Sequestration Effect on the Open-Cyclic Switchable Property of Warfarin Induced by Cyclodextrin: Time-Resolved Fluorescence Study
Abstract
:1. Introduction
2. Results and Discussion
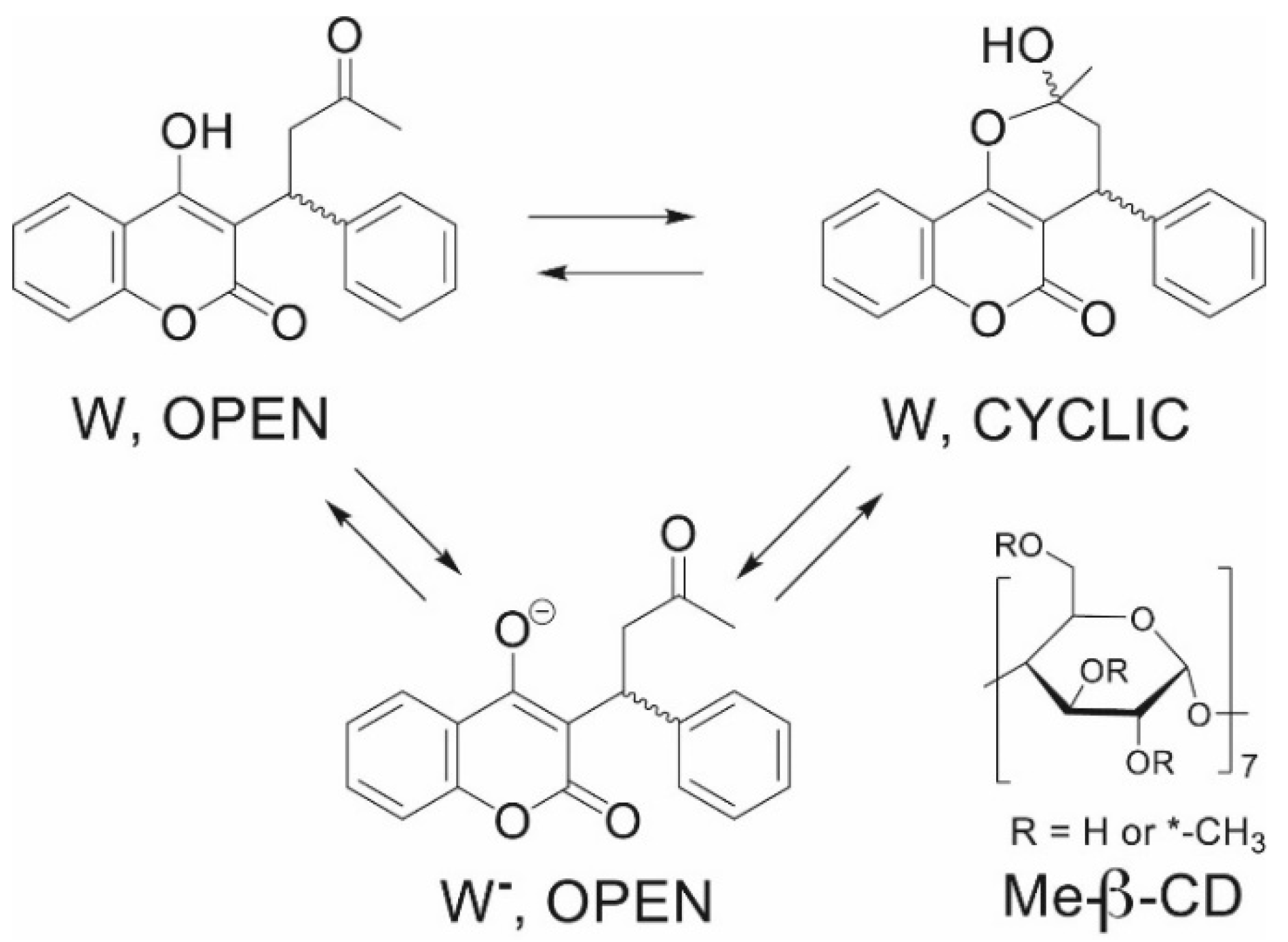
2.1. Interactions of Warfarin with Cyclodextrins
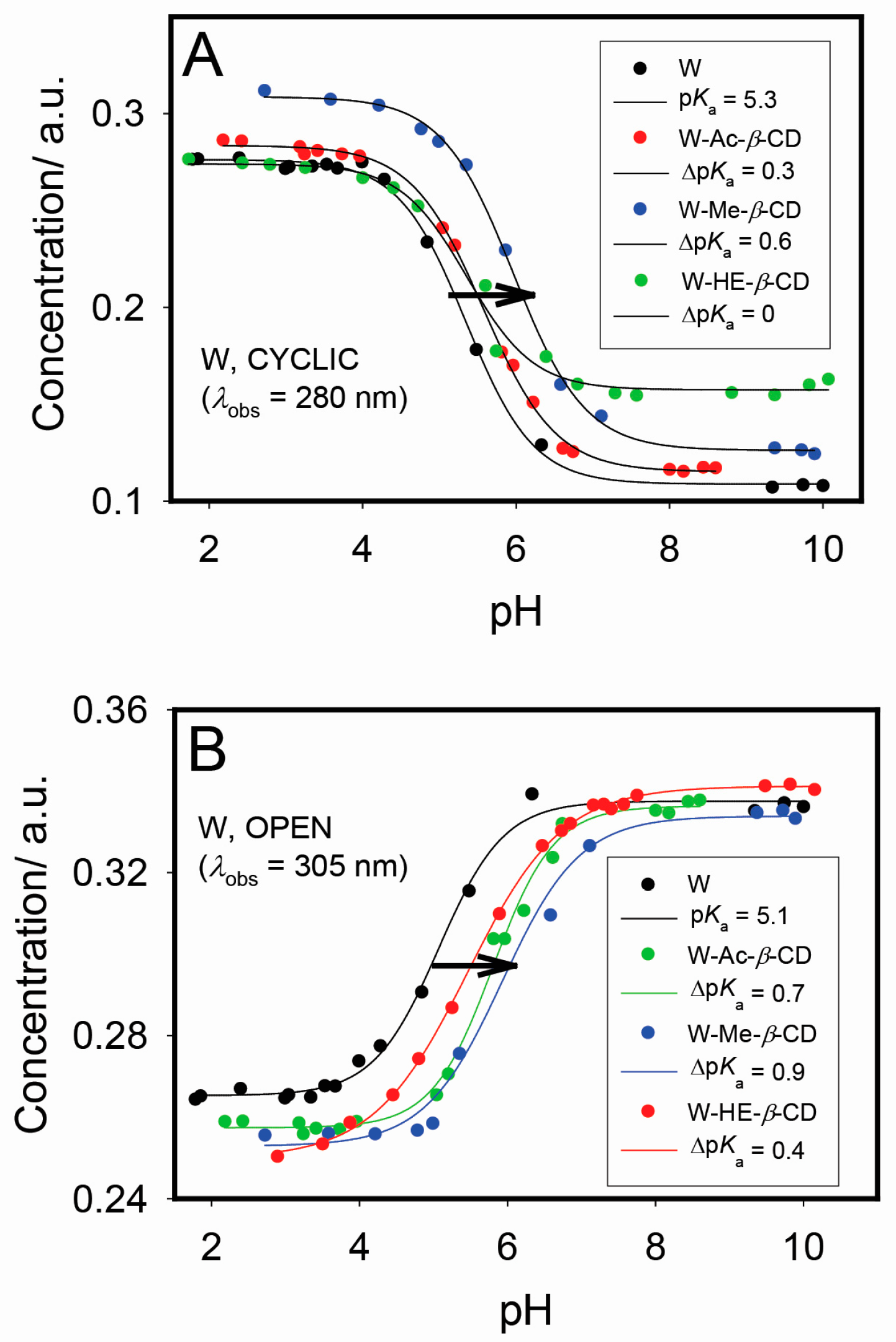
2.2. Optical Measurements and Host-Induced pKa Shifts
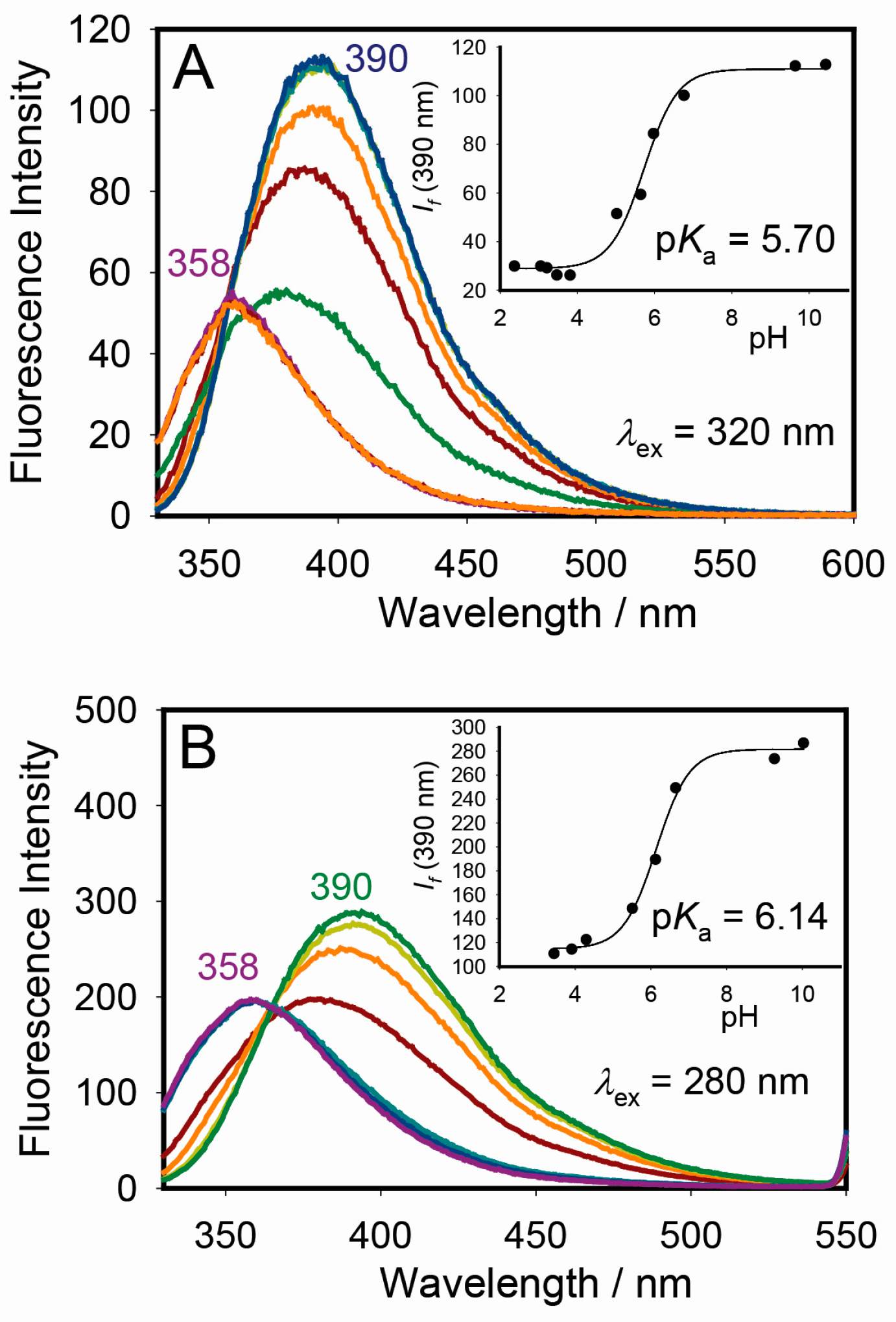
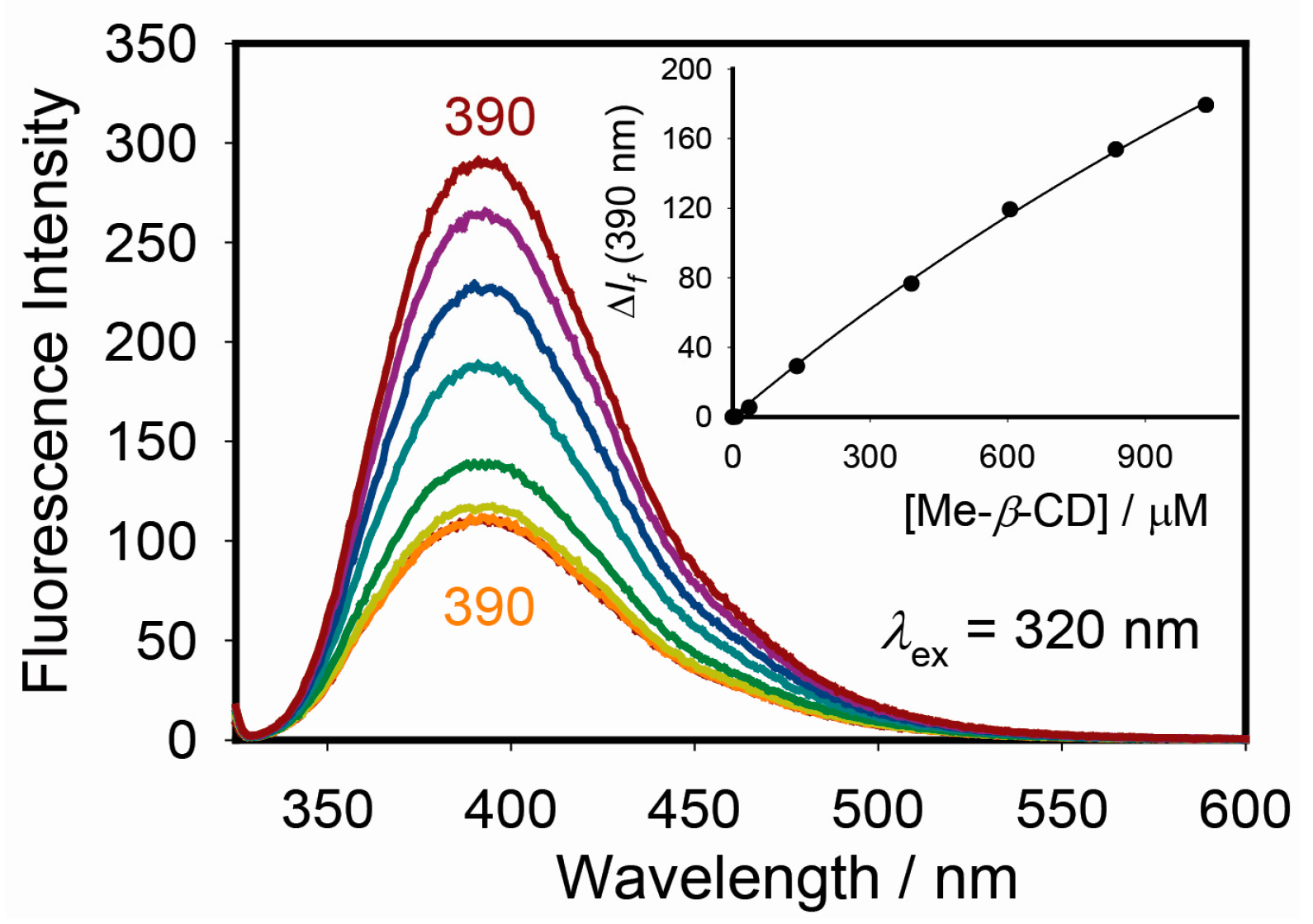
2.3. Excitation, pH, and Cyclodextrin Dependence of Warfarin Steady-State Fluorescence
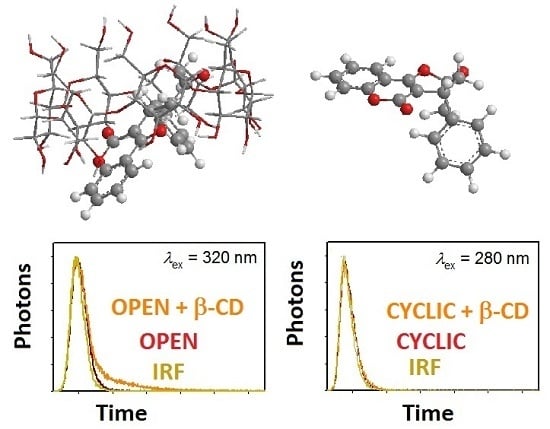
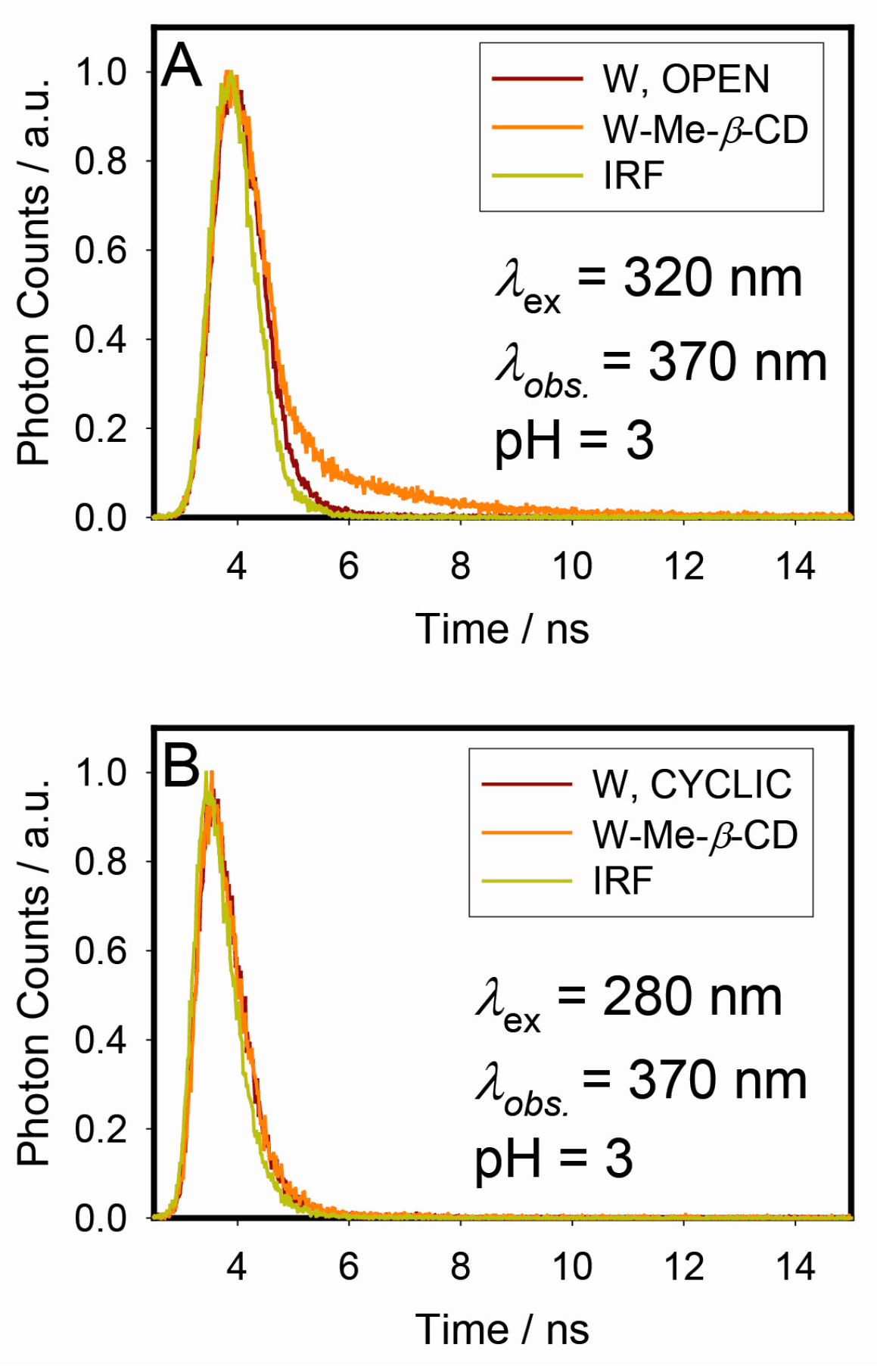
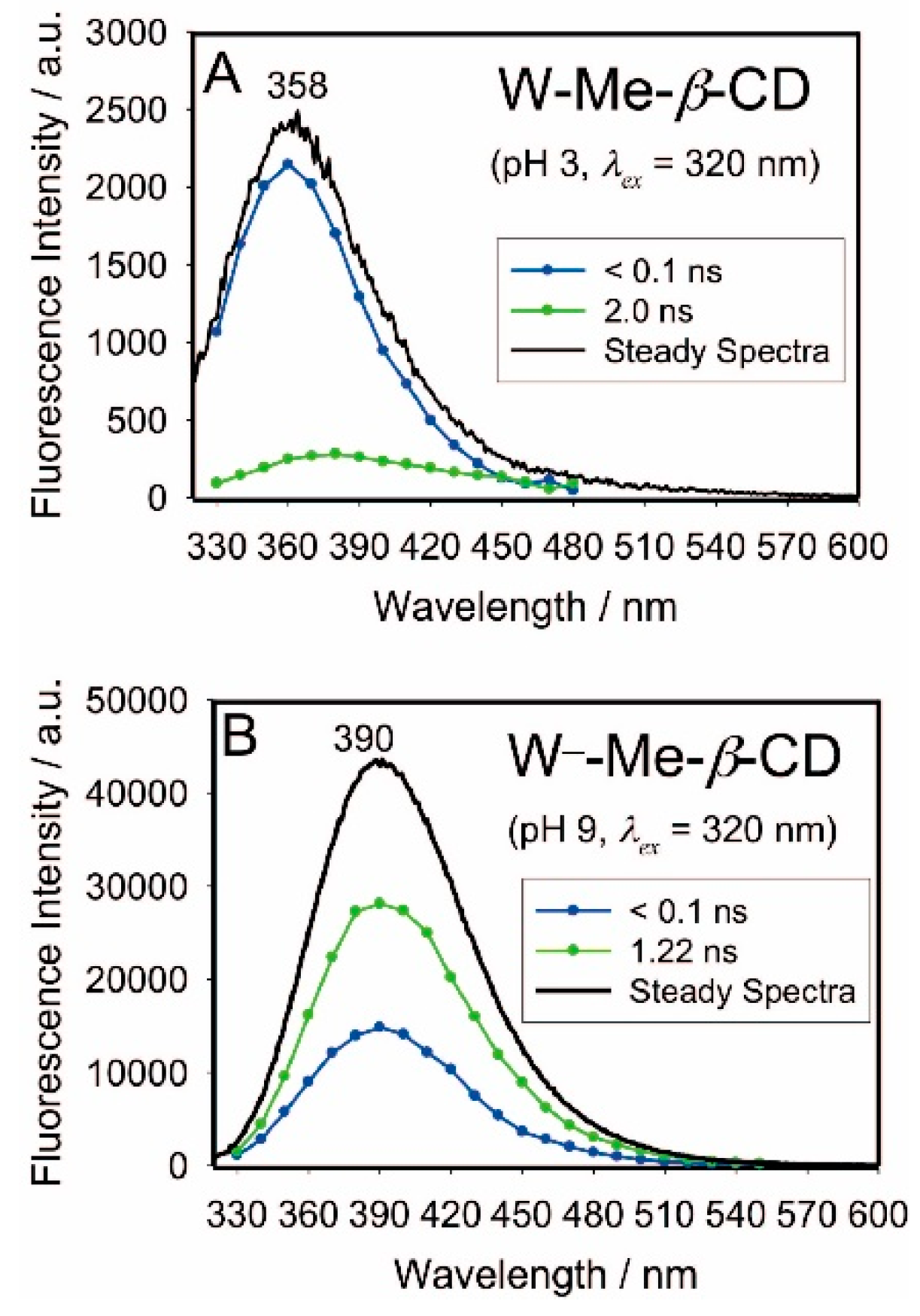
2.4. Fluorescence Lifetime Measurements/Decay-Associated Spectra (DAS)
3. Experimental Section
3.1. Reagents
3.2. Absorption/Steady-State Fluorescence Spectroscopy
3.3. pH-Titration Studies
3.4. Steady-State Binding Titration Studies

3.5. Time-Resolved Fluorescence Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Woods, L.L.; Shamma, S.M. Synthesis of substituted coumarins with fluorescent properties. J. Chem. Eng. Data 1971, 16, 101–102. [Google Scholar] [CrossRef]
- Saleh, N.; Al-Soud, Y.A.; Nau, W.M. Novel fluorescent pH sensor based on coumarin with piperazine and imidazole substituents. Spectrochim. Acta Mol. Biomol. Spectrosc. 2008, 71, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.D. The use of coumarins as environmentally-sensitive fluorescent probes of heterogeneous inclusion systems. Molecules 2009, 14, 210–237. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.; Al-Soud, Y.A.; Al-Kaabi, L.; Ghosh, I.; Nau, W.M. A coumarin-based fluorescent PET sensor utilizing supramolecular pKa shifts. Tetrahedron Lett. 2011, 52, 5249–5254. [Google Scholar] [CrossRef]
- Bristol-Meyers Squibb Company. COUMADIN Medication Guide; Bristol-Meyers Squibb Company: Princeton, NJ, USA, 2007. [Google Scholar]
- Valente, E.J.; Lingafelter, E.C.; Porter, W.R.; Trager, W.F. Structure of warfarin in solution. J. Med. Chem. 1977, 20, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Valente, E.J.; Trager, W.F. Anomalous chiroptical properties of warfarin and phenprocoumon. J. Med. Chem. 1978, 21, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, B.; Rosengren, A.M.; Andersson, P.O.; Nicholls, I.A. The spectrophysics of warfarin: Implications for protein binding. J. Phys. Chem. B 2007, 111, 10520–10528. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, J.M.; Vu, A.; Schultz, J.S.; Vullev, V.I. Fluorescence enhancement of warfarin induced by interaction with β-cyclodextrin. Biotechnol. Prog. 2009, 25, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.M.; Wozniakiewicz, M.; Mitoraj, M.P.; Garnysza, M.; Koscielniaka, P. Modulation of pKa by cyclodextrins; subtle structural changes induce spectacularly different behaviors. RSC Adv. 2015, 5, 77545–77552. [Google Scholar] [CrossRef] [Green Version]
- Datta, S.; Halder, M. Effect of encapsulation in the anion receptor pocket of sub-domain IIA of human serum albumin on the modulation of pKa of warfarin and structurally similar acidic guests: A possible implication on biological activity. J. Photochem. Photobiol. B 2014, 130, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, S.; Kamiya, M. Cyclodextrin inclusion effects on fluorescence and fluorimetricp of the pesticide warfarin. Chemosphere 1997, 34, 783–789. [Google Scholar]
- Heimark, L.D.; Trager, W.F. The preferred solution conformation of warfarin at the active site of cytochrome P-450 based on the CD spectra in octanol/water model system. J. Med. Chem. 1984, 27, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.M.; Sagan, F.; Mitoraj, M.P. On the origin of remarkably different acidity of hydroxycoumarins–joint experimental and theoretical studies. J. Phys. Chem. B 2017, 121, 4554–4561. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.M.; Olechowska, P.; Mitoraj, M.P.; Wozniakiewicz, M.; Koscielniaka, P. Determination of acid dissociation constants of warfarin and hydroxywarfarins by capillary electrophoresis. J. Pharm. Biomed. Anal. 2015, 112, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.; Al-Handawi, M.B.; Bufaroosha, M.S.; Assaf, K.I.; Nau, W.M. Tuning protonation states of tripelennamine antihistamines by cucurbit[7]uril. J. Phys. Org. Chem. 2016, 29, 101–106. [Google Scholar] [CrossRef]
- Dondon, R.; Fery-Forgues, S. Inclusion Complex of Fluorescent 4-hydroxycoumarin derivatives with native β-cyclodextrin: Enhanced stabilization induced by the appended substituentn. J. Phys.Chem. B 2001, 105, 10715–10722. [Google Scholar] [CrossRef]
- Valeur, B. Molecular Fluorescence: Principles and Applications; Wiley-VCH: Weinheim, Germany, 2002; Volume 387, pp. 46–53. [Google Scholar]
- Glotaran Software. A More Advanced form of the Global Analysis Involves Fitting the Time-Resolved Data to Both Parallel and Sequential Compartmental Kinetics Models Using Glotaran Software. The Former Model Assumes Excited Species Decay Mono-Exponentially in Parallel. In the Sequential Model, the Evolution Associated Spectra (EAS) are Obtained upon Fitting the Time-Resolved Data to All Possible Kinetic Steps and Equilibria (Described by Microscopic Rate Constants) for the Transfer of Population of One (Excited-State) Species into Another or the Decay to the Ground State. For More Details on Compartmental Models, See Reviews by Van Stokkum and Co-Workers. Available online: http://www.glotaran.org (accessed on 20 July 2016).
- Badia, R.; Diaz-Garcia, M.E. Cyclodextrin-based optosensor for the determination of warfarin in waters. J. Agric. Food Chem. 1999, 47, 4256–4260. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.X.; Rowell, F.J. Rapid determination of warfarin by sequential injection analysis with cyclodextrin-enhanced fluorescence detection. Anal. Lett. 1998, 31, 891–901. [Google Scholar] [CrossRef]
- Hollifield, H.C.; Winefordner, J.D. Fluorescence and phosphorescence characteristics of anticoagulants: A new approach to direct measurement of drugs in whole blood. Talanta 1967, 14, 103–107. [Google Scholar] [CrossRef]
- Abdulmalik, A.; Hibah, A.; Zainy, B.M.; Makoto, A.; Daisuke, I.; Masaki, O.; Uekama, K.; Fumitoshi, H. Preparation of soluble stable C60/human serum albumin nanoparticles via cyclodextrin complexation and their reactive oxygen production characteristics. Life Sci. 2013, 93, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Bolattin, M.B.; Nandibewoor, S.T.; Joshi, S.D.; Dixit, S.R.; Chimatadar, S.A. Interaction of hydralazine with human serum albumin and effect of β-cyclodextrin on binding: Insights from spectroscopic and molecular docking techniques. Ind. Eng. Chem. Res. 2016, 55, 5454−5464. [Google Scholar] [CrossRef]
- Chen, J.; Ohnmacht, C.M.; Hage, D.S. Characterization of drug interactions with soluble β-cyclodextrin by high-performance affinity chromatography. J. Chromatogr. 2004, 1033, 115–126. [Google Scholar] [CrossRef]
- Thuaud, N.; Sebille, B.; Deratani, A.; Lelievre, G. Determination by high-performance liquid chromatography of the binding properties of charged β-cyclodextrin derivatives with drugs. J. Chromatogr. 1990, 503, 453–458. [Google Scholar] [CrossRef]
- Zingone, G.; Rubessa, F. Preformulation study of the inclusion complex warfarin-β-cyclodextrin. Int. J. Pharm. 2005, 291, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Karadag, O.; Gok, E.; Serdar, A. Inclusion complexation of warfarin with β-cyclodextrins and its influence on absorption kinetics of warfarin in rat. J. Incl. Phenom. Mol. Recognit. Chem. 1995, 20, 23–32. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Drug Forms | λabs/nm | ϵ/M−1·cm–1 | λex/nm | λem/nm | τ1/ns (λem/nm) | τ2/ns (λem/nm) |
|---|---|---|---|---|---|---|
| Free (pH 3) | 305 | 10 680 | 320 | 358 | <0.1 | |
| 282 | 12 820 | 280 | 358 | <0.1 | ||
| Free (pH 9) | 309 | 13 532 | 320 | 390 | <0.1 | |
| Complex (pH 3) | 306 | 10 102 | 320 a | 358 | <0.1 (358) | 2.0 ± 0.4 b (375) |
| Complex (pH 9) | 309 | 13 580 | 320 | 390 | <0.1 (390) | 1.22 ± 0.02 b (390) |
| Drug Forms | λex/nm | ФF (×10–3) a | kr (× 107 s–1) b | knr (×109 s–1) b | τ/ns (Mean) |
|---|---|---|---|---|---|
| W OPEN | 320 | 6.0 ± 0.1 | >6 | >10 | <0.1 |
| W− | 320 | 12 ± 0.1 | >12 | >10 | <0.1 |
| W-Me-β-CD OPEN | 320 | 0.5 ± 0.1 | 0.02 | 0.50 | 2.0 |
| W−-Me-β-CD | 320 | 24 ± 0.1 | 2.0 | 0.81 | 1.22 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Dubaili, N.; Saleh, N. Sequestration Effect on the Open-Cyclic Switchable Property of Warfarin Induced by Cyclodextrin: Time-Resolved Fluorescence Study. Molecules 2017, 22, 1326. https://doi.org/10.3390/molecules22081326
Al-Dubaili N, Saleh N. Sequestration Effect on the Open-Cyclic Switchable Property of Warfarin Induced by Cyclodextrin: Time-Resolved Fluorescence Study. Molecules. 2017; 22(8):1326. https://doi.org/10.3390/molecules22081326
Chicago/Turabian StyleAl-Dubaili, Naji, and Na’il Saleh. 2017. "Sequestration Effect on the Open-Cyclic Switchable Property of Warfarin Induced by Cyclodextrin: Time-Resolved Fluorescence Study" Molecules 22, no. 8: 1326. https://doi.org/10.3390/molecules22081326