The Influence of Lead on Generation of Signalling Molecules and Accumulation of Flavonoids in Pea Seedlings in Response to Pea Aphid Infestation
Abstract
:1. Introduction
2. Results
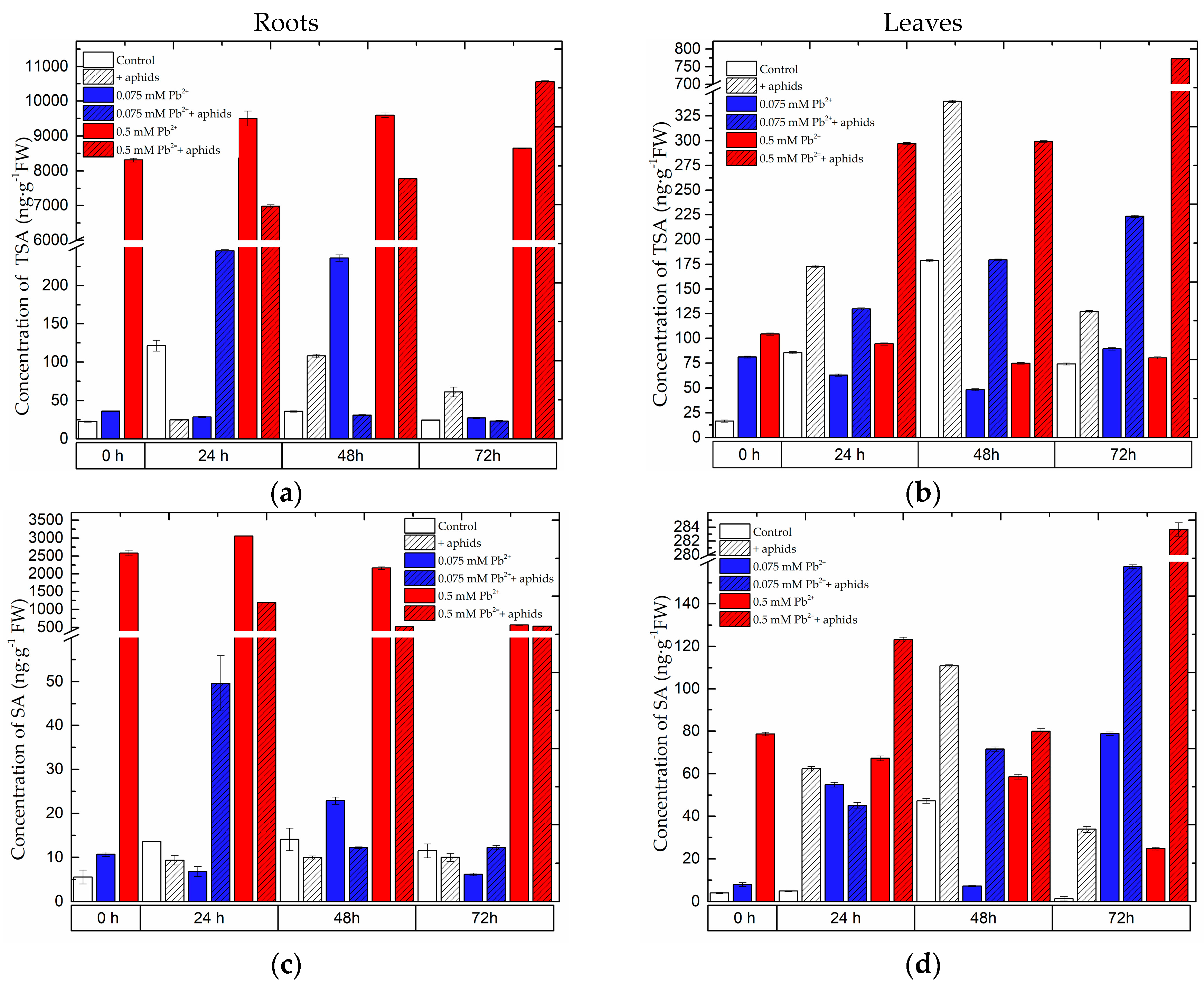
2.1. The Effect of Lead and A. pisum on SA Accumulation in Pea Seedlings
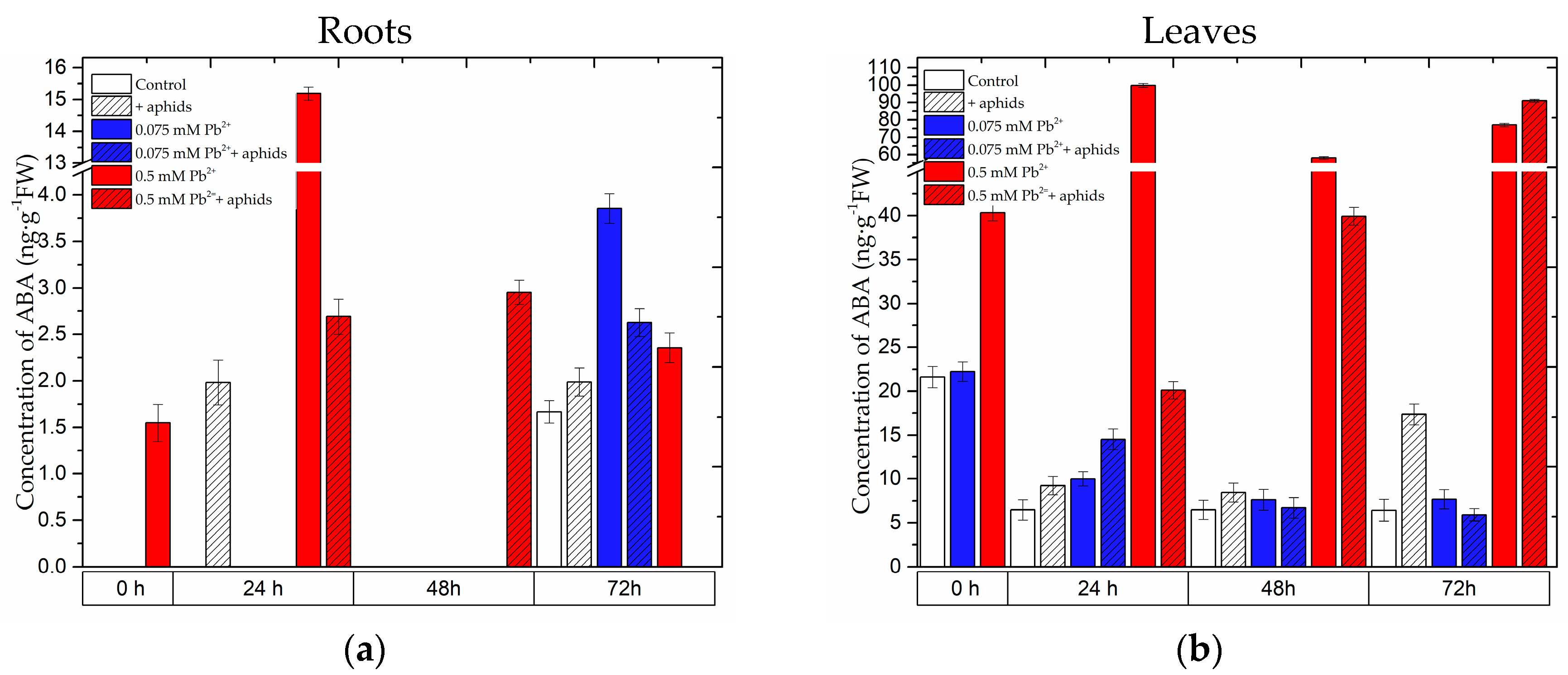
2.2. The Effect of Lead and A. pisum on ABA Accumulation in Pea Seedlings
2.3. The Effect of Lead and A. pisum on Accumulation of Flavonoids in Pea Seedlings
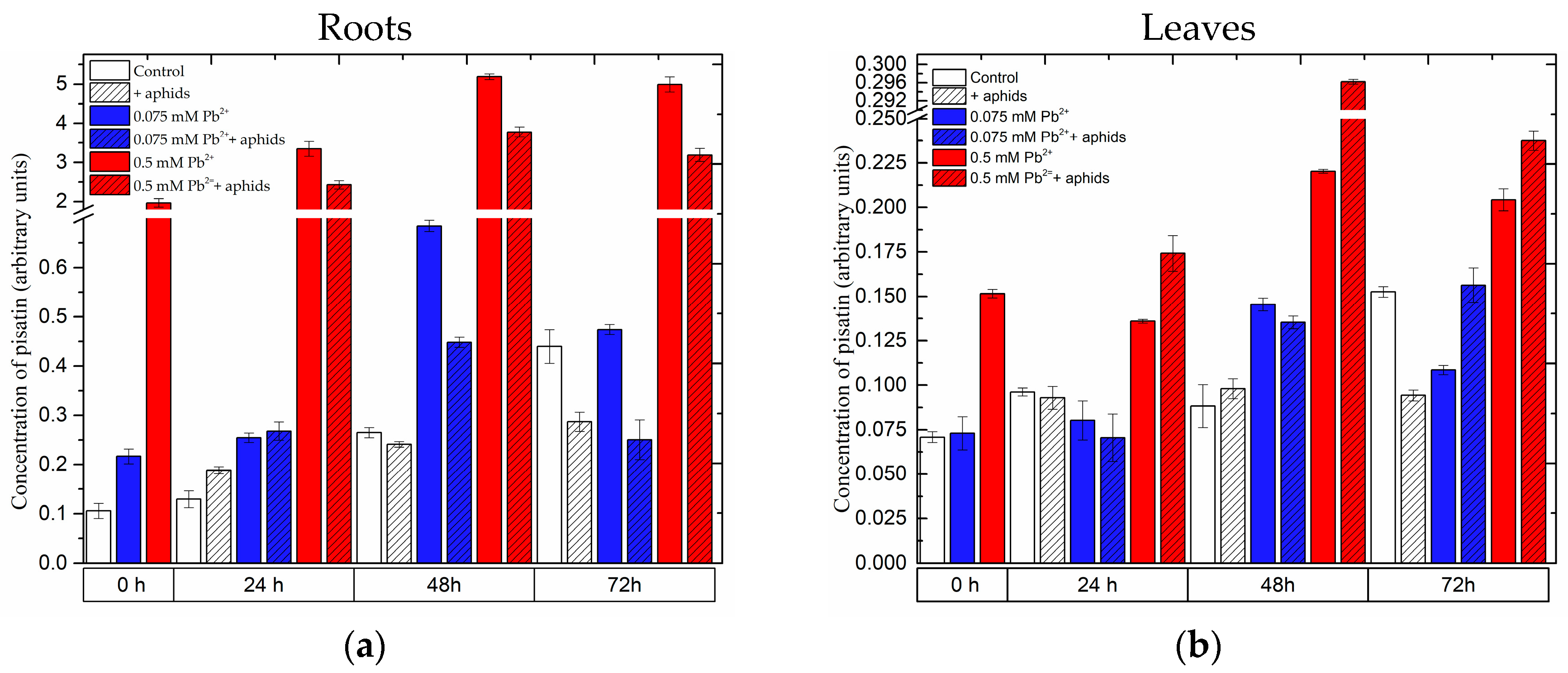
2.3.1. The Effect of Lead and A. pisum on Accumulation of Pisatin in Pea Seedlings
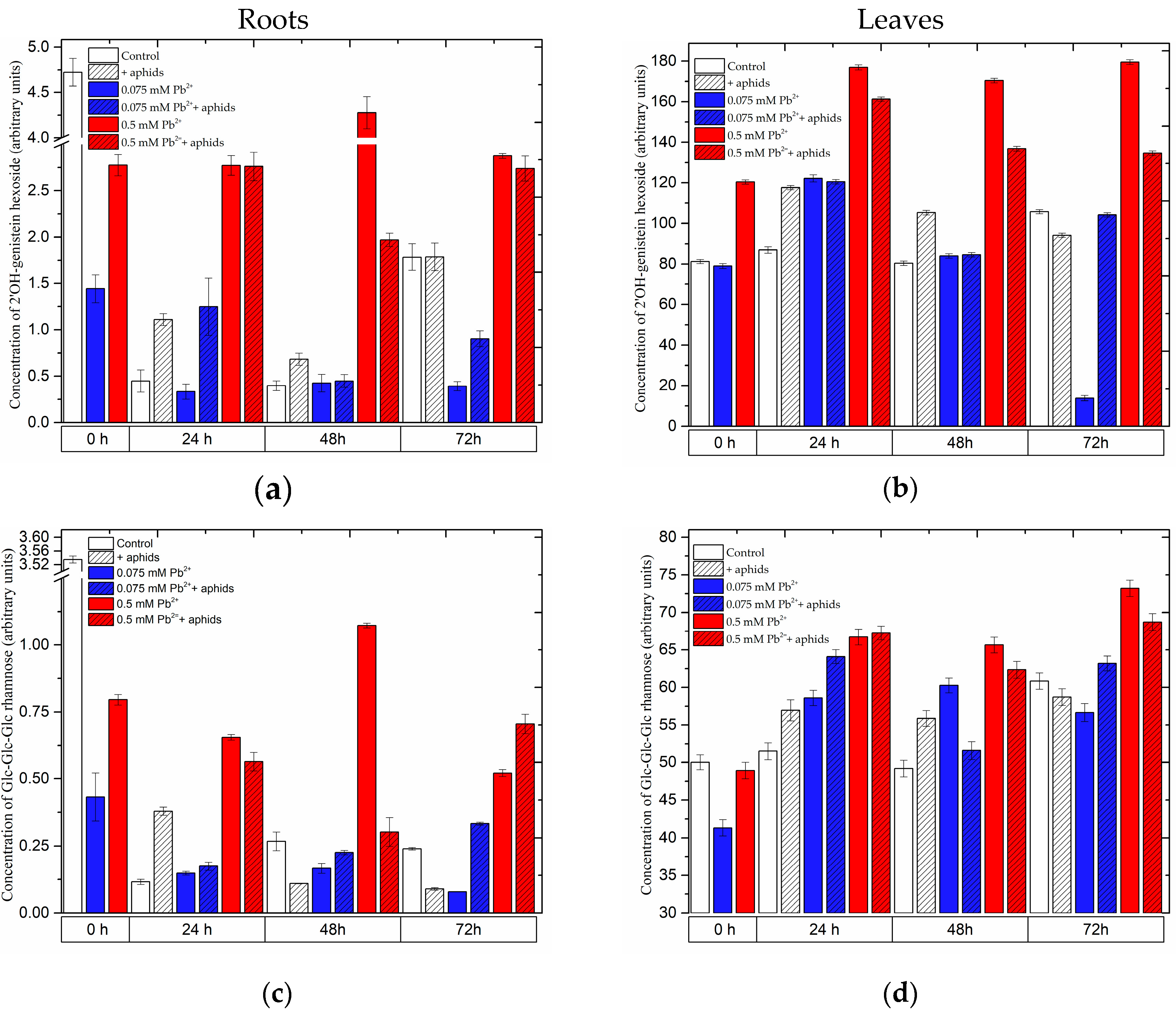
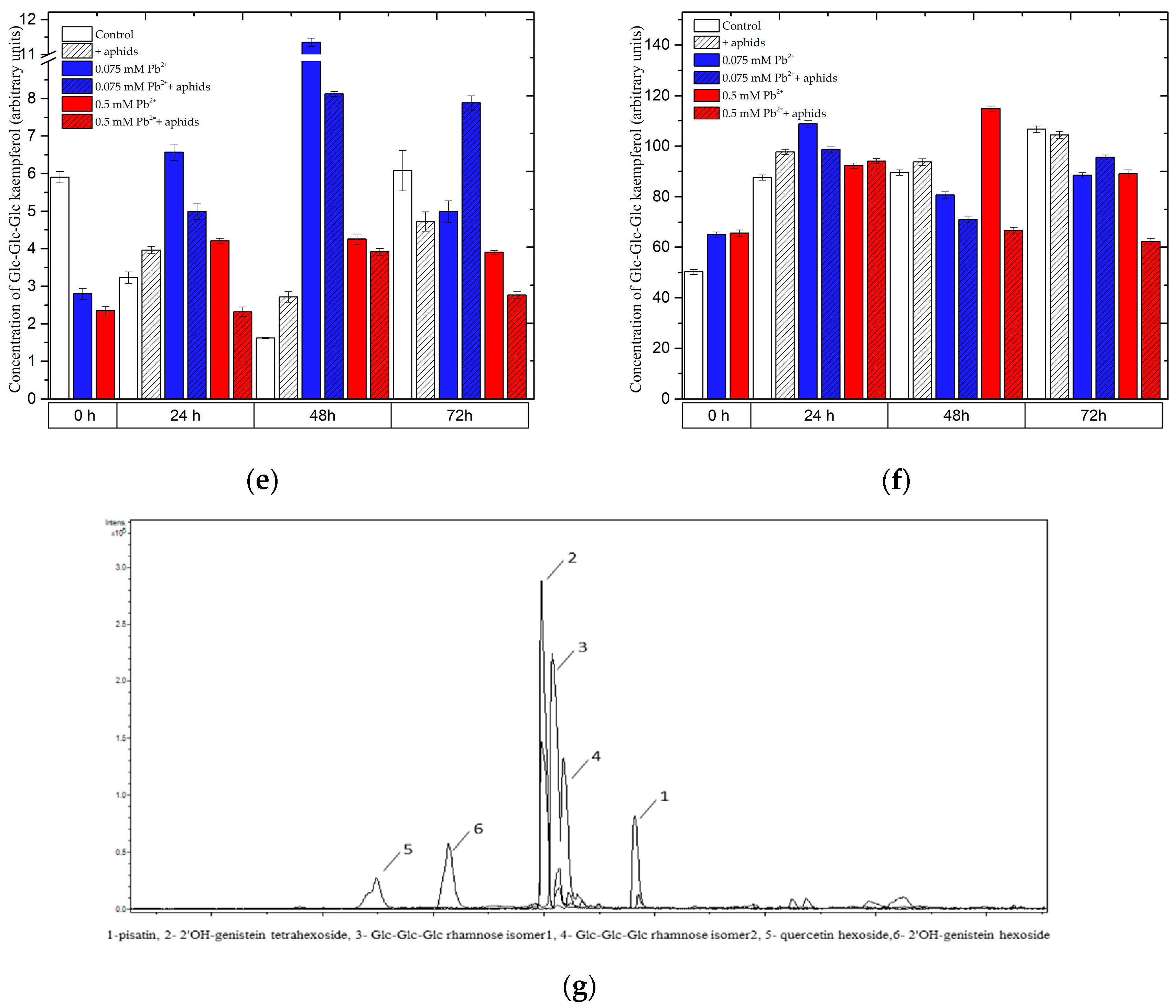
2.3.2. The Effect of Lead and A. pisum on the Level of Isoflavonoid and Flavonoid Glycosides in Pea Seedlings
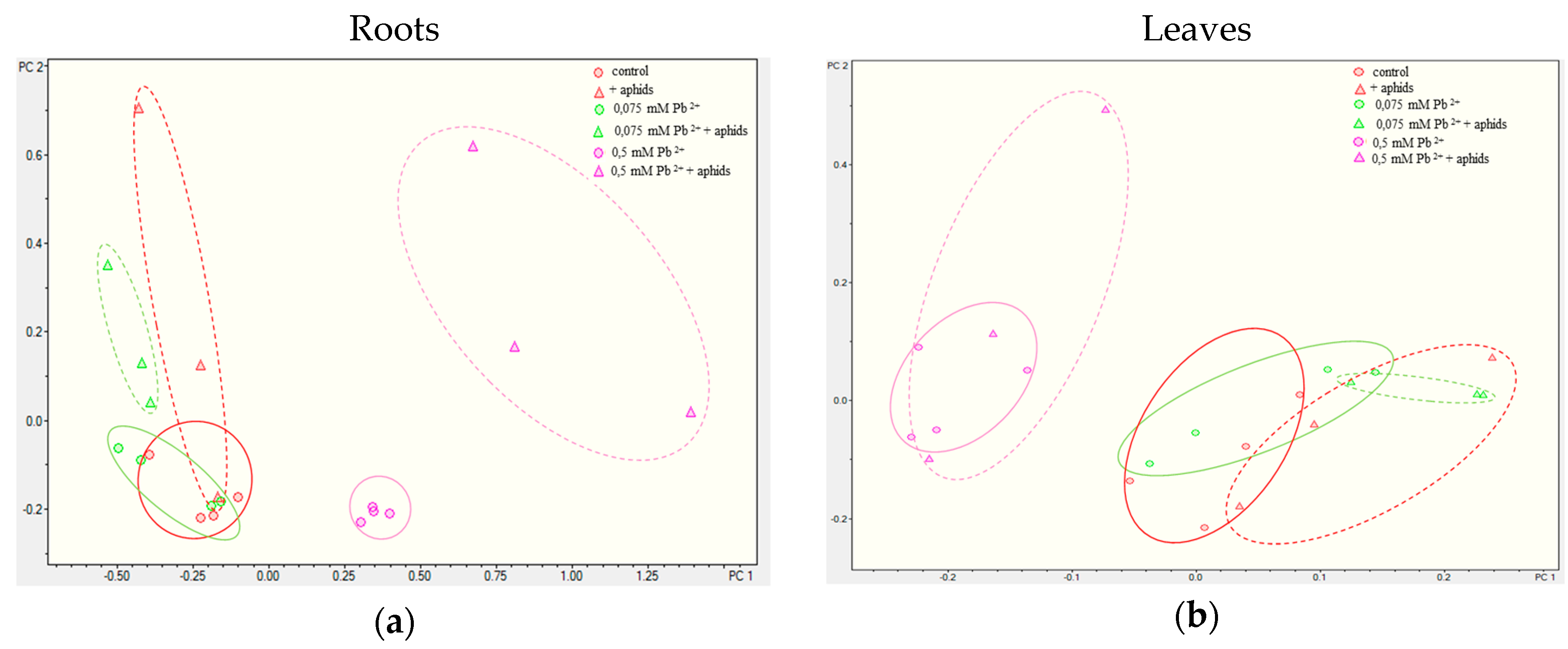
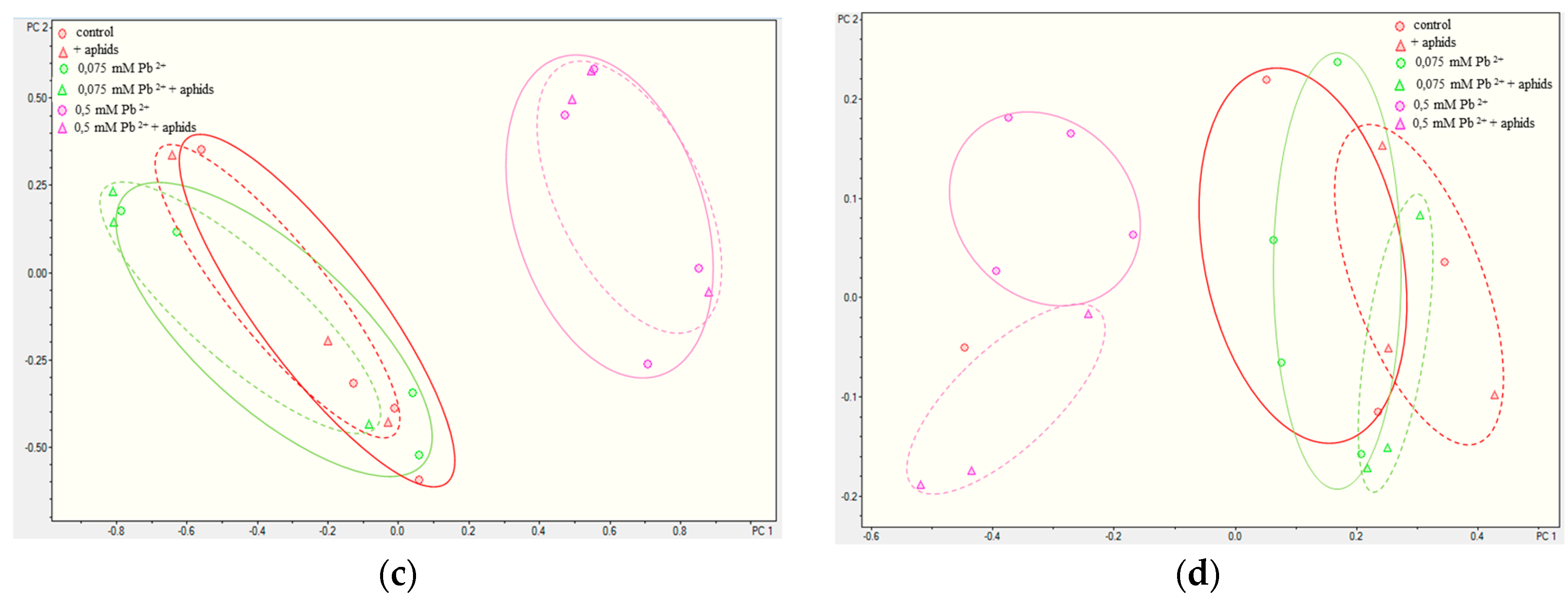
2.3.3. Quantitative Analysis of Metabolites in the Roots and Leaves of Pea Seedlings
2.4. The Effect of Lead and A. pisum on Expression Levels of Phenylalanine Ammonialyase and Chalcone Synthase Genes in Pea Seedlings
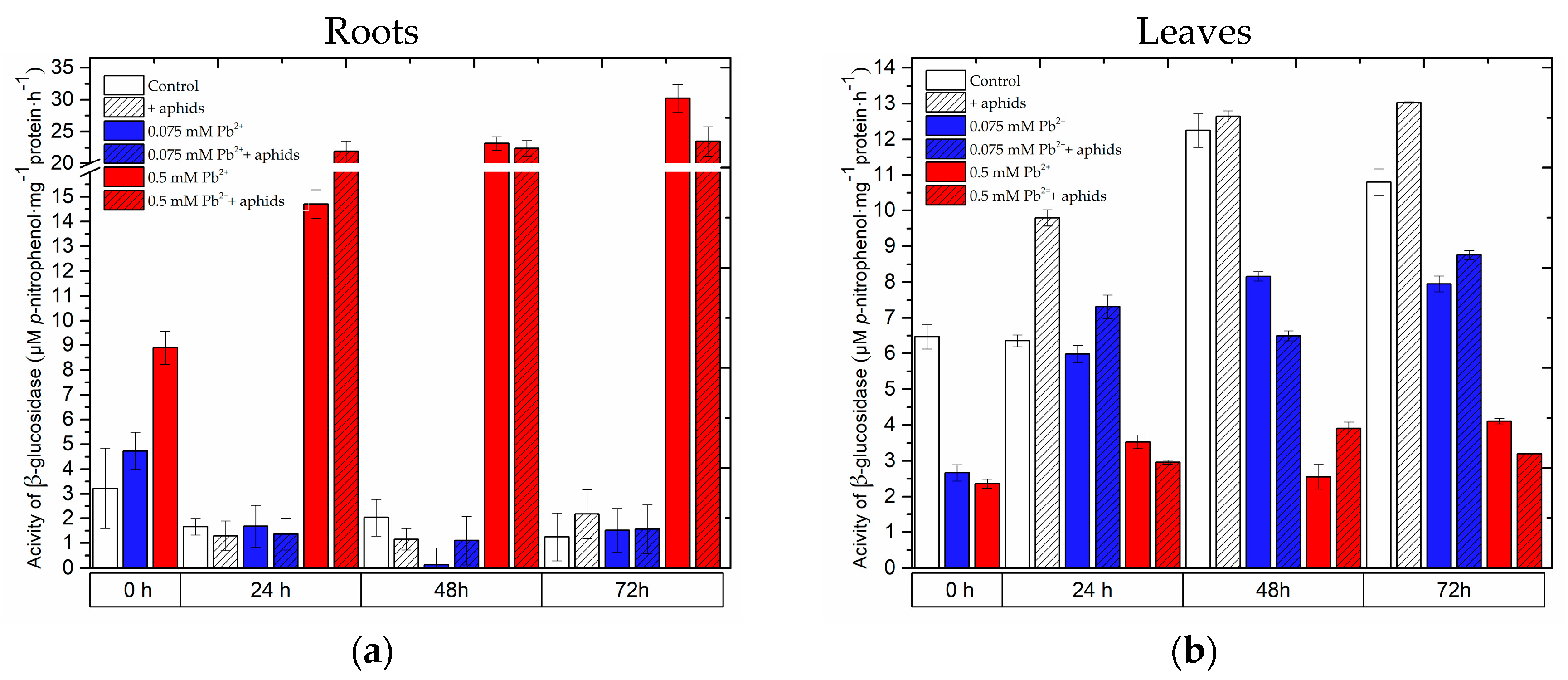
2.5. The Effect of Lead and A. pisum on β-Glucosidase Activity in Pea Seedlings
2.6. The Effect of Lead and A. pisum on Activity of Phenylalanine Ammonialyase in Pea Seedlings
2.7. The Effect of Lead and A. pisum on the Growth of Pea Seedlings
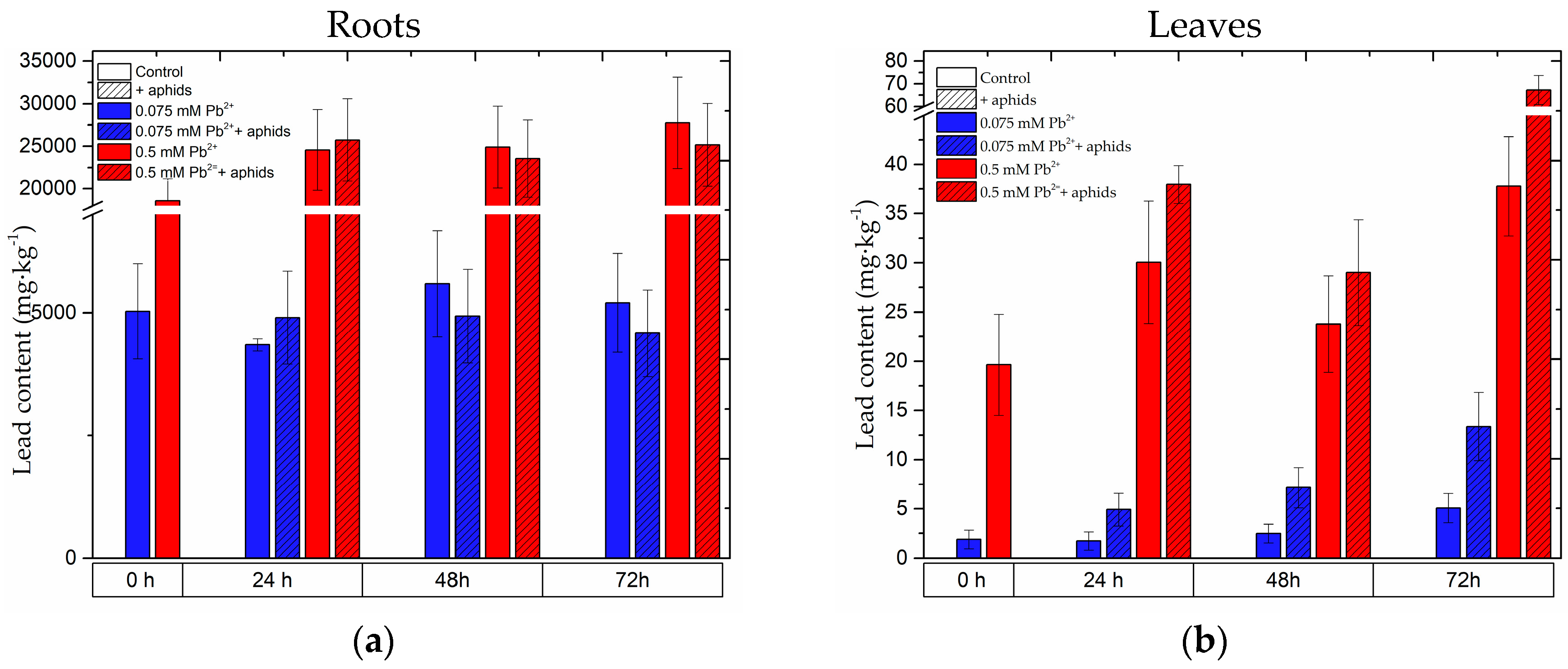
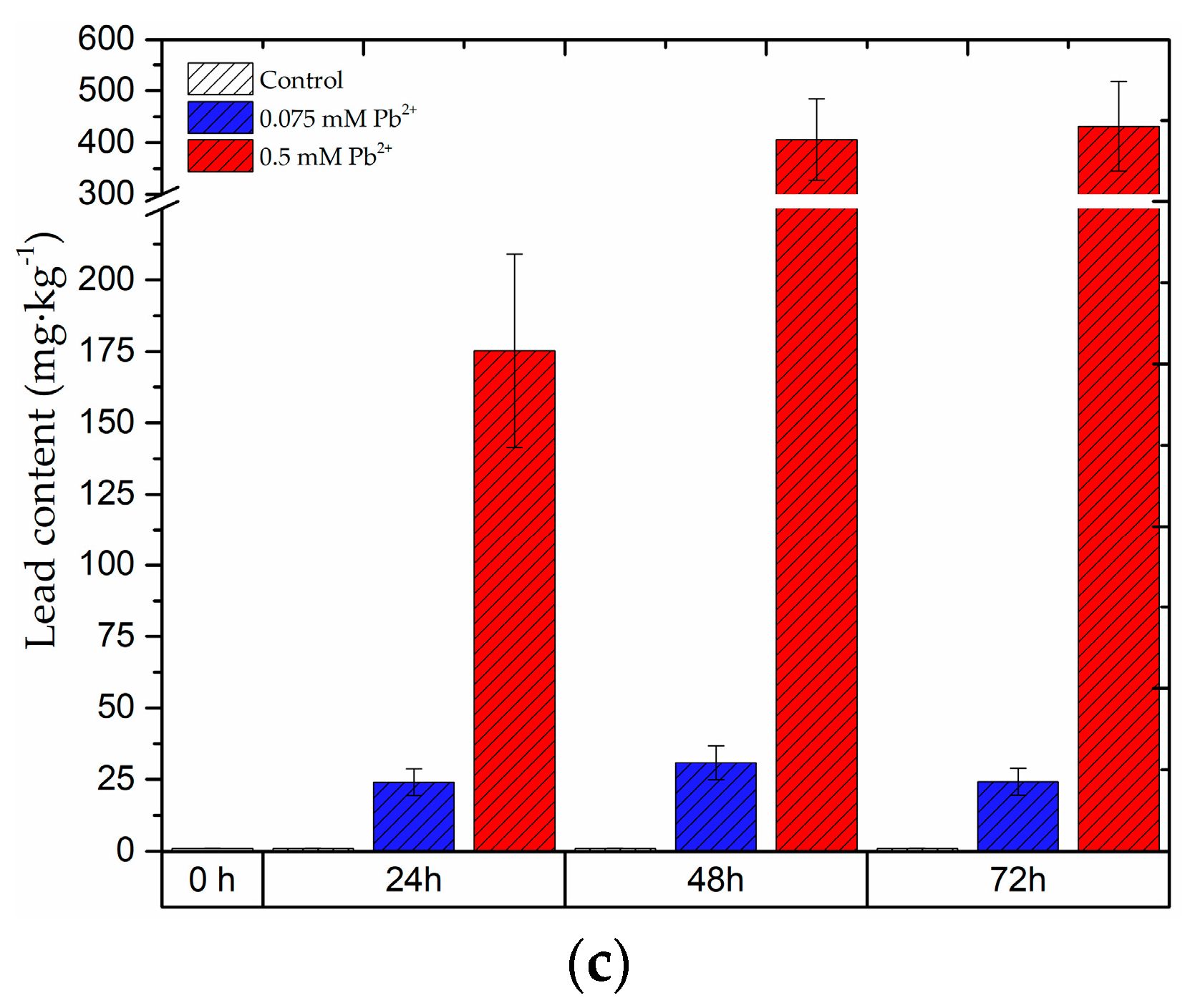
2.8. The Content of Lead in the Roots and Leaves of Pea Seedlings and in Bodies of Pea Aphid
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Aphids and Infestation Experiment
4.3. Detection of Salicylic Acid (SA)
4.4. Detection of Abscisic Acid (ABA)
4.5. Analysis of Flavonoids
4.5.1. Isolation of Phenolic Compounds
4.5.2. Liquid Chromatography–Mass Spectrometry (LC/UV/ESI/MS/MS)
4.5.3. Quantitative Analysis of Metabolites
4.6. Total RNA Extraction and Semiquantitative RT-PCR Analysis
4.7. Extraction and Assay of β 1,3-Glucosidase Activity
4.8. Extraction and Assay of Phenylalanine Ammonialyase (PAL) Activity
4.9. Determination of Lead Content
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| ANOVA | analysis of variance |
| Cd | cadmium |
| CHS | chalcone synthase |
| EDTA | ethylenedinitrilotetraacetic acid |
| ESI | electrospray ionization |
| FW | fresh weight |
| g | gram |
| Glc | glucose |
| h | hour |
| FA | formic acid |
| GC-MS | gas chromatography/mass spectrometry |
| hpi | hour post infestation |
| HPLC | high-performance liquid chromatography |
| IAA | indole-3yl-acetic acid |
| LC/UV/ESI/MS/MS | Liquid chromatography/ultraviolet detection/electrospray–mass spectrometry (tandem mass spectrometry) |
| MS | mass spectrometry |
| MS2 | second stage of mass spectrometry |
| PAL | phenylalanine ammonia-lyase |
| PCA | principial component analysis |
| Pb | lead |
| REACH | Registration, Evaluation, Authorisation and Restriction of Chemicals |
| RT-PCR | Reverse transcription polymerase chain reaction |
| SA | salicylic acid |
| SAG | salicylic acid glucoside |
| SIM | Selected ion monitoring |
| TCA | trichloroacetic acid |
| TSA | total salicylic acid |
| UV-Vis | ultraviolet-visible spectrophotometry |
References
- Maksymiec, W. Signaling responses in plants to heavy metal stress. Acta Physiol. Plant. 2007, 29, 177–187. [Google Scholar] [CrossRef]
- Poschenrieder, Ch.; Tolrà, R.; Barceló, J. Can metal defend plants against biotic stress? Trends Plant Sci. 2006, 11, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, K.B.; Abdelly, C.; Savouré, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Fujita, Y.; Noutoshi, Y.; Takahashi, F.; Narusaka, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Crosstalk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 2006, 9, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Poschenrieder, Ch.; Cabot, C.; Martos, S.; Gallego, B.; Barceló, J. Do toxic ions induce hormesis in plants? Plant Sci. 2013, 212, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.; Maffei, M.E. Calcium and secondary CPK signaling in plants in response to herbivore attack. Biochem. Biophys. Res. Commun. 2010, 400, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.; Ozawa, R.; Maffei, M.E. Recent advances in plant early signaling in response to herbivory. Int. J. Mol. Sci. 2011, 12, 3723–3739. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.Ch.; Drzewiecka, K.; Jeleń, H.; Narożna, D.; Rucińka-Sobkowiak, R.; Kęsy, J.; Floryszak-Wieczorek, J.; Gabryś, B.; Morkunas, I. Differential induction of Pisum sativum defense signaling molecules in response to pea aphid infestation. Plant Sci. 2014, 221–222, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Courot, E.; Clément, Ch.; Ricord, S.; Crouzet, J.; Aziz, A.; Cordelier, S. Molecular engineering of phytoalexins in plants: Benefits and limitations for food and agriculture. J. Agric. Food Chem. 2017, 65, 2643–2644. [Google Scholar] [CrossRef] [PubMed]
- Gondor, O.K.; Pál, M.; Darkó, É.; Janda, T.; Szalai, G. Salicylic acid and sodium salicylate alleviate cadmium toxicity to different extents in maize (Zea mays L.). PLoS ONE 2016, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Arpaia, S.; Cardinali, A.; di Venere, D.; Linsalata, V. Role of endogenous flavonoids in resistance mechanism of Vigna to aphids. J. Agric. Food Chem. 2000, 48, 5316–5320. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.S.J. Flavonoid–insect interactions: Recent advances in our knowledge. Phytochem 2003, 6, 21–30. [Google Scholar] [CrossRef]
- Leitner, M.; Boland, W.; Mithӧfer, A. Direct and indirect defences induced by piercing-sucking and chewing herbivores in Medicago truncatula. New Phytol. 2005, 167, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.S.; Lister, C. Flavonoid functions in plants. In Flavonoids: Chemistry, Biochemistry and Applications; Andersen, Ø.M., Markham, K.R., Eds.; CRC Press: London, UK; New York, NY, USA, 2006; pp. 397–441. ISBN 0-8493-2021-6. [Google Scholar]
- Bernards, M.A.; Båstrup-Spohr, L. Phenylpropanoid metabolism induced by wounding and insect herbivory. In Induced Plant Resistance to Herbivory; Schaller, A., Ed.; Springer: New York, NY, USA, 2008; pp. 189–211. ISBN 978-1-4020-8181-1. [Google Scholar]
- Lev-Yadun, S.; Gould, K.S. Role of anthocyanins in plant defense. In Anthocyanins Biosynthesis, Functions and Applications; Gould, K.S., Davies, K.M., Winefield, C., Eds.; Springer: Berlin, Germany, 2009; pp. 21–48. ISBN 978-0-387-77334-6. [Google Scholar]
- Mewis, I.; Khan, M.A.M.; Glawischnig, E.; Schreiner, M.; Ulrichs, Ch. Water stress and aphid feeding differentially influence metabolite composition in Arabidopsis thaliana (L.). PLoS ONE 2012, 7(11), e48661. [Google Scholar] [CrossRef] [PubMed]
- Prince, D.C.; Drurey, C.; Zipfel, C.; Hogenhout, S.A. The leucine-rich repeat receptor-like kinase Brassinosteroid Insensitive1-Associated Kinase1 and the Cytochrome P450 PHYTOALEXIN DEFICIENT3 contribute to innate immunity to aphids in arabidopsis. Plant Physiol. 2014, 164, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Golan, K.; Sempruch, C.; Górska-Drabik, E.; Czerniewicz, P.; Łagowska, B.; Kot, I.; Kmieć, K.; Magierowicz, K.; Leszczyński, B. Accumulation of amino acids and phenolic compounds in biochemical plants responses to feeding of two different herbivorous arthropod pests. Arthropod-Plant Interact. 2017, 1–8. [Google Scholar] [CrossRef]
- Morkunas, I.; Woźniak, A.; Formela, M.; Mai, V.Ch.; Marczak, Ł.; Narożna, D.; Borowiak-Sobkowiak, B.; Kühn, Ch.; Grimm, B. Pea aphid infestation induces changes in flavonoids, antioxidative defence, soluble sugars and sugar transporter expression in leaves of pea seedlings. Protoplasma 2015, 253, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.; Kogan, M.; Paxton, J.D. Effect of soybean phytoalexins on the herbivorus insects mexican bean bettle and soybean looper. J. Chem. Ecol. 1983, 9, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Russel, G.B.; Sutherland, O.R.W.; Hutchins, R.F.N.; Chrostmas, P.E. Vestitol: A phytoalexin with insect feeding-deterrent activity. J. Chem. Ecol. 1978, 4, 571–579. [Google Scholar] [CrossRef]
- Sutherland, O.; Russell, G.; Biggs, D.; Lane, G. Insect feeding deterrent activity of phytoalexin isoflavonoids. Biochem. Syst. Ecol. 1980, 8, 73–75. [Google Scholar] [CrossRef]
- Morimoto, M.; Kumeda, S.; Komai, K. Insect antifeedant flavonoids from Gnaphalium affin. J. Agric. Food Chem. 2000, 48, 1888–1891. [Google Scholar] [CrossRef] [PubMed]
- Onyilagha, J.C.; Lazorko, J.; Gruber, M.Y.; Soroka, J.J.; Erlandson, M.A. Effect of flavonoids on feeding preference and development of the crucifer pest Mamestra configurata Walker. J. Chem. Ecol. 2004, 30, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.S.; Wang, C.Z.; Loon, J.J.A. Chemosensory basis of behavioural plasticity in response to deterrent plant chemicals in the larva of the small cabbage white butterfly Pieris rapae. J. Insect Physiol. 2009, 55, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Goławska, S.; Łukasik, I. Antifeedant activity of luteolin and genistein against the pea aphid, Acyrthosiphon pisum. J. Pest Sci. 2012, 85, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.S.J. Importance of flavonoids in insect-plant interactions: Feeding and oviposition. Phytochemistry 2001, 56, 245–252. [Google Scholar] [CrossRef]
- Ateyyat, M.; Abu-Romman, S.; Abu-Darwish, M.; Ghabeish, I. Impact of flavonoids against woolly apple aphid, Eriosoma lanigerum (Hausmann) and its sole parasitoid, Aphelinus mali (Hald.). J. Agric. Sci. 2012, 4, 227–236. [Google Scholar] [CrossRef]
- Diaz Napal, G.N.; Palacios, S.M. Bioinsecticidal effect of flavonoids pinocembrin and quercetin against Spodoptera frugiperda. J. Pest Sci. 2015, 88, 629–635. [Google Scholar] [CrossRef]
- Diaz, J.; Bernal, A.; Pomar, F.; Merino, F. Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L.) seedling in response to copper stress and its relation to lignification. Plant Sci. 2001, 161, 179–188. [Google Scholar] [CrossRef]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Smeets, K.; Cuypers, A.; Lambrechts, A.; Semane, B.; Hoet, P.; Laere, A.V.; Vangorsveld, J. Induction of oxidative stress and antioxidative mechanisms in Phaseolus vulgaris after Cd application. J. Plant Physiol. Biochem. 2005, 43, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, M.; Abdel-Farid, I.B.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Healthy and unhealthy plants: The effect of stress on the metabolism of Brassicaceae. Environ. Exp. Bot. 2009, 67, 23–33. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Kontek, R.; Janas, K.M. Antioxidant enzymes activity and phenolic compounds content in red cabbage seedlings expose to copper stress. Ecotoxicol. Env. Saf. 2009, 72, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Pawlak-Sprada, S.; Arasimowicz-Jelonek, M.; Podgórska, M.; Deckert, J. Activation of phenylpropanoid pathway in legume plants expose to heavy metals: Part I. Effects of cadmium and lead on phenylalanine ammonia-lyase gene expression, enzyme activity and lignin content. Acta Biochim. Pol. 2011, 58, 211–216. [Google Scholar] [PubMed]
- Rastgoo, L.; Alemzadeh, A. Biochemical responses of Gouan (Aeluropus littoralis) to heavy metals stress. Aust. J. Crop Sci. 2011, 5, 375–383. [Google Scholar]
- Ashraf, U.; Kanu, A.S.; Deng, Q.; Mo, Z.; Pan, S.; Tian, H.; Tang, X. Lead (Pb) toxicity; physio-biochemical mechanisms, grain yield, quality, and Pb distribution proportions in scented rice. Front. Plant Sci. 2017, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Silvestre, J.; Pinelli, E.; Kallerhoff, J.; Kaemmerer, M.; Tarigo, A.; Shahid, M.; Guiresse, M.; Pradere, P.; Dumat, C. A field study of lead phytoextraction by various scented Pelargonium cultivars. Chemosphere 2008, 71, 2187–2192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzu, G.; Sobanska, S.; Sarret, G.; Munoz, M.; Dumat, C. Foliar lead uptake by lettuce exposed to atmospheric fallouts. Environ. Sci. Technol. 2010, 44, 1036–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzywy, I.; Krzywy, E.; Pastuszak-Gabinowska, M.; Brodkiewicz, A. Lead- is there something to be afraid of? Ann. Acad. Med. Stetin. 2010, 56, 118–128. [Google Scholar] [PubMed]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead uptake, toxicity, and detoxification in plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: Berlin, Germany, 2011; Volume 213, pp. 113–136. ISBN 978-1-4419-9859-0. [Google Scholar]
- Morkunas, I.; Marczak, Ł.; Stachowiak, J.; Stobiecki, M. Sucrose-induced lupine defense against Fusarium oxysporum: Sucrose-stimulated accumulation of isoflavonoids as a defense response of lupine to Fusarium oxysporum. Plant Physiol. Biochem. 2005, 43, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zheng, Z.; Huang, J.; Lai, Z.; Fan, B. Biosynthesis of salicylic acid in plants. Plant Signal. Behav. 2009, 4, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Pan, Q.; Yang, H.; Zhan, J.; Huang, W. The plasma membrane H+-ATPase is related to the development of salicylic acid-induced thermotolerance in pea leaves. Planta 2009, 229, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Bostock, R.M. Interactions between abscisic acid-mediated responses and plant resistance to pathogens and insects. Ecology 2004, 85, 48–58. [Google Scholar] [CrossRef]
- Metwally, A.; Finkermeier, I.; Georgi, M.; Dietz, K.J. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol. 2003, 132, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Horvath, E.; Szalai, G.; Janda, T. Induction of abiotic stress tolerance by salicylic acid signalling. J. Plant Growth Reg. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Leng, P.; Yuan, B.; Guo, Y.; Chen, P. The role of abscisic acid in fruit ripening and responses to abiotic stress. J. Exp. Bot. 2014, 65, 4577–4588. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed]
- Pickett, J.A.; Poppy, G.M. Switching on plant genes by external chemical signals. Trends Plant Sci. 2001, 6, 137–139. [Google Scholar] [CrossRef]
- Ton, J.; Flors, V.; Mauch-Mani, B. The multifacede role of ABA in disease resistance. Trends Plant Sci. 2009, 14, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Jasmonate and salicylate induce expression of herbivore cytochrome P450 genes. Nature 2002, 419, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; He, Z.; Yang, X. Role of soil rhizobacteria in phytoremediation of heavy metal contaminated soils. J. Zhejiang Univ. Sci. B 2007, 192–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, C.; Li, L.P.; Sun, Z.Y. Effects of exogenous salicylic acid on growth and H2O2– metabolizing enzymes in rice seedlings under lead stress. J. Environ. Sci. 2007, 19, 44–49. [Google Scholar] [CrossRef]
- López-Orenes, A.; Martinez-Pérez, A.; Calderón, A.A.; Ferrer, M.A. Pb-induced response in Zygophyllum fabago plants are organ-dependent and modulated by salicylic acid. Plant Physiol. Biochem. 2014, 84, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, E.M.F.; Mahfouz, H.M. Effects of salicylic acid elicitor against aphids on wheat and detection of infestation using infrared thermal imaging technique in Ismailia. Pestic. Phytomed. (Belgrade) 2015, 30, 91–97. [Google Scholar] [CrossRef]
- El-Khawas, S.A. Priming Pisum sativum with salicylic acid against the leafminer Liriomyza trifolii. Afr. J. Agric. Res. 2012, 34, 4731–4737. [Google Scholar]
- Janda, T.; Gondor, O.K.; Yordanova, R.; Szalai, G.; Pál, M. Salicylic acid and photosynthesis: Signalling and effects. Acta Physiol. Plant. 2014, 36, 2537–2546. [Google Scholar] [CrossRef] [Green Version]
- Krantev, A.; Yordanova, R.; Janda, T.; Szalai, G.; Popova, L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 2008, 165, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Zengin, F. Exogenous treatment with salicylic acid alleviating copper toxicity in bean seedlings. Proc. Natl. Acad. Sci. India Sec. B Biol. Sci. 2014, 84, 749–755. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Yang, S.; Chen, Y. Salicylic acid alleviates cadmium-induced inhibition of growth and photosynthesis through upregulating antioxidant defense system in two melon cultivars (Cucumis melo L.). Protoplasma 2015, 252, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Horváth, E.; Pál, M.; Szalai, G.; Páldi, E.; Janda, T. Exogenous 4-hydroxybenzoic acid and salicylic acid modulate the effect of short-term drought and freezing stress on wheat plants. Biol. Plant. 2007, 51, 480–487. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in Plants. CRC Crit. Rev. Plant. Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Tuteja, N. Abscisic acid and abiotic stress signaling. Plant Signal. Behav. 2007, 2, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Danquah, A.; de Zelicourt, A.; Colcombet, J.; Hirt, H. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol. Adv. 2014, 32, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Rauser, W.E.; Dumbroff, E.B. Effects of excess cobalt, nickel and zinc on the water relations of Phaseolus vulgaris. Environ. Exp. Bot. 1981, 21, 249–255. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Gunsé, B.; Barceló, J. Influence of cadmium on water relations, stomatal resistance, and abscisic acid content in expanding bean leaves. Plant Physiol. 1989, 90, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Hollenbach, B.; Schreiber, L.; Hartung, W.; Dietz, K.J. Cadmium leads to stimulated expression of the lipid transfer protein genes in barley: Implications for the involvement of lipid transfer proteins in wax assembly. Planta 1997, 203, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Stroiński, A.; Giżewska, K.; Zielezińska, M. Abscisic acid is required in transduction of cadmium signal to potato roots. Biol. Plant. 2013, 57, 121–127. [Google Scholar] [CrossRef]
- Fediuc, E.; Lips, H.; Erdei, L. O-acetylserine (thiol) lyase activity in Pragmites and Typha plants under cadmium and NaCl stress conditions and the involvement of ABA in the stress response. J. Plant. Physiol. 2005, 162, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Stroiński, A.; Chadzinikolau, T.; Giżewska, K.; Zielezińska, M. ABA or cadmium induced phytochelatin synthesis in potato tubers. Biol. Plant. 2010, 54, 117–120. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Khan, A.L.; Kim, D.-H.; Lee, S.-Y.; Kim, K.-M.; Waqas, M.; Jung, H.-Y.; Shin, J.-H.; Kim, J.-G.; Lee, I.-J. Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant. Biol. 2014, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Munzuro, Ö.; Fikriye, K.Z.; Yahyagil, Z. The abscisic acid levels of wheat (Triticum aestivum L. cv. Çakmak 79) seeds that were germinated under heavy metal (Hg++, Cd++, Cu++) stress. J. Sci. 2008, 21, 1–7. [Google Scholar]
- Wang, Y.; Wang, Y.; Kai, W.; Zhao, B.; Chen, P.; Sun, L.; Ji, K.; Li, Q.; Dai, S.; Sun, Y.; et al. Transcriptional regulation of abscisic acid signal core components during cucumber seed germination and under Cu2+, Zn2+, NaCl and simulated acid rain stresses. Plant Physiol. Biochem. 2014, 76, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Atici, Ö.; Ağar, G.; Battal, P. Changes in phytohormone contents in chickpea seeds germinating under lead or zinc stress. Biol. Plant. 2005, 49, 215–222. [Google Scholar] [CrossRef]
- Monni, S.; Uhlig, C.; Hansen, E.; Magel, E. Ecophysiological responses of Empetrum nigrum to heavy metal pollution. Environ. Pollut. 2001, 112, 121–129. [Google Scholar] [CrossRef]
- Jeong, S.; Goto-Yamamoto, N.; Kobayashi, S.; Esaka, M. Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Sci. 2004, 167, 247–252. [Google Scholar] [CrossRef]
- Wheeler, S.; Loveys, B.; Ford, C.; Davies, C. The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Aust. J. Grape Wine Res. 2009, 15, 195–204. [Google Scholar] [CrossRef]
- Giribaldi, M.; Geny, L.; Delrot, S.; Schubert, A. Proteomic analysis of the effects of ABA treatments on ripening Vitis vinifera berries. J. Exp. Bot. 2010, 61, 2447–2458. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Sadamatsu, K.; Goto-Yamamoto, N. Abscisic acid stimulated ripening and gene expression in berry skins of the Cabernet Sauvignon grape. Funct. Integr. Genom. 2010, 10, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Cantín, C.M.; Fidelibus, M.W.; Crisosto, C.H. Application of abscisic acid (ABA) at veraison advanced red color development and maintained postharvest quality of ‘Crimson Seedless’ grapes. Postharvest Biol. Technol. 2007, 46, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Woźniak, A.; Formela, M.; Bilman, P.; Grześkiewicz, K.; Bednarski, W.; Marczak, Ł.; Narożna, D.; Dancewicz, K.; Mai, V.Ch.; Borowiak-Sobkowiak, B.; et al. The dynamics of the defense strategy of pea induced by exogenous nitric oxide in response to aphid infestation. Int. J. Mol. Sci. 2017, 18, 329. [Google Scholar] [CrossRef] [PubMed]
- Sytykiewicz, H. Influence of bird cherry-oat aphid (Rhaphalosiphum padi (Linnaeus, 1758))/Hemiptera, Aphidoidea/feeding on the activity of β-glucosidase within tissues of its primary host. Aphids Hem. Insects 2008, 14, 155–164. [Google Scholar]
- Harborne, J.B. Nature, distribution and function of plant flavonoids. In Plant Flavonoids in Biology and Medicine: Biochemical, Pharmacological, and Structure-Activity Relationships. Progress in Clinical and Biological Research; Cody, V., Middleton, E., Harborne, J., Eds.; Alan R. Liss: New York, NY, USA, 1978; p. 1. ISBN 0-8451-5063-4. [Google Scholar]
- Jeandet, P. Phytoalexins: Current progress and future prospects. Molecules 2015, 20, 2770–2774. [Google Scholar] [CrossRef]
- Jeandet, P.; Clément, C.; Courut, E.; Cordelier, S. Modulation of phytoalexin biosynthesis in engineered plants for disease resistance. Int. J. Mol. Sci. 2013, 14, 14136–14170. [Google Scholar] [CrossRef] [PubMed]
- Adrian, M.; Jeandet, P.; Douillet-Breuil, A.C.; Tesson, L.; Bessis, R. Stilbene content of mature Vitis vinifera berries in response to UV-C elicitation. J. Agric. Food Chem. 2000, 48, 6103–6105. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, S.; Singh, V.P.; Prasad, S.M.; Maurya, J.N. Modulation of manganese toxicity in Pisum sativum L. seedlings by kinetin. Sci Hortic. 2010, 126, 467–474. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, G.H.; Liu, P.; Song, J.M.; Xu, G.D.; Cai, M.Z. Morphological and physiological responses of root tip cells to Fe2+ toxicity in rice. Acta Physiol Plant. 2011, 33, 683–689. [Google Scholar] [CrossRef]
- Gautam, S.; Anjani, K.; Srivastava, N. In vitro evaluation of excess copper affecting seedlings and their biochemical characteristics in Carthamus tinctorius L. (variety PBNS−12). Physiol. Mol. Biol. Plants. 2016, 22, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.V.; Kartashov, A.V.; Ivanova, A.I.; Savochkin, Y.V.; Kuznetsov, V.V. Effects of zinc on Scots pine (Pinus sylvestris L.) seedlings grown in hydroculture. Plant Physiol. Biochem. 2016, 102, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Kalaji, H.M.; Jajoo, A. Investigation of deleterious effects of chromium phytotoxicity and photosynthesis in wheat plant. Photosynthetica 2016, 54, 1–9. [Google Scholar] [CrossRef]
- Gwóźdź, E.A.; Przymusiński, R.; Rucińska, R.; Deckert, J. Plant responses to heavy metals: Molecular and physiological aspects. Acta Physiol. Plant. 1997, 19, 459–465. [Google Scholar] [CrossRef]
- Rucińska, R.; Waplak, S.; Gwóźdź, E. Free radical formation and activity of antioxidant enzymes in lupin roots exposed to lead. Plant. Physiol. Biochem. 1999, 37, 187–194. [Google Scholar] [CrossRef]
- Małecka, A.; Piechalak, A.; Tomaszewska, B. Reactive oxygen species production and antioxidative defense system in pea root tissues treated with lead ions: The whole roots level. Acta Physiol. Plant. 2009, 31, 1053–1063. [Google Scholar] [CrossRef]
- Shahid, M.; Pinelli, E.; Dumat, C. Review of Pb availability and toxicity to plants in relations with metal speciation; role of synthetic and natural organic ligands. J. Hazard. Mater. 2012, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Abbas, N.; Arshad, F.; Akram, M.; Khan, Z.I.; Ahmad, K.; Mansha, M.; Mirzaei, F. Effects of diverse doses of lead (Pb) on different growth attributes of Zea mays L. Agric. Sci. 2013, 4, 262–265. [Google Scholar]
- Malar, S.; Vikram, S.S.; Favas, P.J.C.; Perumal, V. Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhornia crassipes (Mart.)]. Bot. Stud. 2014, 55, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Małecka, A.; Piechalak, A.; Morkunas, I.; Tomaszewska, B. Accumulation of lead in root cells of Pisum sativum. Acta Physiol. Plant. 2008, 30, 629–637. [Google Scholar] [CrossRef]
- Krzesłowska, M.; Rabęda, I.; Basińska, A.; Lewandowski, M.; Mellerowicz, E.J.; Napieralska, A.; Samardakiewicz, S.; Woźny, A. Pectinous cell wall thickenings fotmation—A common defense of plants to cope with Pb. Environ. Pollut. 2016, 214, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Rabęda, I.; Bilski, H.; Mellerowicz, E.J.; Napieralska, A.; Suski, Sz.; Woźny, A.; Krzesłowska, M. Colocalization of low-methylesterified pectins and Pb deposits in the apoplast of Aspen roots exposed to lead. Environ. Pollut. 2015, 205, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Erofeeva, E.A. Hormesis and paradoxical effects of wheat seedling (Triticum Aestivum L.) Parameters upon exposure to different pollutants in a wide range of doses. Dose-Response 2014, 12, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Liu, Z.; Chen, W.; Ye, Y.; Yu, S.; He, H. Hormesis effects induced by cadmium on growth and photosyntheticperformance in hypperaccumulator, Jonicera japonica Thunb. J. Plant Growth Regul. 2015, 34, 13–21. [Google Scholar] [CrossRef]
- Görür, G. Effects of heavy metals accumulation in host plants to cabbage aphid (Brevicoryne brassicae)—morphology. Ekologia (Bratislava) 2006, 25, 314–321. [Google Scholar]
- Görür, G. Developmental instability in cabbage aphid (Brevicoryne brassicae) populations exposed to heavy metal accumulated host plants. Ecol. Indic. 2006, 6, 743–748. [Google Scholar] [CrossRef]
- Mai, V.C.; Bednarski, W.; Borowiak-Sobkowiak, B.; Wilkaniec, B.; Samardakiewicz, S.; Morkunas, I. Oxidative stress in pea seedling leaves in response to Acyrthosiphon pisum infestation. Phytochemistry 2013, 93, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Yalpani, N.; León, J.; Lawton, M.A.; Raskin, I. Pathway of salicylic acid biosynthesis in healthy and virus-inoculated tobacco. Plant Physiol. 1993, 103, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Vine, J.H.; Niton, D.; Plummer, J.A.; Baleriola-Lucas, C.; Mullins, M.G. Simultaneous quantitation of indole-3-acetic acid and abscisic acid in small samples of plant tissue by gas chromatography/mass spectrometry/selected ion monitoring. Plant Physiol. 1987, 85, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Formela, M.; Floryszak-Wieczorek, J.; Marczak, Ł.; Narożna, D.; Nowak, W.; Bednarski, W. Cross-talk interactions of exogenous nitric oxide and sucrose modulates phenylpropanoid metabolism in yellow lupine embryo axes infected with Fusarium oxysporum. Plant Sci. 2013, 211, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Stobiecki, M.; Wojtaszek, P.; Gulewicz, K. Application of solid phase extraction for profiling of quinolisidine alkaloids and phenolic compounds in Lupinus albus. Phytochem. Anal. 1997, 8, 153–158. [Google Scholar] [CrossRef]
- Morkunas, I.; Stobiecki, M.; Marczak, Ł.; Stachowiak, J.; Narożna, D.; Remlein-Starosta, D. Changes in carbohydrate and isoflavonoid metabolism in yellow lupine in response to infection by Fusarium oxysporum during the stages of seed germination and early seedling growth. Physiol. Mol. Plant Pathol. 2010, 75, 46–55. [Google Scholar] [CrossRef]
- Nichols, E.J.; Beckman, J.M.; Hadwiger, L.A. Glycosidic enzyme activity in pea tissue and pea-Fusarium solani interactions. Plant Physiol. 1980, 66, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Kozłowska, M.; Ratajczak, L.; Marczak, Ł. Role of sucrose in the development of Fusarium wilt in lupine embryo axes. Physiol. Mol. Plant Pathol. 2007, 70, 25–37. [Google Scholar] [CrossRef]
- Cahill, D.M.; McComb, J.A. A comparison of changes in phenylalanine ammonia-lyase activity, lignin and phenolic synthesis in the roots of Eucalyptus calophylla (field resistant) and E. marginata (susceptible) when infected with Phytophtora cinnamon. Physiol. Mol. Plant Pathol. 1992, 40, 315–332. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available |




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźniak, A.; Drzewiecka, K.; Kęsy, J.; Marczak, Ł.; Narożna, D.; Grobela, M.; Motała, R.; Bocianowski, J.; Morkunas, I. The Influence of Lead on Generation of Signalling Molecules and Accumulation of Flavonoids in Pea Seedlings in Response to Pea Aphid Infestation. Molecules 2017, 22, 1404. https://doi.org/10.3390/molecules22091404
Woźniak A, Drzewiecka K, Kęsy J, Marczak Ł, Narożna D, Grobela M, Motała R, Bocianowski J, Morkunas I. The Influence of Lead on Generation of Signalling Molecules and Accumulation of Flavonoids in Pea Seedlings in Response to Pea Aphid Infestation. Molecules. 2017; 22(9):1404. https://doi.org/10.3390/molecules22091404
Chicago/Turabian StyleWoźniak, Agnieszka, Kinga Drzewiecka, Jacek Kęsy, Łukasz Marczak, Dorota Narożna, Marcin Grobela, Rafał Motała, Jan Bocianowski, and Iwona Morkunas. 2017. "The Influence of Lead on Generation of Signalling Molecules and Accumulation of Flavonoids in Pea Seedlings in Response to Pea Aphid Infestation" Molecules 22, no. 9: 1404. https://doi.org/10.3390/molecules22091404






