, T.; Xiang, H.; Zhang, X.; Fang, K.; Wu, G.; Yan, M.; Xue, N.; Chen, M.; Xie, T.; et al. Origin and Formation Mechanism Investigation of Compound Precipitation from the Traditional Chinese Prescription Huang-Lian-Jie-Du-Tang by Isothermal Titration Calorimetry. Molecules 2017, 22, 1456. https://doi.org/10.3390/molecules22091456
Wang H, Li T, Xiang H, Zhang X, Fang K, Wu G, Yan M, Xue N, Chen M, Xie T, et al. Origin and Formation Mechanism Investigation of Compound Precipitation from the Traditional Chinese Prescription Huang-Lian-Jie-Du-Tang by Isothermal Titration Calorimetry. Molecules. 2017; 22(9):1456. https://doi.org/10.3390/molecules22091456
Chicago/Turabian StyleWang, Hui, Tong Li, Hongjun Xiang, Xinyu Zhang, Kang Fang, Gaorong Wu, Mengmeng Yan, Nannan Xue, Meng Chen, Tianxin Xie, and et al. 2017. "Origin and Formation Mechanism Investigation of Compound Precipitation from the Traditional Chinese Prescription Huang-Lian-Jie-Du-Tang by Isothermal Titration Calorimetry" Molecules 22, no. 9: 1456. https://doi.org/10.3390/molecules22091456





