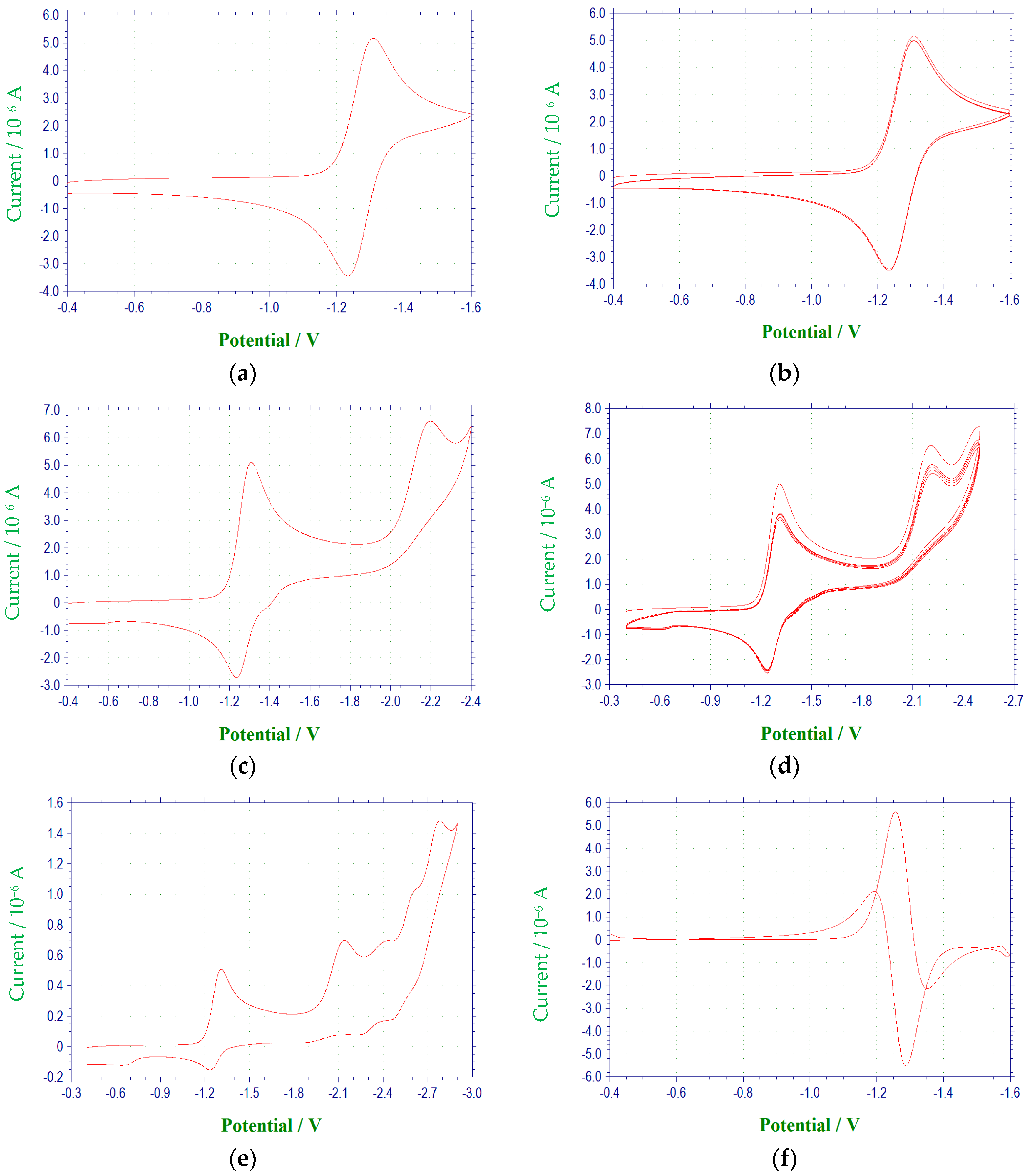
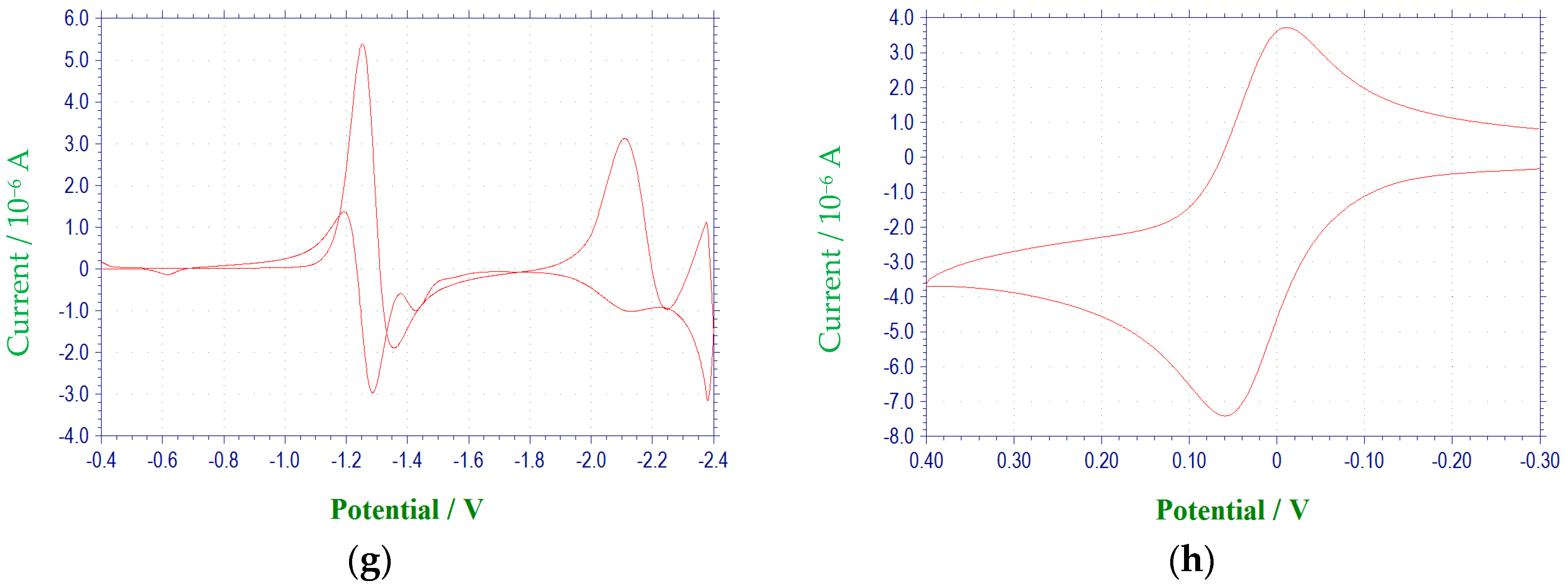
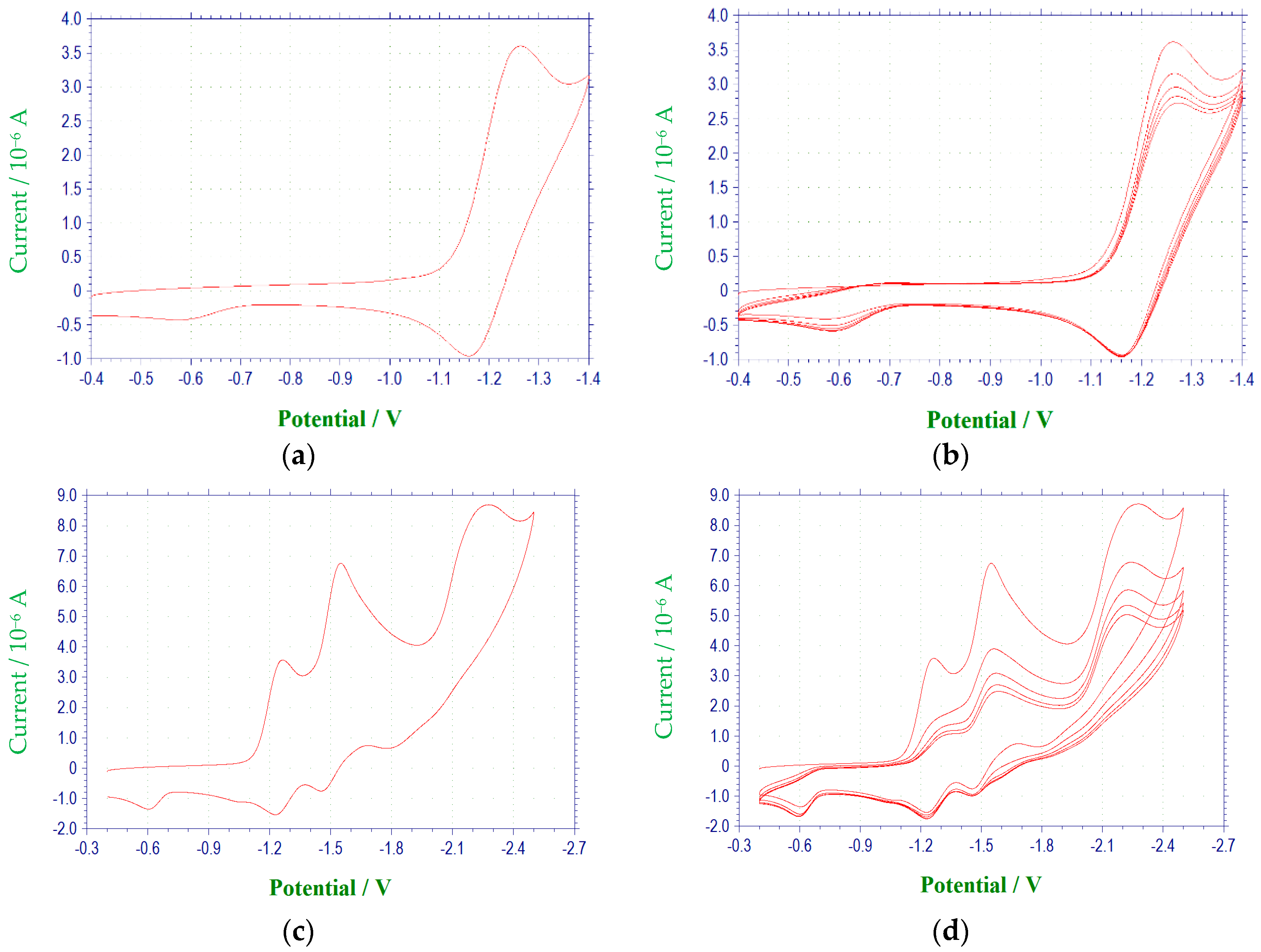
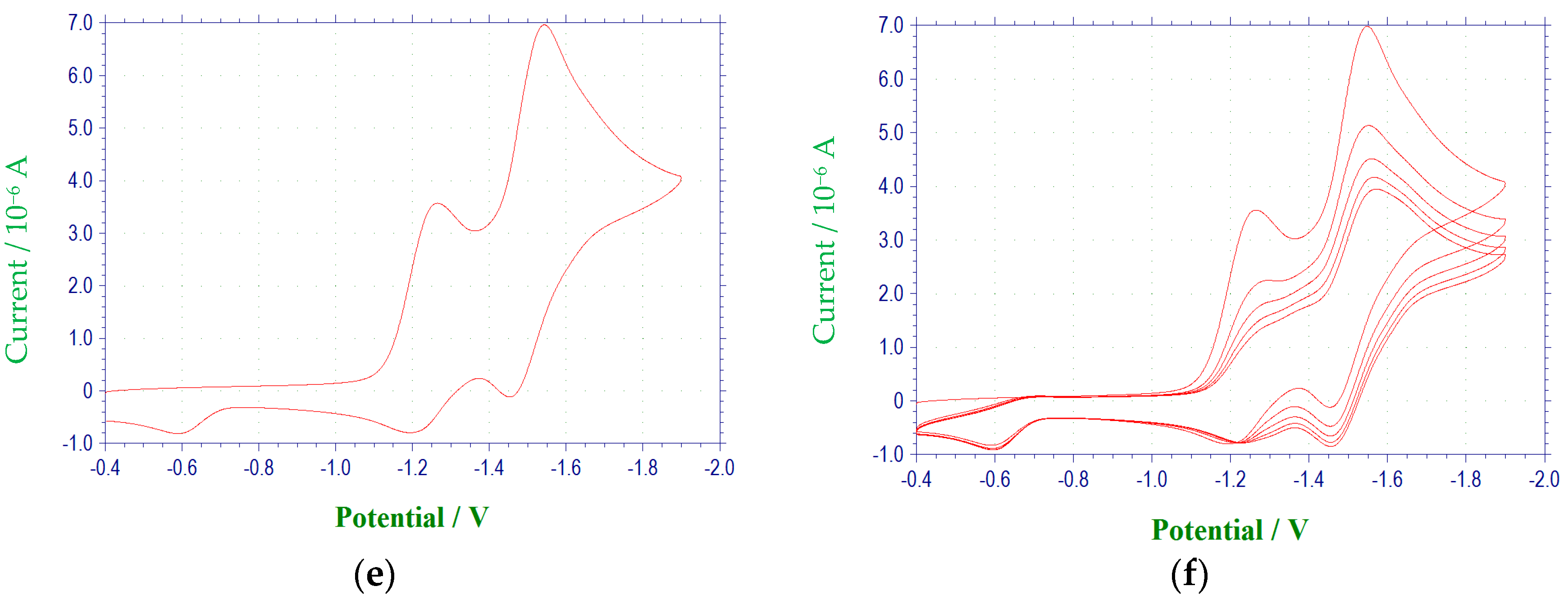
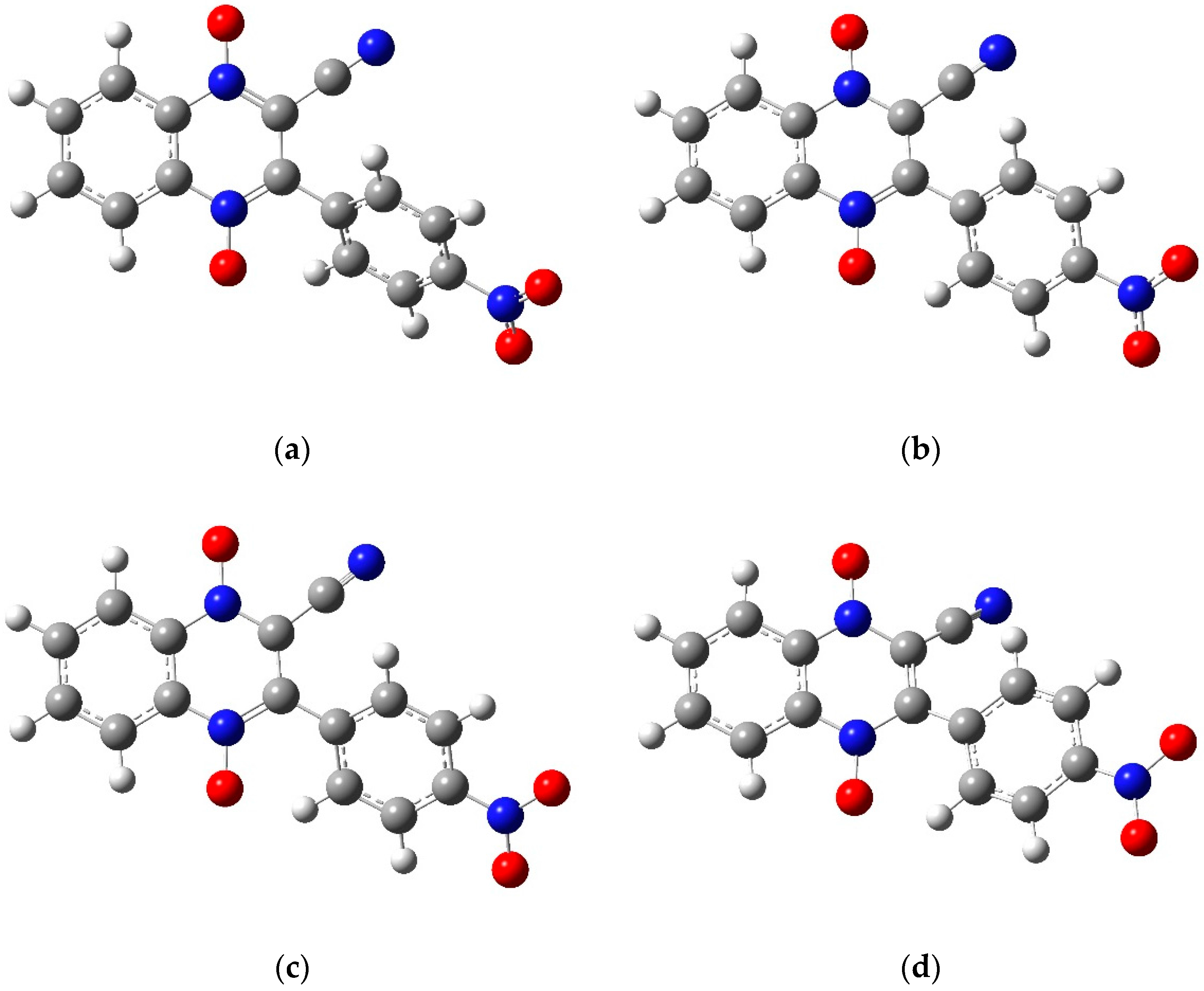
2.1. Electrochemical Behavior
The compounds included in this study are 3-aryl-quinoxaline-2-carbonitrile 1,4-di-
N-oxide derivatives (
Table 1) that have been evaluated for their biological activities as hypoxic selective anti-tumor agents [
21]. The derivatives possess varying substituents in the 3 and 7 positions of the basic quinoxaline structure. The redox properties of these substances were investigated via cyclic voltammetry and first derivative cyclic voltammetry in DMF using a platinum disc-working electrode. The reductions observed for these compounds were found to be diffusion controlled based on current functions that were relatively independent of scan rate and linearity in the plots of cathodic peak current versus the square root of scan rate [
23,
24]. Electrochemical data for scans obtained at 100 mV/s are summarized in
Table 2 and
Table 3, and representative voltammograms are shown in
Figure 1 and
Figure 2. All redox potentials reported in this study are relative to the (Fc/Fc
+) redox couple.
The electrochemical characteristics of a number of quinoxaline di-
N-oxide derivatives have been reported previously [
10,
17,
18,
19,
20,
25,
26,
27]. The first voltammetric wave observed, representing the reduction of a
N-oxide functionality to form a radical anion [
27], was reversible or quasireversible for all derivatives studied, with the exception of
3c. (
Figure 3) E
1/2 values for this reduction process ranged from −1.154 V to −1.333 V. Values of ΔE
p and E
pc − E
1/2 for this wave were typically greater than the theoretical values of 57 mV and −28.5 mV [
23,
24], respectively, for a reversible, one electron reduction. Estimates of the number of electrons involved in this reduction process based on the observed values for ΔE
p and E
pc − E
1/2 verify the one electron nature of this reduction. Likewise, calculated i
pa/i
pc ratios for derivatives
1a,
1c,
1d,
2a–
2d,
3a,
3b,
3d and
4a–
4d were close to one at all scan rates, indicating that relatively stable reduction products were formed within the time frame of the experiment [
23]. For compounds
1b,
1e,
2e,
3e and
4e, current ratios were significantly less than one, i.e., 0.2 to 0.3, indicating kinetic or other complications [
23]. For compound
3c, the first reduction process was irreversible with E
pc = −1.401 V. Although the first reduction for quinoxaline di-
N-oxide derivatives is typically reversible or quasireversible in aprotic solvents, examples of irreversibility in this process for some quinoxaline di-
N-oxides has been noted previously under conditions similar to those used in this study, i.e., voltammetry at a platinum working electrode in DMF [
17].
The relationship between quinoxaline structure and reduction potential may be observed via examination of the data in
Table 2 and
Table 3. Replacement of a H atom in the 3-/4-position of the 3-aryl group or 7-position of the quinoxaline ring with an electron donating group which increases the electron density in the conjugated system generally resulted in a negative shift in potential (E
1/2 or E
pc), making the reduction more difficult (cf.
1a vs.
1b,
1a vs.
2a,
1a vs.
3a). Replacement of a H atom in those same positions with an electron withdrawing group which removes electron density from the conjugated system resulted in a more facile reduction by shifting the potential in the positive direction (cf.
1a vs.
1c,
1a vs.
1d,
1a vs.
1e, and
1a vs.
4a). Thus, the change in electron density that occurs with a change in substituent is transmitted through the conjugated system to the electroactive heterocyclic ring.
The 20 compounds studied can be broken down into different analogues based on structure, as evident from
Table 1. The reduction potentials for the various derivatives within each analogous series fit the modified Hammett equation, ΔE
1/2 = ρ
π,Rσ
x [
28] with correlation coefficients that ranged from 0.92 to 0.99. In this equation, ρ
π,R is a measure of the extent to which the electrode reaction is affected by the polar effects of the substituents, whereas σ
x is a measure of the electronic effect that a substituent has on a molecule, and thus the redox potential in this case. The average of the sum of σ
m−x and σ
p−x [
29], i.e., (σ
m−x + σ
p−x)/2, was used in place of the total polar substituent constant σ
x in the Hammett plots, as recommended for the quinoxaline system [
30] (
Figure 4). The results observed are consistent with facilitation of reduction by a positive charge at the electroactive site [
28], and are in agreement with previous studies of the electrochemical properties of quinoxaline-di-
N-oxides [
10,
17,
18,
19,
25,
26,
28].
A voltammetric wave representing the reduction of the second N-oxide functionality was observed for all derivatives studied, with the exception of 3c, at potentials between −1.97 and −2.6 V. This process was irreversible for each of the quinoxaline derivatives. Additional irreversible reduction waves were observed at more negative potentials for compound 1a at −2.5 (sh), −2.7 (sh), and −2.829 V. The last reduction observed appeared close to background reduction. The currents for these processes would indicate one-electron reductions in each case. However, these processes were not studied in further detail.
Compounds
1e,
2e,
3e and
4e possess a nitro group in the
para position of the 3-aryl group. In each case, a quasireversible reduction process was observed between the two voltammetric waves representing the reductions of the
N-oxide moieties, with half-wave potentials ranging from −1.516 to −1.563 V. ΔE
p and E
pc − E
1/2 values at 100 mV/s for this wave ranged from 0.063 to 0.096 V and −0.031 to −0.048 V, respectively. In addition, the i
pa/i
pc ratios for these derivatives at 100 mV/s were between 0.6 and 0.7. Comparison of cathodic peak currents for this process to those for the first
N-oxide reduction, as well as using ΔE
p and E
pc − E
1/2 for estimates, indicates that this process involves one electron. This wave may be attributed to reduction of the nitro group. Previous investigations into the electrochemical properties of the nitro group have shown that this functional group undergoes one electron reduction to form a radical anion in aprotic media [
31,
32,
33,
34]. It seems reasonable to assume that a nitro radical anion was formed during the reductions of these derivatives as well.
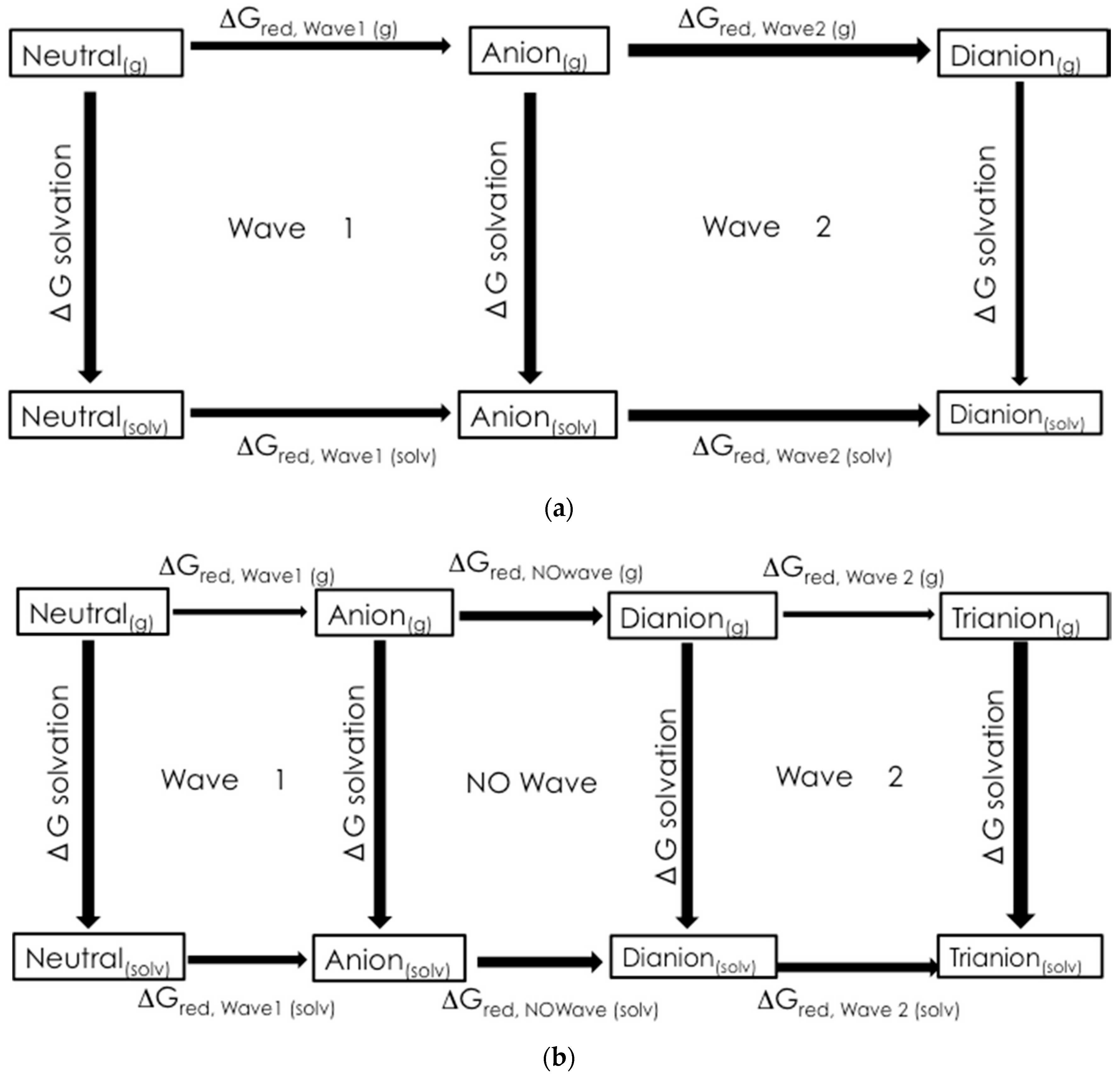
2.2. Preliminary Computational Study
The computational half-cell potentials for molecules
1a–
1e from
Table 1 were calculated using Guassian 09 and are listed in
Table 4.
The optimization energy, thermal correction factor, and solvation energy were calculated for the first wave with the extra electron of the radical anion on both the carbon attached to the benzene ring and the carbon attached to the cyano group. The energies and thermal correction factors were found to be identical regardless of which of these carbon atoms the radical was located on (results not shown, manuscript in preparation). This result seems to indicate that the extra electron isn’t isolated to a single carbon, but instead is in some sort of resonance, as shown in
Figure 3. Future computational work will investigate this observation further. For the remainder of this paper, we will only present the calculations with the radical on the carbon neighboring the cyano group. In
Table 5, the computational half-cell potentials are compared to the ferrocene/ferrocinium (Fc/Fc
+) redox couple, firstly with the standard hydrogen half-cell potential set to zero, and secondly with the ferrocene/ferrocinium reduction potential set to zero. These half-cell potentials are analyzed relative to ferrocene to provide a direct comparison to the experimental half wave potentials (E
1/2). For derivatives
1a–
1d, while the computational and experimental values do not agree quantitatively, they have a strong qualitative agreement for the first and second reduction waves.
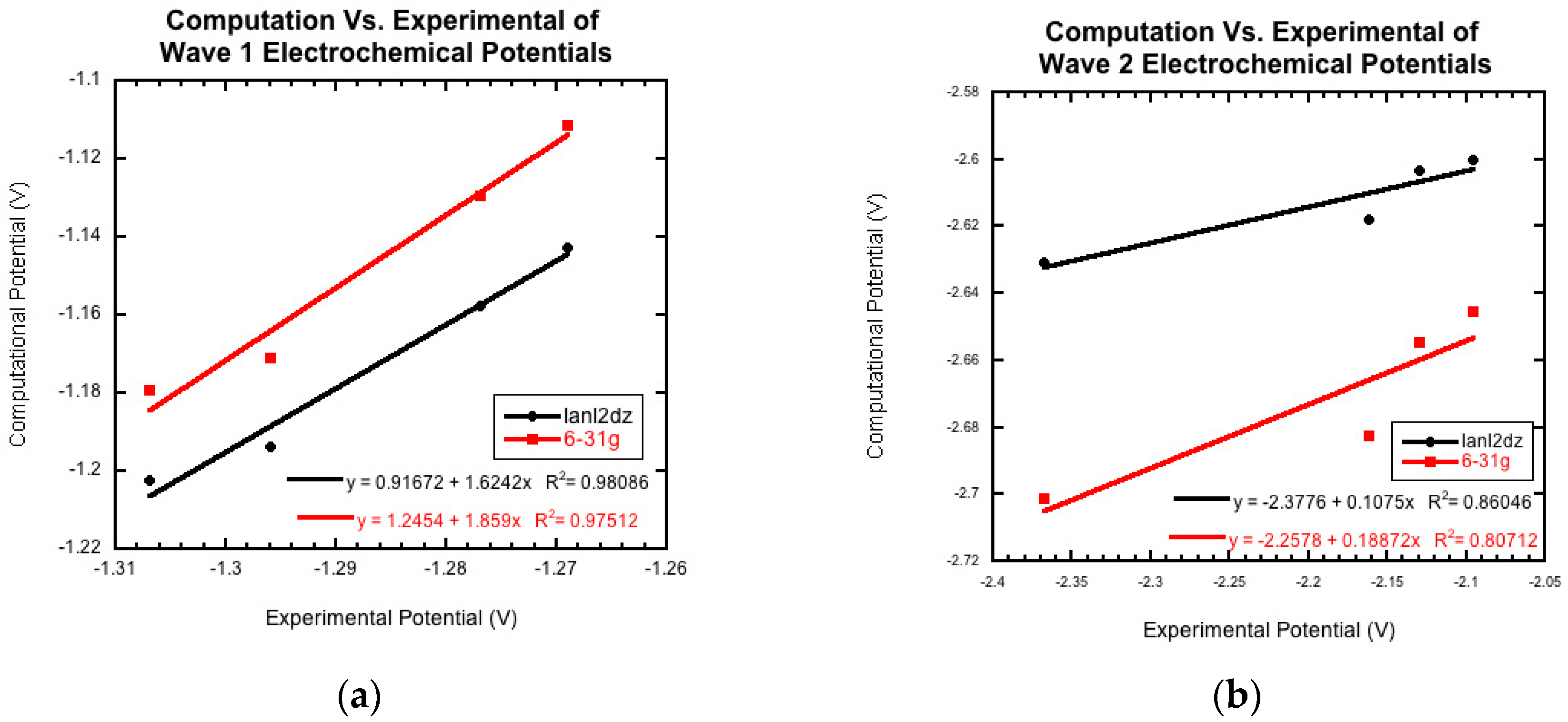
Figure 5a compares the two computational basis set calculations, lanld2z (black) and 6-31g (red), to the experimental reduction potentials (E
1/2) for wave 1. For both basis sets, there is a strong positive correlation.
Figure 5b shows the comparison of the two computational basis set calculations, lanl2dz (black) and 6-31g (red), compared to the experimental peak potentials (E
pc) for wave 2. While there is still good qualitative agreement, the correlation between the computational and experimental data decreased compared to wave 1. For the second wave, the computational values could demonstrate more accurate predictions than those generated electrochemically since this wave was found to be irreversible for all derivatives.
Molecule
1e was not included in
Figure 5. This nitro group-containing derivative displayed drastically different calculated potentials from those of derivatives
1a–
1d and from the experimental value. The origin of these differences will be investigated in future computational research.
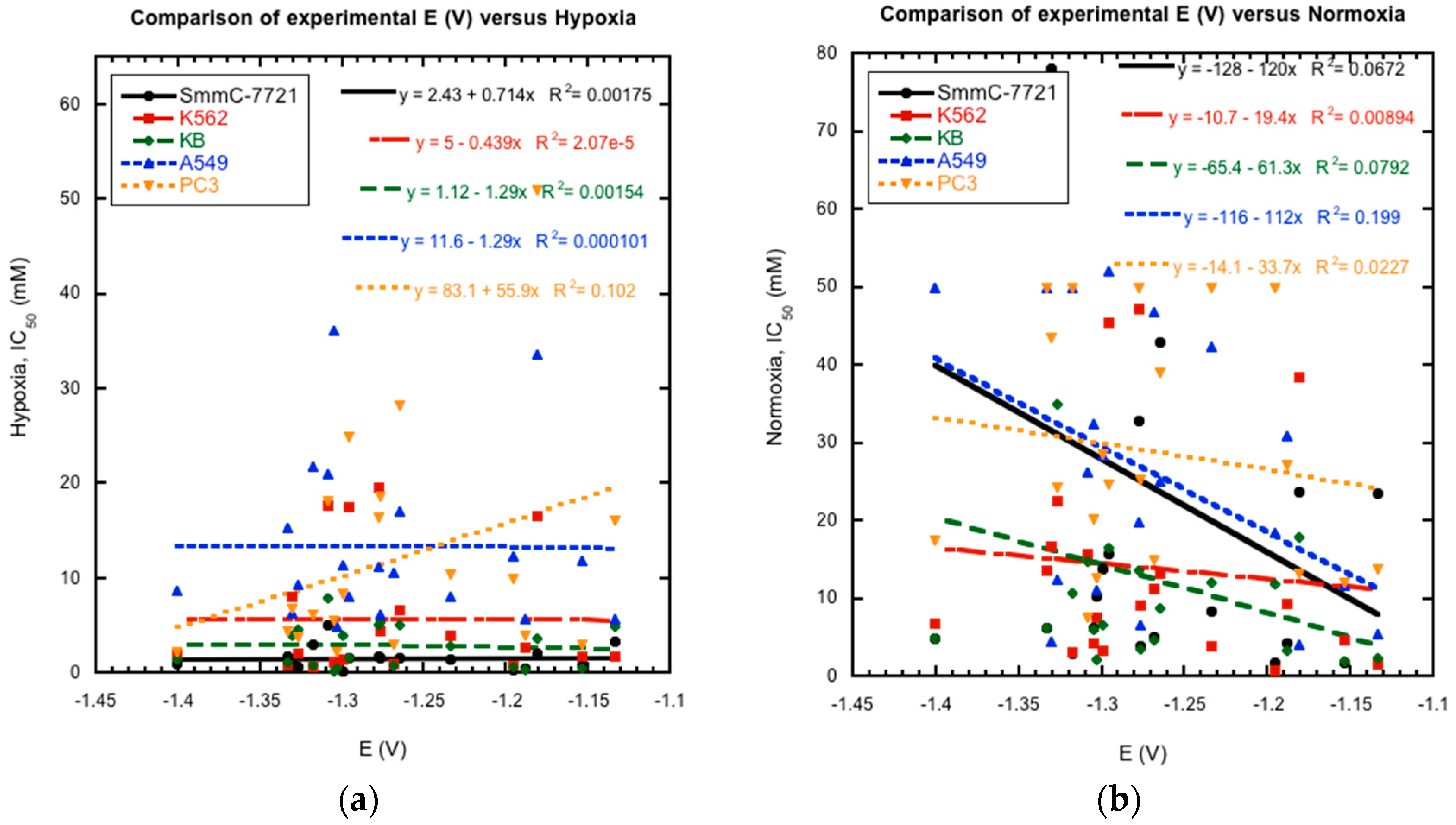
2.3. Reduction Potentials versus Cytotoxicity
Previous studies of substituted quinoxaline di-
N-oxides have demonstrated a link between reduction potential and certain biological activities, i.e., the compounds with higher activities generally have less negative reduction potentials and are easier to reduce [
10,
17,
18,
19]. Thus, possible links between reduction potential and anti-tumor activity were investigated for the current series of compounds. Comparison of their reported cytotoxicity against cancer cell lines in hypoxia and normoxia [
21] versus their measured E
1/2 values as a whole does not show a clear and direct correlation between activity and reduction potential (
Figure 6). Plots of reduction potential versus cytotoxicity as a whole show no clear patterns. Comparison of smaller subsets within the data also is inconclusive. For example, derivatives
4a and
4b were shown to possess better hypoxic activity against cancer cell lines than the un-substituted derivatives
1a and
1b [
21]. And the former are also more easily reduced by over 100 mV. However, derivatives
1e and
2e are more easily reduced than derivatives
1c and
2c, respectively, but have lower hypoxic activities. In addition, the most potent compound with the highest reported activities,
2c, was not the most easily reduced derivative. These results do not rule out bioreduction in the mechanism of action of these compounds against cancer cells. However, they indicate that other factors besides bioreduction may play a more important role for the in vivo mechanism of action of these compounds, such as metabolism, stereochemistry, membrane permeability, bioactivation, DNA binding, and diffusion [
35,
36].