7-Dialkylaminocoumarin Oximates: Small Molecule Fluorescent “Turn-On” Chemosensors for Low-Level Water Content in Aprotic Organic Solvents
Abstract
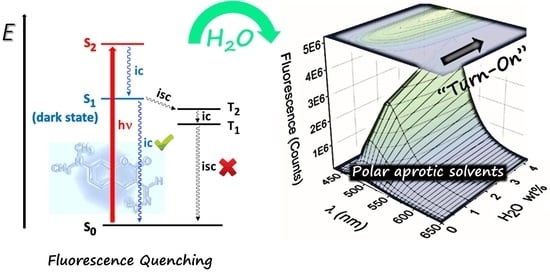
:1. Introduction
2. Results and Discussion
2.1. Spectral Characteristics
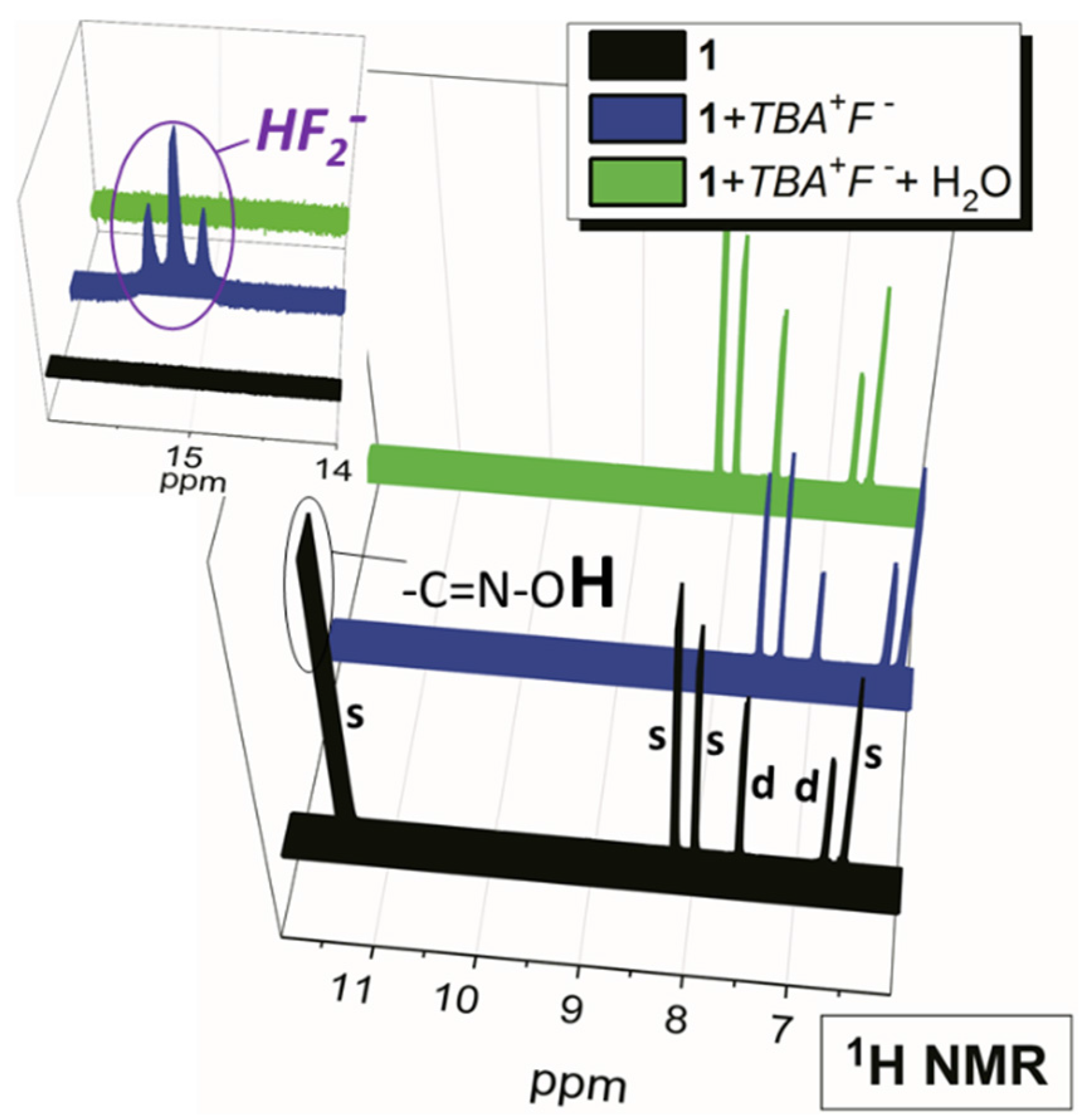
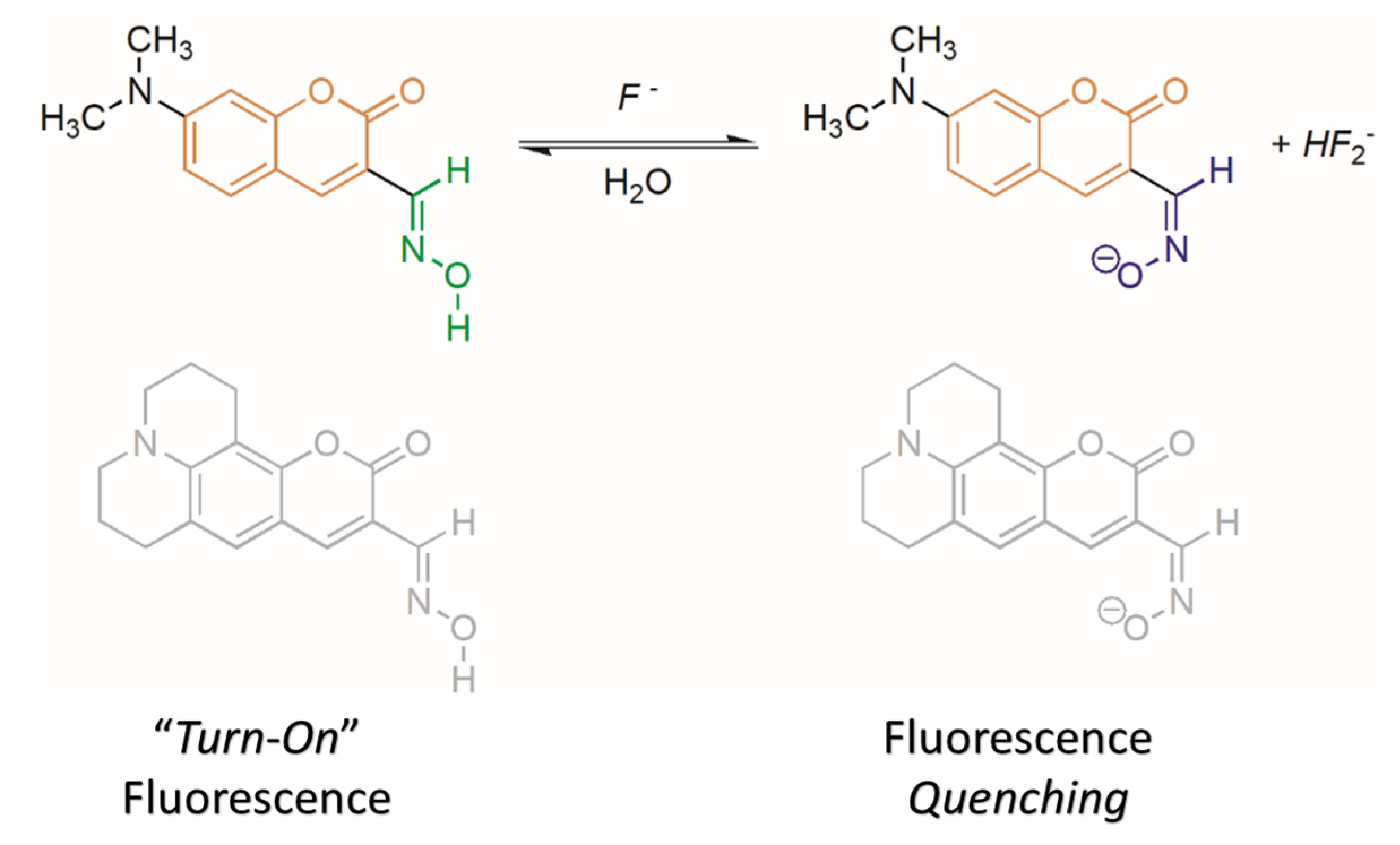
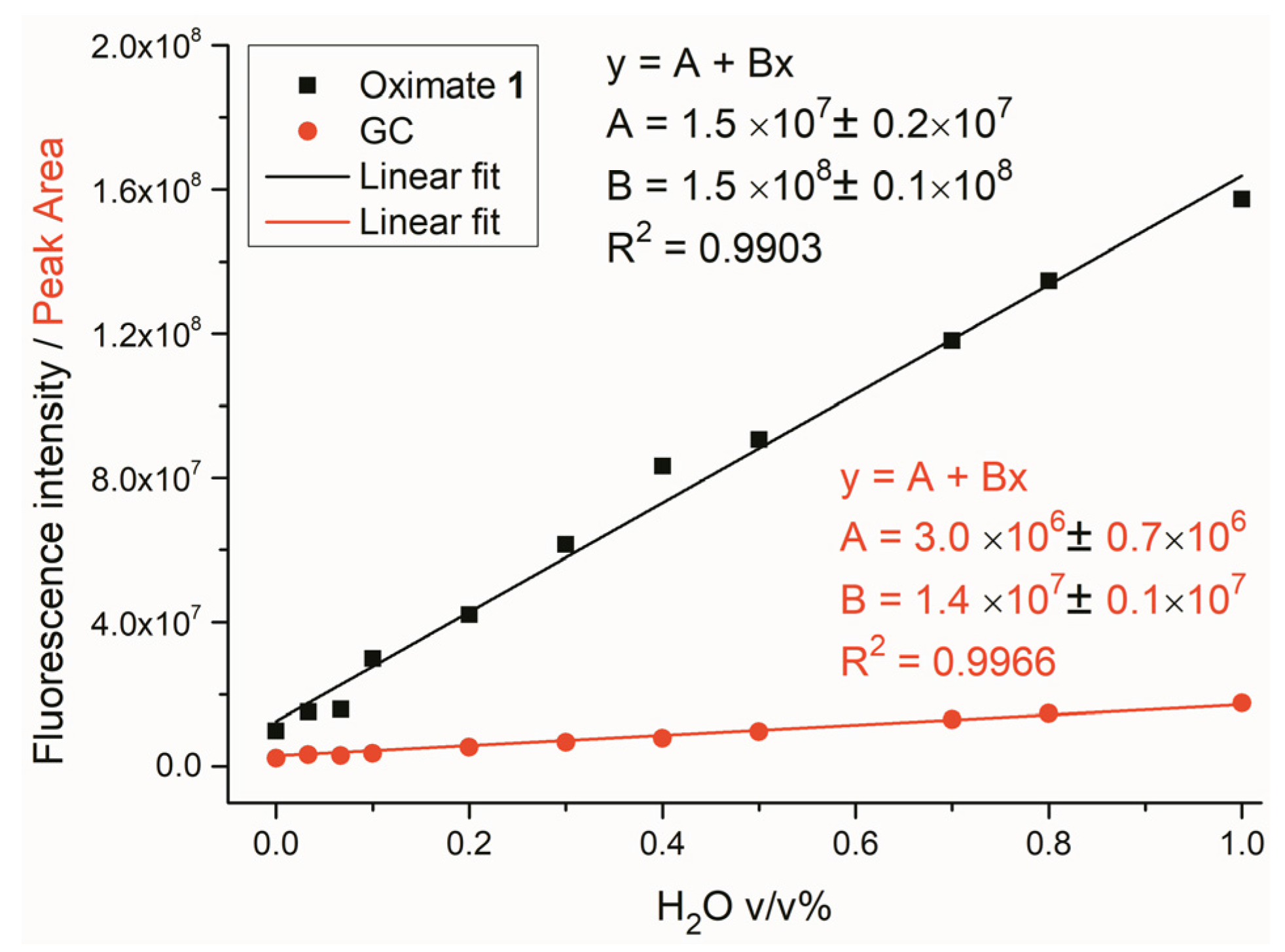
2.2. Water Sensing
3. Materials and Methods
3.1. General Information
3.2. Synthesis
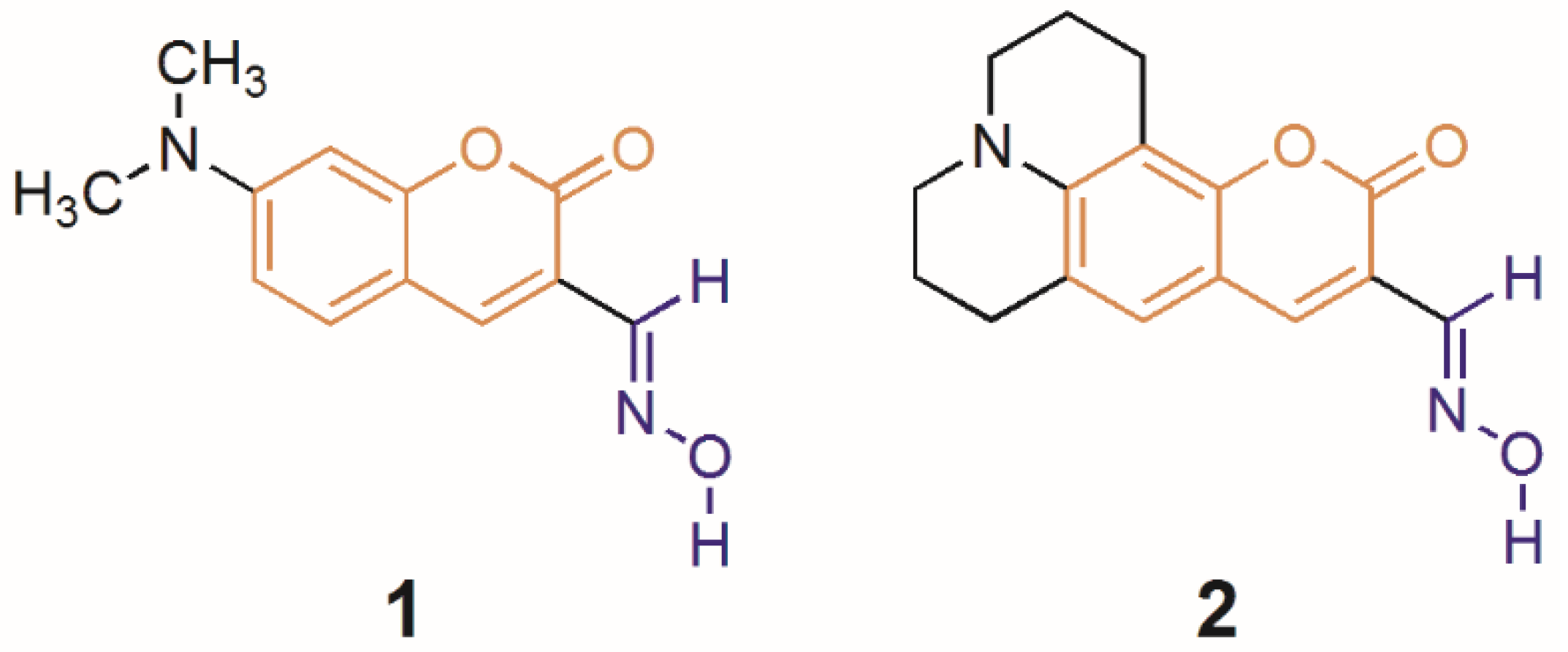
3.2.1. Synthesis of Coumarin Oximes 1 and 2
3.2.2. Product Characterization
3.3. Spectroscopic Measurements
3.4. Titration Experiments
3.4.1. Materials
3.4.2. General Method
3.4.3. Detection and Quantification Limits
3.5. Quantum-Chemical Calculations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling Recognition Events with Fluorescent Sensors and Switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.M.F.; Tse, O.L. Humidity-sensitive optode membrane based on a fluorescent dye immobilized in gelatin film. Anal. Chim. Acta 1999, 378, 127–134. [Google Scholar] [CrossRef]
- Citterio, D.; Minamihashi, K.; Kuniyoshi, Y.; Hisamoto, H.; Sasaki, S.; Suzuki, K. Optical determination of low-level water concentrations in organic solvents using fluorescent acridinyl dyes and dye-immobilized polymer membranes. Anal. Chem. 2001, 73, 5339–5345. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.-G.; Guan, A.-L.; Zeng, G.-M.; Liu, Y.-G.; Li, Z.-W. Fluorescence water sensor based on covalent immobilization of chalcone derivative. Anal. Chim. Acta 2006, 577, 264–270. [Google Scholar] [CrossRef] [PubMed]
- de Silva, A.P.; Moody, T.S.; Wright, G.D. Fluorescent PET (Photoinduced Electron Transfer) sensors as potent analytical tools. Analyst 2009, 134, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Luo, F.; Chen, X.; Yao, W.; Yin, J.; Yao, Z.; Wang, L. Fluorometric determination of water in organic solvents using europium ion-based luminescent nanospheres. Microchim. Acta 2009, 166, 163–167. [Google Scholar] [CrossRef]
- Niu, C.; Li, L.; Qin, P.; Zeng, G.; Zhang, Y. Determination of water content in organic solvents by naphthalimide derivative fluorescent probe. Anal. Sci. 2010, 26, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, V.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Y.; Peng, X.; Yoon, J. Fluorescent and colorimetric probes for detection of thiols. Chem. Soc. Rev. 2010, 39, 2120–2135. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Han, Y.K.; Kang, J. A new chromogenic water sensing system utilizing deprotonation and protonation of anion receptor. Bull. Korean Chem. Soc. 2011, 32, 4244–4246. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Ge, J.; Zhang, H.; Wang, P. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 2011, 40, 3483–3495. [Google Scholar] [CrossRef] [PubMed]
- Karuppannan, S.; Chambron, J.-C. Supramolecular chemical sensors based on pyrene monomer-excimer dual luminescence. Chem. Asian J. 2011, 6, 964–984. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Li, Y.; Wu, J.; Liu, Y.; Fang, G.; Wang, S.; Zhang, Y. Highly sensitive fluorescent sensing for water based on poly(m-aminobenzoic acid). Chem. Commun. 2012, 48, 3009–3011. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ji, S.; Chen, Y.; Guo, H.; Yang, P. Excited state intramolecular proton transfer (ESIPT): From principal photophysics to the development of new chromophores and applications in fluorescent molecular probes and luminescent materials. Phys. Chem. Chem. Phys. 2012, 14, 8803–8817. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, M.G.; Im, H.G.; Ahn, S.; Shim, I.W.; Chang, S.K. Chromogenic signalling of water content in organic solvents by hydrazine-acetate complexes. Dyes Pigm. 2012, 92, 1199–1203. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, T.; Wang, G.; Chang, X.; Xue, D.; Belfield, K.D.; Fang, Y. A butterfly-shaped pyrene derivative of cholesterol and its uses as a fluorescent probe. J. Phys. Chem. 2013, 117, 5659–5667. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Lee, W.-J.; Kim, J.N.; Kim, H.J. An off-on fluorescent sensor for detecting a wide range of water content in organic solvents. Bull. Korean Chem. Soc. 2013, 34, 2261–2266. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Li, X.; Ågren, H.; Su, J. Highly sensitive detection of low-level water content in organic solvents and cyanide in aqueous media using novel solvatochromic AIEE fluorophores. RSC Adv. 2015, 5, 12191–12201. [Google Scholar] [CrossRef]
- Huang, D.; Bing, Y.; Hong, W.; Lai, C.; Guo, Q.; Niu, C. An optical-fiber sensor based on time-gated fluorescence for detecting water content in organic solvents. Anal. Methods 2015, 7, 4621–4628. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Li, M.; Liu, C.; Yang, W.; Liu, S.; Yang, C. Two sensitive fluorescent bopim probes with tunable tict character for low-level water detection in organic solvents. J. Fluoresc. 2016, 26, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, L.; Cao, D. The synthesis and highly sensitive detection of water content in THF using a novel solvatochromic AIE polymer containing diketopyrrolopyrrole and triphenylamine. New J. Chem. 2016, 40, 6706–6713. [Google Scholar] [CrossRef]
- Schäfel, K.; Ihmels, H. Ratiometric detection of water in acetonitrile with 9-hydroxybenzo[b]quinolizinium as fluorosolvatochromic probe. J. Fluoresc. 2017, 27, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Verwilst, P.; Kim, W.Y.; Kim, J.S. Fluorescent and colorimetric sensors for the detection of humidity or water content. Chem Soc. Rev. 2016, 45, 1242–1256. [Google Scholar] [CrossRef] [PubMed]
- Cigáň, M.; Gašpar, J.; Gáplovská, K.; Holekšiová, J.; Jakusová, K.; Donovalová, J.; Garaj, V.; Stankovičová, H. Coumarin phenylsemicarbazones: Sensitive colorimetric and fluorescent “turn-on” chemosensors for low-level water content in aprotic organic solvents. New J. Chem. 2016, 40, 8946–8953. [Google Scholar] [CrossRef]
- Moon, J.O.; Kim, Y.H.; Choi, M.G.; Chang, S.-K. Colorimetric signaling of water content in acetonitrile by phenolic dye-fluoride complexes. Bull. Korean Chem. Soc. 2011, 32, 3517–3520. [Google Scholar] [CrossRef]
- Wagner, B.D. The use of Coumarins as environmentally-sensitive fluorescent probes of heterogeneous inclusion systems. Molecules 2009, 14, 210–237. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cai, L.; Chen, Z. Coumarin-Derived Fluorescent Chemosensors. In Advances in Chemical Sensors; Wang, W., Ed.; InTech: Rijeka, Croatia, 2012; ISBN 978-953-307-792-5. [Google Scholar]
- Park, S.; Kim, H.-J. Highly selective chemodosimeter for cyanide based on a doubly activated Michael acceptor type of coumarin thiazole fluorophore. Sens. Actuator B Chem. 2012, 161, 317–321. [Google Scholar] [CrossRef]
- Kwon, H.; Lee, K.; Kim, H.-J. Coumarin-malonitrile conjugate as a fluorescence turn-on probe for biothiols and its cellular expression. Chem. Commun. 2011, 47, 1773–1775. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-K.; Lee, D.-N.; Kim, H.-J. Highly selective fluorescent sensor for homocysteine and cysteine. Tetrahedron Lett. 2008, 49, 4879–4881. [Google Scholar] [CrossRef]
- Kim, G.-J.; Kim, H.-J. Highly selective and sensitive fluorescence turn-on probe for proline. Tetrahedron Lett. 2010, 51, 4670–4672. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.-F.; Yang, S.-H.; Yang, W.-C.; Yang, G.-F. A Coumarin-based fluorescent probe for selective and sensitive detection of thiophenols and its application. Anal. Chem. 2014, 86, 3037–3042. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Xu, Q.; Liu, Y.; Wei, H.; Tang, Y.; Lin, W. Coumarin-based turn-on fluorescence probe for specific detection of glutathione over cysteine and homocysteine. ACS Appl. Mater. Interfaces 2015, 7, 12809–12813. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Li, J.; Yang, L.; Hou, C.; Ni, T.; Yang, Z.; Qian, X.; Li, C. The Synthesis of a coumarin carbohydrazide dinuclear copper complex based fluorescence probe and its detection of thiols. PLoS ONE 2016, 11, e0148026. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-Y.; Hsu, C.-Y.; Chen, W.-C.; Wei, L.-F.; Wu, S.-P. A hypochlorous acid turn-on fluorescent probe based on HOCl-promoted oxime oxidation and its application in cell imaging. Sens. Actuator B Chem. 2014, 196, 203–207. [Google Scholar] [CrossRef]
- Lee, S.K.; Choi, M.G.; Chang, S.-K. Signaling of chloramine: A fluorescent probe for trichloroisocyanuric acid based on deoximation of a coumarin oxime. Tetrahedron Lett. 2014, 55, 7047–7050. [Google Scholar] [CrossRef]
- Wallace, K.J.; Fagbemi, R.I.; Folmer-Andersen, F.J.; Morey, J.; Lynth, V.M.; Anslyn, E.V. Detection of chemical warfare simulants by phosphorylation of a coumarin oximate. Chem. Commun. 2006, 3886–3888. [Google Scholar] [CrossRef] [PubMed]
- Dale, T.J.; Rebek, J., Jr. Hydroxy oximes as organophosphorus nerve agent sensors. Angew. Chem. Int. Ed. 2009, 48, 7850–7852. [Google Scholar] [CrossRef] [PubMed]
- Donovalová, J.; Cigáň, M.; Stankovičová, H.; Gašpar, J.; Danko, M.; Gaplovský, A.; Hrdlovič, P. Spectral properties of substituted coumarins in solution and polymer matrices. Molecules 2012, 17, 3259–3276. [Google Scholar] [CrossRef] [PubMed]
- Escudero, D. Revising Intramolecular Photoinduced electron transfer (pet) from first-principles. Acc. Chem. Res. 2016, 49, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wu, Y.-W.; Chai, W.-X.; Tao, Y.-S.; Jiang, C.; Wang, Q.-H. Fluorescence quenching of a europium coordination compound for the detection of trace amounts of water: Uncovering the response mechanism by structural confirmation. Eur. J. Inorg. Chem. 2015, 2264–2271. [Google Scholar] [CrossRef]
- Rostami, A.; Taylor, M.S. Polymers for anion recognition and sensing. Macromol. Rapid Commun. 2012, 33, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, O.; Cassels, B.K.; Mena, N.; Nuñez, M.T.; Yañez, O.; Caballero, J. A coumarinylaldoxime as a specific sensor for Cu2+ and its biological application. Tetrahedron Lett. 2014, 55, 873–876. [Google Scholar] [CrossRef]
- Foster, J.P.; Weinhold, F. Natural hybrid orbitals. J. Am. Chem. Soc. 1980, 102, 7211–7218. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F. Natural localized molecular orbitals. J. Chem. Phys. 1985, 83, 1736–1740. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1998, 88, 899–926. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
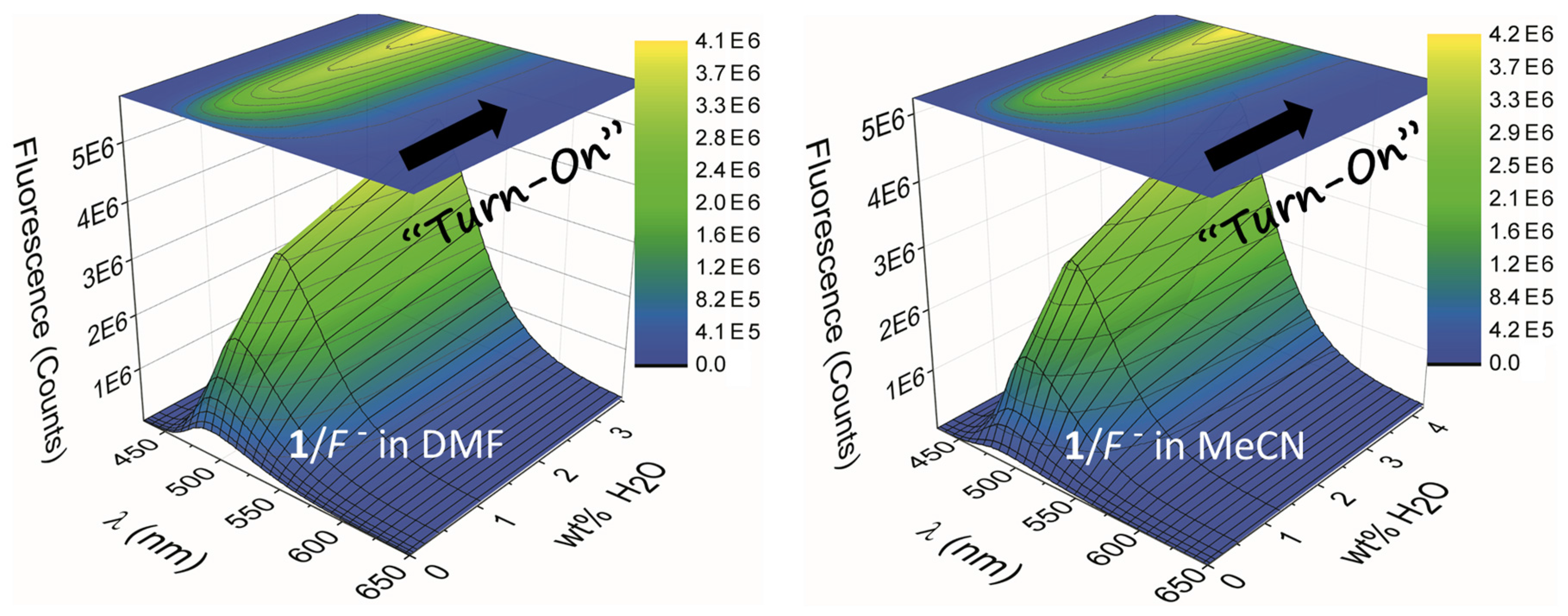



| Compd. | λA (nm) | Log εA | λF (nm) | ΦF | τ (ns) | χ2 |
|---|---|---|---|---|---|---|
| MeCN | ||||||
| 1 | 413 | 4.27 | 487 | 0.80 | τ1 = 1.6 (10%) | 1.111 |
| τ2 = 3.5 (90%) | ||||||
| 2 | 438 | 4.28 | 504 | 0.87 | τ1 = 0.5 (2%) | 1.050 |
| τ2 = 3.6 (98%) | ||||||
| DMF | ||||||
| 1 | 414 | 4.52 | 491 | 0.87 | τ1 = 0.3 (3%) | 1.171 |
| τ2 = 3.3 (97%) | ||||||
| 2 | 442 | 4.55 | 507 | 0.95 | τ1 = 0.5 (2%) | 1.196 |
| τ2 = 3.6 (98%) | ||||||
| DMSO | ||||||
| 1 | 419 | 4.54 | 497 | 0.88 | τ1 = 1.5 (8%) | 1.007 |
| τ2 = 3.3 (92%) | ||||||
| 2 | 442 | 4.51 | 512 | 1.00 | τ = 3.6 (100%) | 1.130 |
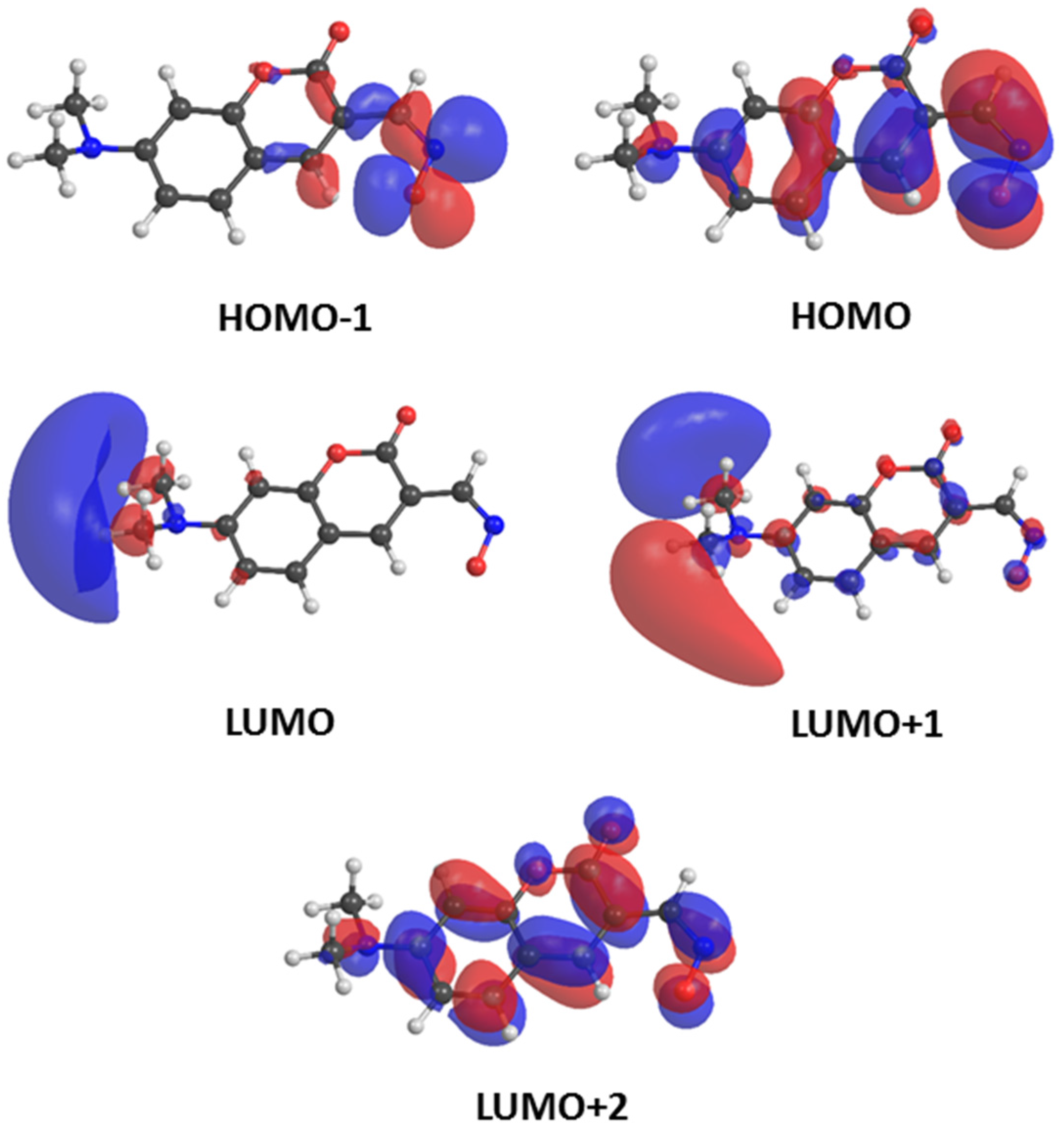
| 7-Dimethylaminocoumarin Oximate 1 | ||||
|---|---|---|---|---|
| Excited State | Orbital Contributions | Energy | Oscillator Strength | |
| [eV] | [nm] | |||
| T1 | HOMO → LUMO (2%) | 1.31 | 946 | 0.0000 |
| HOMO → LUMO+1 (10%) | ||||
| HOMO → LUMO+2 (77%) | ||||
| T2 | HOMO−1 → LUMO+1 (7%) | 1.36 | 914 | 0.0000 |
| HOMO−1 → LUMO+2 (54%) | ||||
| S1 | HOMO−1 → LUMO+1 (8%) | 1.80 | 686 | 0.0003 |
| HOMO−1 → LUMO+2 (61%) | ||||
| S2 | HOMO → LUMO (12%) | 3.06 | 406 | 0.5360 |
| HOMO → LUMO+1 (10%) | ||||
| HOMO → LUMO+2 (73%) | ||||
| LOD (3σ/S) | LOQ (10σ/S) | |||
|---|---|---|---|---|
| wt % | v/v % | wt % | v/v % | |
| MeCN | ||||
| 1/F− | 0.0014 | 0.0012 | 0.0048 | 0.0039 |
| 2/F− | 0.0175 * | 0.0137 * | 0.0583 * | 0.0457 * |
| DMF | ||||
| 1/F− | 0.0059 | 0.0046 | 0.0196 | 0.0153 |
| 2/F− | 0.0046 | 0.0044 | 0.0154 | 0.0146 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cigáň, M.; Horváth, M.; Filo, J.; Jakusová, K.; Donovalová, J.; Garaj, V.; Gáplovský, A. 7-Dialkylaminocoumarin Oximates: Small Molecule Fluorescent “Turn-On” Chemosensors for Low-Level Water Content in Aprotic Organic Solvents. Molecules 2017, 22, 1340. https://doi.org/10.3390/molecules22081340
Cigáň M, Horváth M, Filo J, Jakusová K, Donovalová J, Garaj V, Gáplovský A. 7-Dialkylaminocoumarin Oximates: Small Molecule Fluorescent “Turn-On” Chemosensors for Low-Level Water Content in Aprotic Organic Solvents. Molecules. 2017; 22(8):1340. https://doi.org/10.3390/molecules22081340
Chicago/Turabian StyleCigáň, Marek, Miroslav Horváth, Juraj Filo, Klaudia Jakusová, Jana Donovalová, Vladimír Garaj, and Anton Gáplovský. 2017. "7-Dialkylaminocoumarin Oximates: Small Molecule Fluorescent “Turn-On” Chemosensors for Low-Level Water Content in Aprotic Organic Solvents" Molecules 22, no. 8: 1340. https://doi.org/10.3390/molecules22081340