Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications
Abstract
:1. Introduction
2. Synthesis and Biofunctionalization
2.1. Synthesis
2.2. Biofunctionalization
3. Au NPs as Drug Delivery Carriers
3.1. Au NPs for Drug Delivery
3.2. Au NPs for Gene Delivery
3.3. Au NPs for Protein Delivery
3.4. Au NPs for Vaccine Delivery
3.5. The Release of a Drug from Au NPs via External Stimulus
4. Au NPs as Molecular Nanoprobes
4.1. Au NP-Based Molecular Diagnostics
4.2. Au NP-Based Molecular Imaging
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Akhter, S.; Ahmad, I.; Ahmad, M.Z.; Ramazani, F.; Singh, A.; Rahman, Z.; Kok, R.J. Nanomedicines as cancer therapeutics: Current status. Curr. Cancer Drug Targets 2013, 13, 362–378. [Google Scholar] [CrossRef] [PubMed]
- Verissimo, T.V.; Santos, N.T.; Silva, J.R.; Azevedo, R.B.; Gomes, A.J.; Lunardi, C.N. In vitro cytotoxicity and phototoxicity of surface-modified gold nanoparticles associated with neutral red as a potential drug delivery system in phototherapy. Mater. Sci. Eng. C 2016, 65, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. How toxic are gold nanoparticles? The state-of-the-art. Nano Res. 2015, 8, 1771–1799. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Focella, T.M.; Smilowitz, H.M. Gold nanoparticles: A new X-ray contrast agent. Br. J. Radiol. 2005, 79, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zhang, X.; Liang, X.J. Gold nanoparticles: Emerging paradigm for targeted drug delivery system. Biotechnol. Adv. 2013, 31, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Julkapli, N.M.; Yehye, W.A.; Basirun, W.J.; Bhargava, S.K. Graphene-gold nanoparticles hybrid-synthesis, functionalization, and application in a electrochemical and surface-enhanced raman scattering biosensor. Materials 2016, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, H.; Zeng, D.; San, L.; Mi, X. DNA nanotechnology mediated gold nanoparticle conjugates and their applications in biomedicine. Chin. J. Chem. 2016, 34, 299–307. [Google Scholar] [CrossRef]
- Razzaque, S.; Hussain, S.Z.; Hussain, I.; Tan, B. Design and utility of metal/metal oxide nanoparticles mediated by thioether end-functionalized polymeric ligands. Polymers 2016, 8, 156. [Google Scholar] [CrossRef]
- Austin, L.A.; Mackey, M.A.; Dreaden, E.C.; El-Sayed, M.A. The optical, photothermal, and facile surface chemical properties of gold and silver nanoparticles in biodiagnostics, therapy, and drug delivery. Arch. Toxicol. 2014, 88, 1391–1417. [Google Scholar] [CrossRef] [PubMed]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Ali, M.R.; Snyder, B.; El-Sayed, M.A. Synthesis and optical properties of small Au nanorods using a seedless growth technique. Langmuir 2012, 28, 9807–9815. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Bhattacharya, J.; Mukherjee, A.; Ghosh, A.; Santra, C.; Dasgupta, A.K.; Karmakar, P. In vitro structural and functional evaluation of gold nanoparticles conjugated antibiotics. Nanoscale Res. Lett. 2007, 2, 614–622. [Google Scholar] [CrossRef]
- Gu, H.; Ho, P.L.; Tong, E.; Wang, L.; Xu, B. Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett. 2003, 3, 1261–1263. [Google Scholar] [CrossRef]
- Chen, Y.H.; Tsai, C.Y.; Huang, P.Y.; Chang, M.Y.; Cheng, P.C.; Chou, C.H.; Wu, C.L. Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Mol. Pharmaceut. 2007, 4, 713–722. [Google Scholar] [CrossRef] [PubMed]
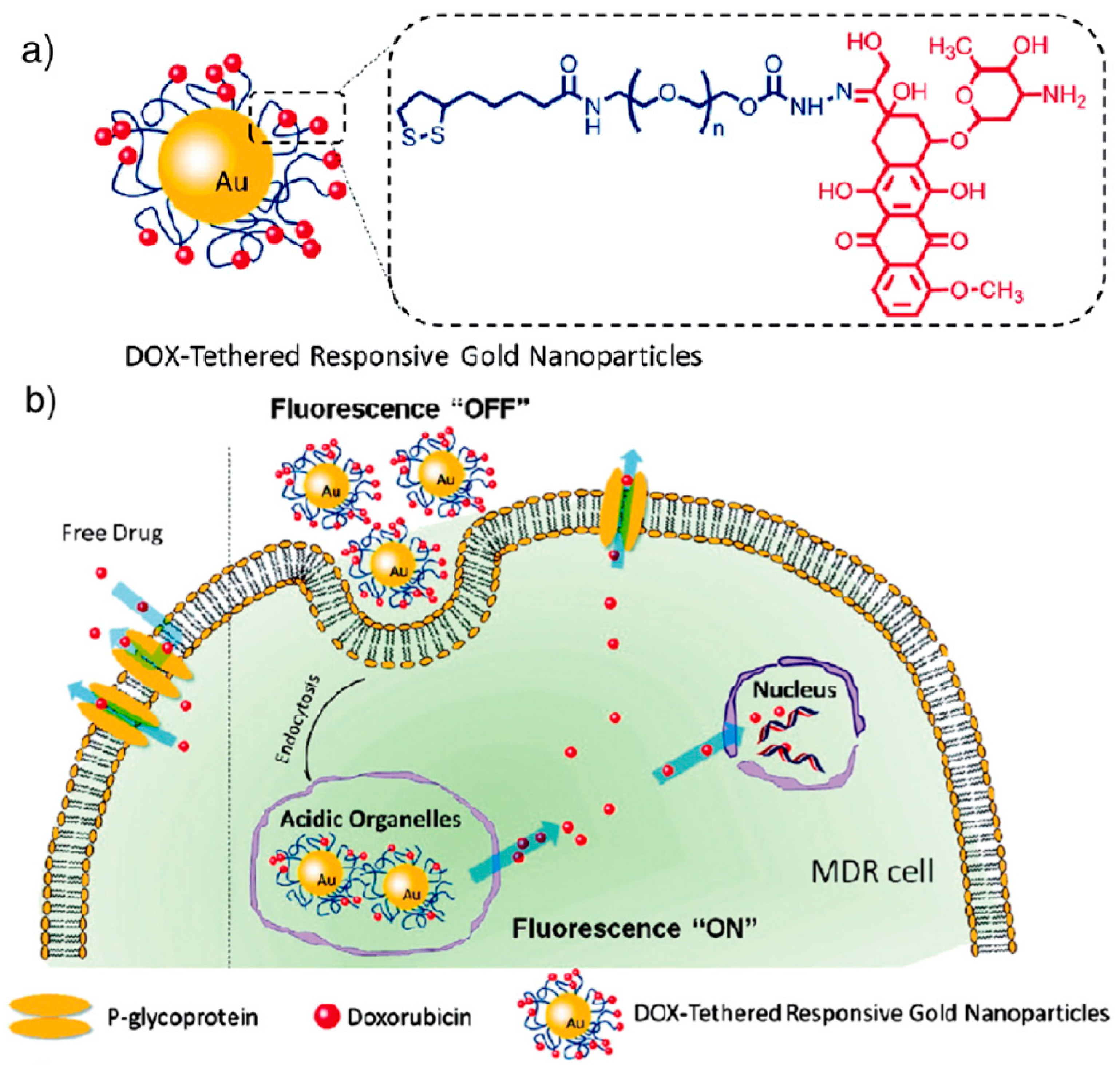
- Wang, F.; Wang, Y.C.; Dou, S.; Xiong, M.H.; Sun, T.M.; Wang, J. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. ACS Nano 2011, 5, 3679–3692. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.D.; Nativo, P.; Smith, J.A.; Stirling, D.; Edwards, P.R.; Venugopal, B.; Wheate, N.J. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J. Am. Chem. Soc. 2010, 132, 4678–4684. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, D.; Fu, W.; Li, J.; Crasto, C.; Jones, G.; DiMarzio, C.; Amiji, M. Surface functionalization of gold nanoparticles using hetero-bifunctional poly (ethylene glycol) spacer for intracellular tracking and delivery. Int. J. Nanomed. 2006, 1, 51–57. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Patra, C.R.; Earl, A.; Wang, S.; Katarya, A.; Lu, L.; Mukherjee, P. Attaching folic acid on gold nanoparticles using noncovalent interaction via different polyethylene glycol backbones and targeting of cancer cells. Nanomed. Nanotechnol. 2007, 3, 224–238. [Google Scholar] [CrossRef]
- Gu, Y.J.; Cheng, J.; Lin, C.C.; Lam, Y.W.; Cheng, S.H.; Wong, W.T. Nuclear penetration of surface functionalized gold nanoparticles. Toxicol. Appl. Pharmacol. 2009, 237, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.D. Human gene therapy comes of age. Nature 1992, 357, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Schulz, R.; Swantek, P.; Kunstman, K.; Malim, M.H.; Wolinsky, S.M. Gold nanoparticle-mediated gene delivery induces widespread changes in the expression of innate immunity genes. Gene Ther. 2012, 19, 347–353. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, C.M.; Esposito, E.A.; Boal, A.K.; Simard, J.M.; Martin, C.T.; Rotello, V.M. Inhibition of DNA transcription using cationic mixed monolayer protected gold clusters. J. Am. Chem. Soc. 2001, 123, 7626–7629. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Martin, C.T.; Rotello, V.M. Stability of gold nanoparticle-bound DNA toward biological, physical, and chemical agents. Chem. Biol. Drug Des. 2006, 67, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Giljohann, D.A.; Thaxton, C.S.; Lytton-Jean, A.K.; Han, M.S.; Mirkin, C.A. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science 2006, 312, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
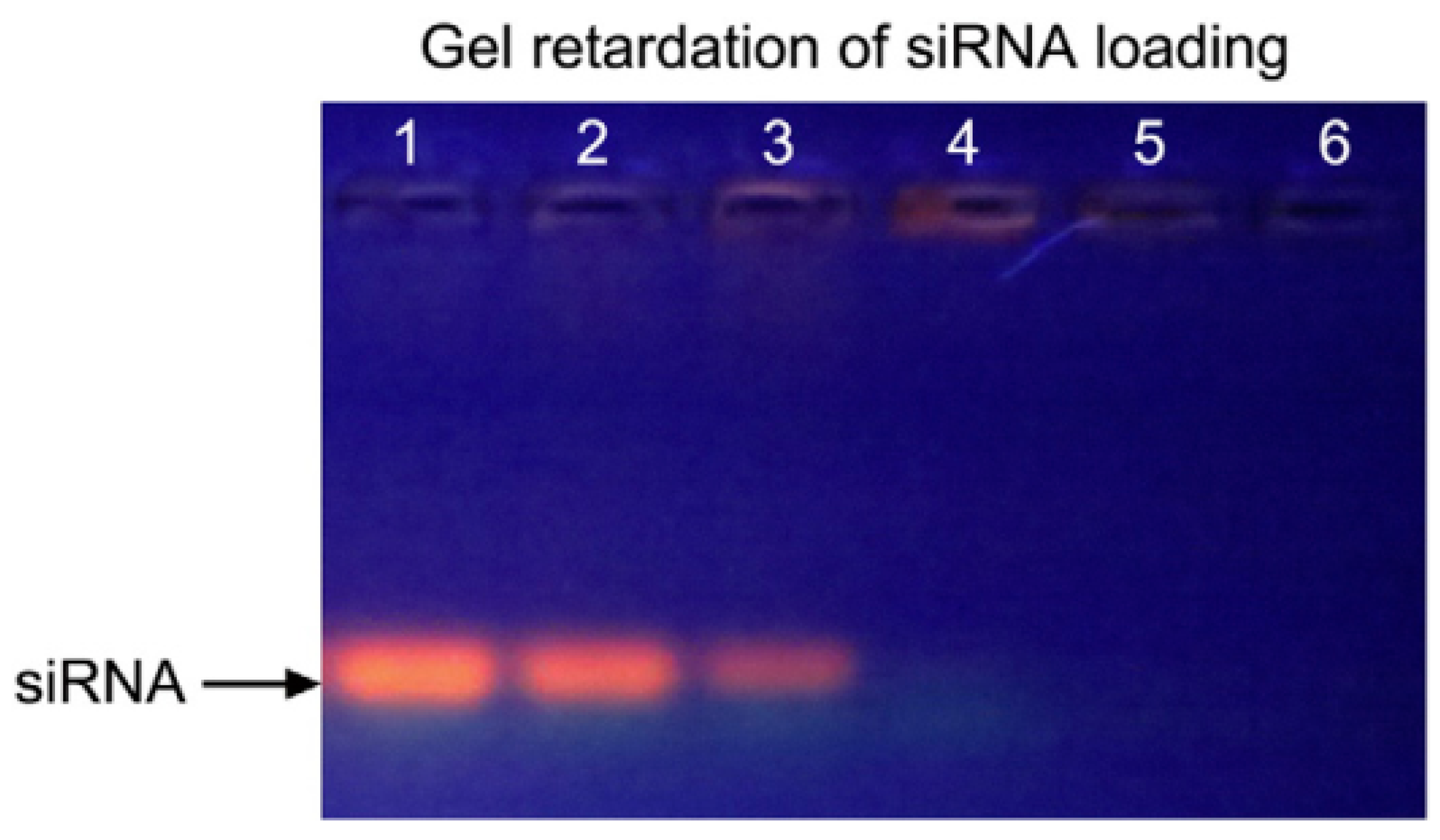
- Bonoiu, A.C.; Mahajan, S.D.; Ding, H.; Roy, I.; Yong, K.T.; Kumar, R.; Prasad, P.N. Nanotechnology approach for drug addiction therapy: Gene silencing using delivery of gold nanorod-siRNA nanoplex in dopaminergic neurons. Proc. Natl. Acad. Sci. USA 2009, 106, 5546–5550. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Huang, Q.; Jin, Y. Gold nanorod delivery of LSD1 siRNA induces human mesenchymal stem cell differentiation. Mater. Sci. Eng. C 2015, 54, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, S.; Liu, L.; Deng, D.; Xia, N. Simple, sensitive and selective detection of dopamine using dithiobis (succinimidylpropionate)-modified gold nanoparticles as colorimetric probes. Analyst 2012, 137, 3794–3799. [Google Scholar] [CrossRef] [PubMed]
- Bhumkar, D.R.; Joshi, H.M.; Sastry, M.; Pokharkar, V.B. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm. Res. 2007, 24, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Simard, J.M.; Worrall, J.W.; Rotello, V.M. Tunable reactivation of nanoparticle-inhibited β-galactosidase by glutathione at intracellular concentrations. J. Am. Chem. Soc. 2004, 126, 13987–13991. [Google Scholar] [CrossRef] [PubMed]
- Schäffler, M.; Sousa, F.; Wenk, A.; Sitia, L.; Hirn, S.; Schleh, C.; Salmona, M. Blood protein coating of gold nanoparticles as potential tool for organ targeting. Biomaterials 2014, 35, 3455–3466. [Google Scholar] [CrossRef] [PubMed]
- Demenev, V.A.; Shchinova, M.A.; Ivanov, L.I.; Vorobeva, R.N.; Zdanovskaia, N.I.; Nebaikina, N.V. Perfection of methodical approaches to designing vaccines against tick-borne encephalitis. Vopr. Virusol. 1996, 41, 107–110. [Google Scholar] [PubMed]
- Xu, L.; Liu, Y.; Chen, Z.; Li, W.; Wang, L.; Wu, X.; Ji, Y.; Zhao, Y.; Ma, L.; Shao, Y.; et al. Surface-engineered gold nanorods: Promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett. 2012, 12, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Shiang, Y.C.; Ou, C.M.; Chen, S.J.; Ou, T.Y.; Lin, H.J.; Huang, C.C.; Chang, H.T. Highly efficient inhibition of human immunodeficiency virus type 1 reverse transcriptase by aptamers functionalized gold nanoparticles. Nanoscale 2013, 5, 2756–2764. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, C. Role of nanotechnology in HIV/AIDS vaccine development. Adv. Drug Deliv. Rev. 2016, 103, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Comber, J.D.; Bamezai, A. Gold nanoparticles (AuNPs): A new frontier in vaccine delivery. J. Nanomed. Biother. Discov. 2015, 5. [Google Scholar] [CrossRef]
- Sun, B.; Xia, T. Nanomaterial-based vaccine adjuvants. J. Mater. Chem. B 2016, 4, 5496–5509. [Google Scholar] [CrossRef]
- Ilinskaya, A.N.; Dobrovolskaia, M.A. Understanding the immunogenicity and antigenicity of nanomaterials: Past, present and future. Toxicol. Appl. Pharmacol. 2016, 299, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.A.; Khlebtsov, N.G. Immunological properties of gold nanoparticles. Chem. Sci. 2017, 8, 1719–1735. [Google Scholar] [CrossRef] [PubMed]
- Neto, L.M.M.; Kipnis, A.; Junqueira-Kipnis, A.P. Role of metallic nanoparticles in vaccinology: Implications for infectious disease vaccine development. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Muñoz Javier, A.; Kreft, O.; Köhler, K.; Piera Alberola, A.; Möhwald, H.; Sukhorukov, G.B. Laser-induced release of encapsulated materials inside living cells. Angew. Chem. Int. Ed. 2006, 45, 4612–4617. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Delive. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Sreejivungsa, K.; Suchaichit, N.; Moosophon, P.; Chompoosor, A. Light-regulated release of entrapped drugs from photoresponsive gold nanoparticles. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Z.; Yu, H.; Chen, X.; Feng, B.; Cui, Z.; Wang, J. Treatment of metastatic breast cancer by combination of chemotherapy and photothermal ablation using doxorubicin-loaded DNA wrapped gold nanorods. Biomaterials 2014, 35, 8374–8384. [Google Scholar] [CrossRef] [PubMed]
- Angelatos, A.S.; Radt, B.; Caruso, F. Light-responsive polyelectrolyte/gold nanoparticle microcapsules. J. Phys. Chem. B 2005, 109, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, X.; Zhou, M.; Tian, B.; Yu, C.; Jie, J.; Zhang, X. Functional core/shell drug nanoparticles for highly effective synergistic cancer therapy. Adv. Healthc. Mater. 2014, 3, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, J.; Ju, E.J.; Park, S.S.; Choi, J.; Lee, J.H.; Kim, C. Multifunctional hollow gold nanoparticles designed for triple combination therapy and CT imaging. J. Control. Release 2015, 207, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; C. Samia, A.; Meyers, J.D.; Panagopoulos, I.; Fei, B.; Burda, C. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J. Am. Chem. Soc. 2008, 130, 10643–10647. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Yamagata, M.; Takahashi, H.; Niidome, Y.; Yamada, S.; Katayama, Y.; Niidome, T. Stabilizing of plasmid DNA in vivo by PEG-modified cationic gold nanoparticles and the gene expression assisted with electrical pulses. J. Control. Release 2006, 111, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Hwang, K.; Lan, T.; Lu, Y. A DNAzyme-gold nanoparticle probe for uranyl ion in living cells. J. Am. Chem. Soc. 2013, 135, 5254–5257. [Google Scholar] [CrossRef] [PubMed]
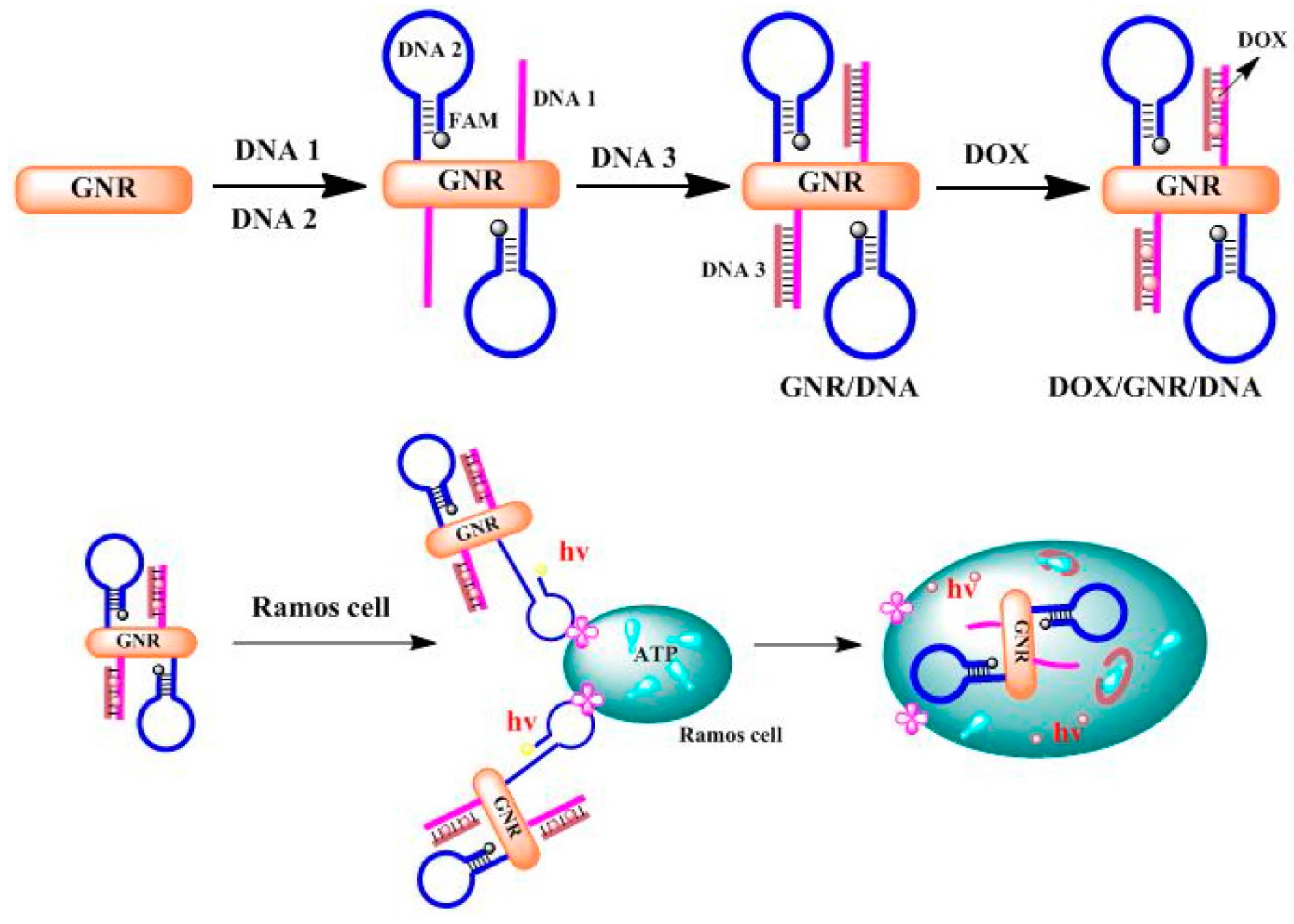
- Guo, Y.; Li, S.; Liu, J.; Yang, G.; Sun, Z.; Wan, J. Double functional aptamer switch probes based on gold nanorods for intracellular ATP detection and targeted drugs transportation. Sens. Actuators B Chem. 2016, 235, 655–662. [Google Scholar] [CrossRef]
- Kong, F.Y.; Xu, M.T.; Xu, J.J.; Chen, H.Y. A novel lable-free electrochemical immunosensor for carcinoembryonic antigen based on gold nanoparticles-thionine-reduced graphene oxide nanocomposite film modified glassy carbon electrode. Talanta 2011, 85, 2620–2625. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.Y.; Xu, M.T.; Xu, J.J.; Chen, H.Y. Gold nanoparticle/DNA/methylene blue nanocomposites for the ultrasensitive electrochemical detection of carcinoembryonic antigen. Electrochim. Acta 2011, 56, 9386–9390. [Google Scholar] [CrossRef]
- Huefner, A.; Septiadi, D.; Wilts, B.D.; Patel, I.I.; Kuan, W.L.; Fragniere, A.; Mahajan, S. Gold nanoparticles explore cells: Cellular uptake and their use as intracellular probes. Methods 2014, 68, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Indrasekara, A.S.D.S.; Paladini, B.J.; Naczynski, D.J.; Starovoytov, V.; Moghe, P.V.; Fabris, L. Dimeric gold nanoparticle assemblies as tags for SERS-based cancer detection. Adv. Healthc. Mater. 2013, 2, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Wang, L.; Zhang, Y.; Su, L.; Shen, A.; Tan, J.; Hu, J. Nuclear targeted nanoprobe for single living cell detection by surface-enhanced Raman scattering. Bioconj. Chem. 2009, 20, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Choi, S.H.; Hyeon, T. Nano-Sized CT Contrast Agents. Adv. Mater. 2013, 25, 2641–2660. [Google Scholar] [CrossRef] [PubMed]
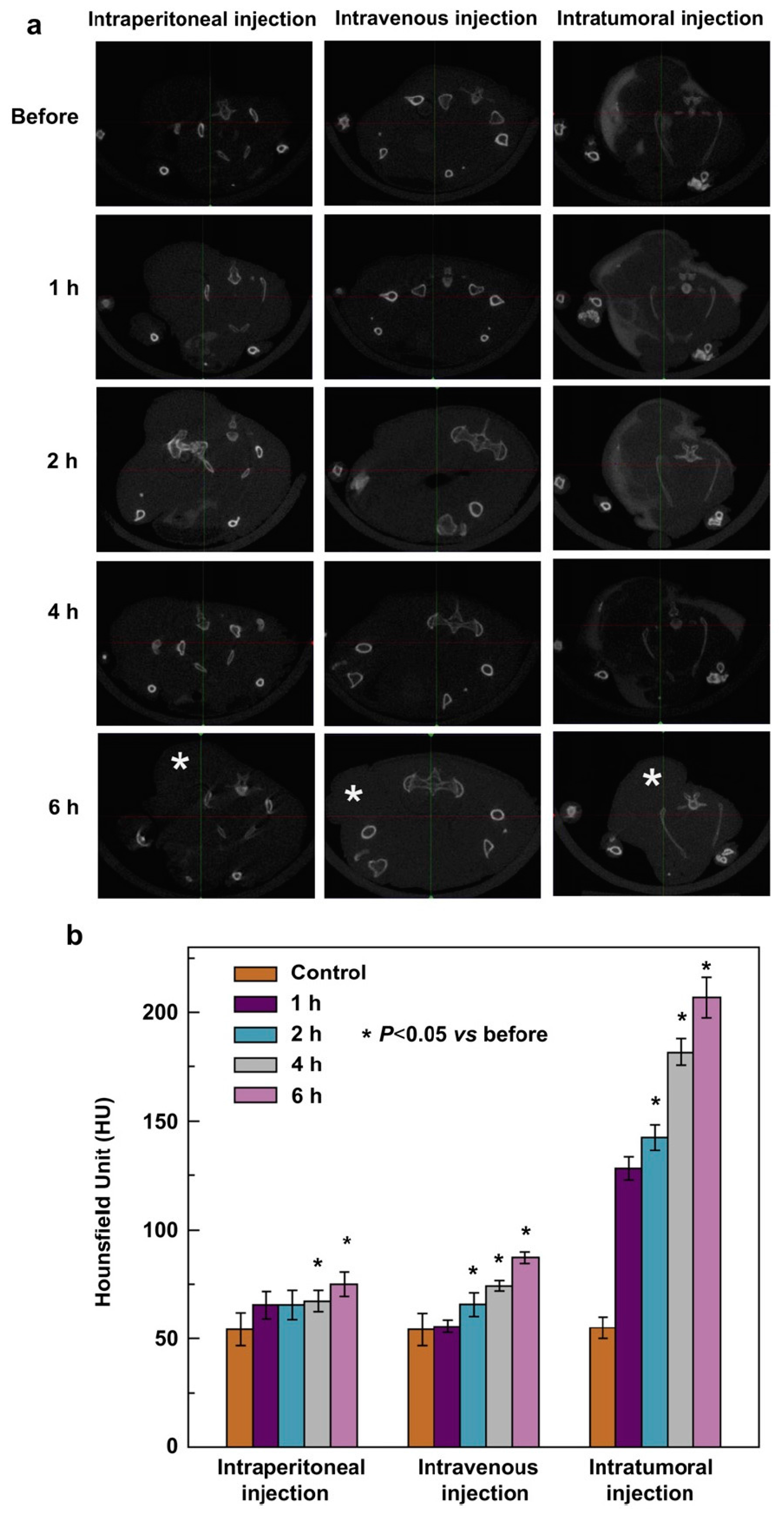
- Jing, L.; Liang, X.; Deng, Z.; Feng, S.; Li, X.; Huang, M.; Dai, Z. Prussian blue coated gold nanoparticles for simultaneous photoacoustic/CT bimodal imaging and photothermal ablation of cancer. Biomaterials 2014, 35, 5814–5821. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jeong, Y.Y.; Jon, S. A drug-loaded aptamer-gold nanoparticle bioconjugate for combined CT imaging and therapy of prostate cancer. ACS Nano 2010, 4, 3689–3696. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, L.; Peng, C.; Guo, R.; Shen, M.; Shi, X.; Zhang, G. Computed tomography imaging of cancer cells using acetylated dendrimer-entrapped gold nanoparticles. Biomaterials 2011, 32, 2979–2988. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zheng, L.; Chen, Q.; Shen, M.; Guo, R.; Wang, H.; Shi, X. PEGylated dendrimer-entrapped gold nanoparticles for in vivo blood pool and tumor imaging by computed tomography. Biomaterials 2012, 33, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, L.; Peng, C.; Shen, M.; Shi, X.; Zhang, G. Folic acid-modified dendrimer-entrapped gold nanoparticles as nanoprobes for targeted CT imaging of human lung adencarcinoma. Biomaterials 2013, 34, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Li, K.; Cai, H.; Chen, Q.; Shen, M.; Huang, Y.; Shi, X. Multifunctional dendrimer-entrapped gold nanoparticles for dual mode CT/MR imaging applications. Biomaterials 2013, 34, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, C.; Zhang, X.; Huo, S.; Jin, S.; An, F.F.; Guo, F. In vivo tumor-targeted dual-modal fluorescence/CT imaging using a nanoprobe co-loaded with an aggregation-induced emission dye and gold nanoparticles. Biomaterials 2015, 42, 103–111. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, F.-Y.; Zhang, J.-W.; Li, R.-F.; Wang, Z.-X.; Wang, W.-J.; Wang, W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445. https://doi.org/10.3390/molecules22091445
Kong F-Y, Zhang J-W, Li R-F, Wang Z-X, Wang W-J, Wang W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules. 2017; 22(9):1445. https://doi.org/10.3390/molecules22091445
Chicago/Turabian StyleKong, Fen-Ying, Jin-Wei Zhang, Rong-Fang Li, Zhong-Xia Wang, Wen-Juan Wang, and Wei Wang. 2017. "Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications" Molecules 22, no. 9: 1445. https://doi.org/10.3390/molecules22091445



