Ultrafast and Slow Cholinergic Transmission. Different Involvement of Acetylcholinesterase Molecular Forms †
Abstract
:1. Introduction
2. Ultra-Fast Cholinergic Transmission
2.1. Two Different Molecular Forms of Acetylcholinesterase Are Associated with Muscle Function
2.2. The Case of Nerve-Electroplaque Junction
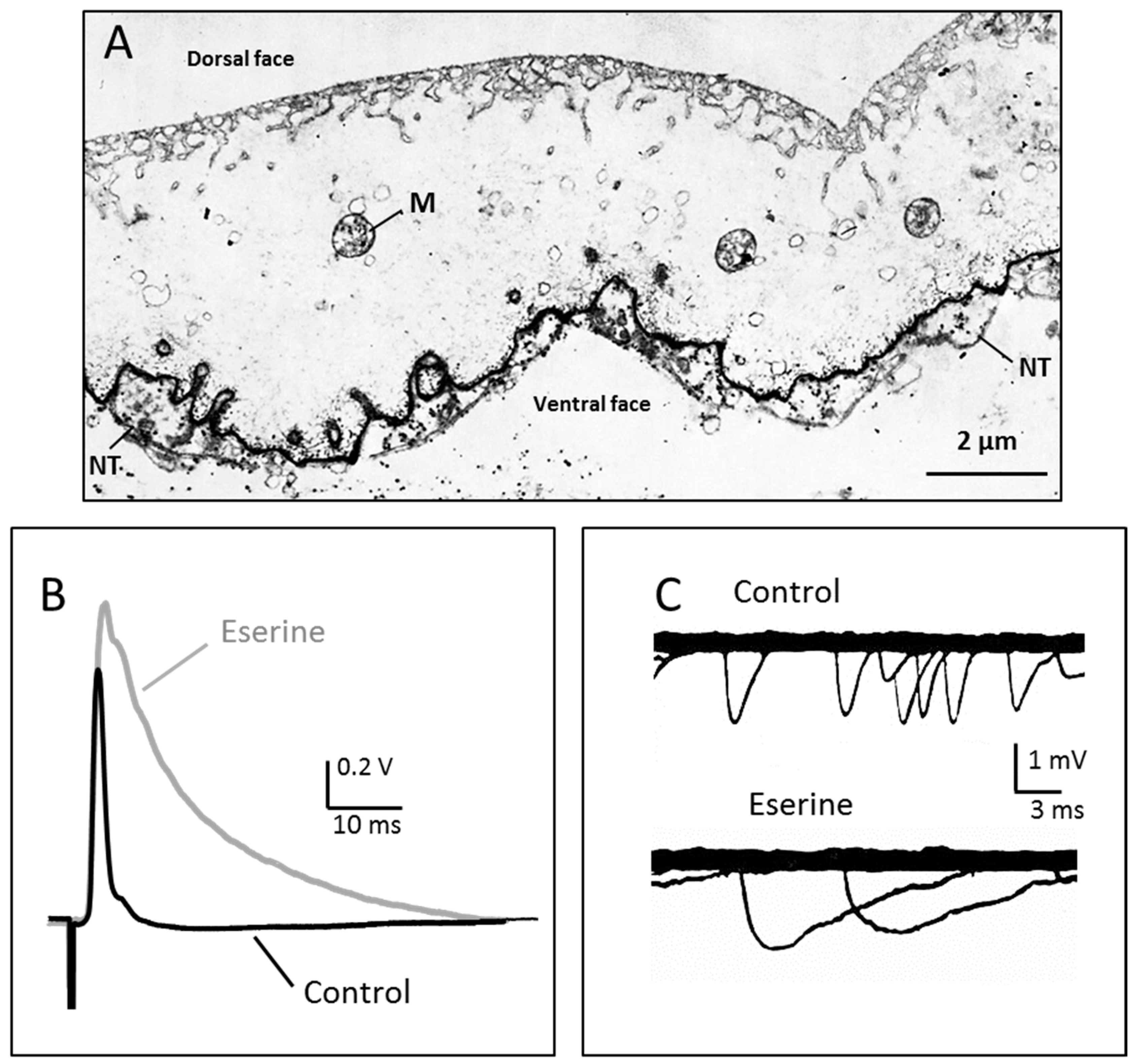
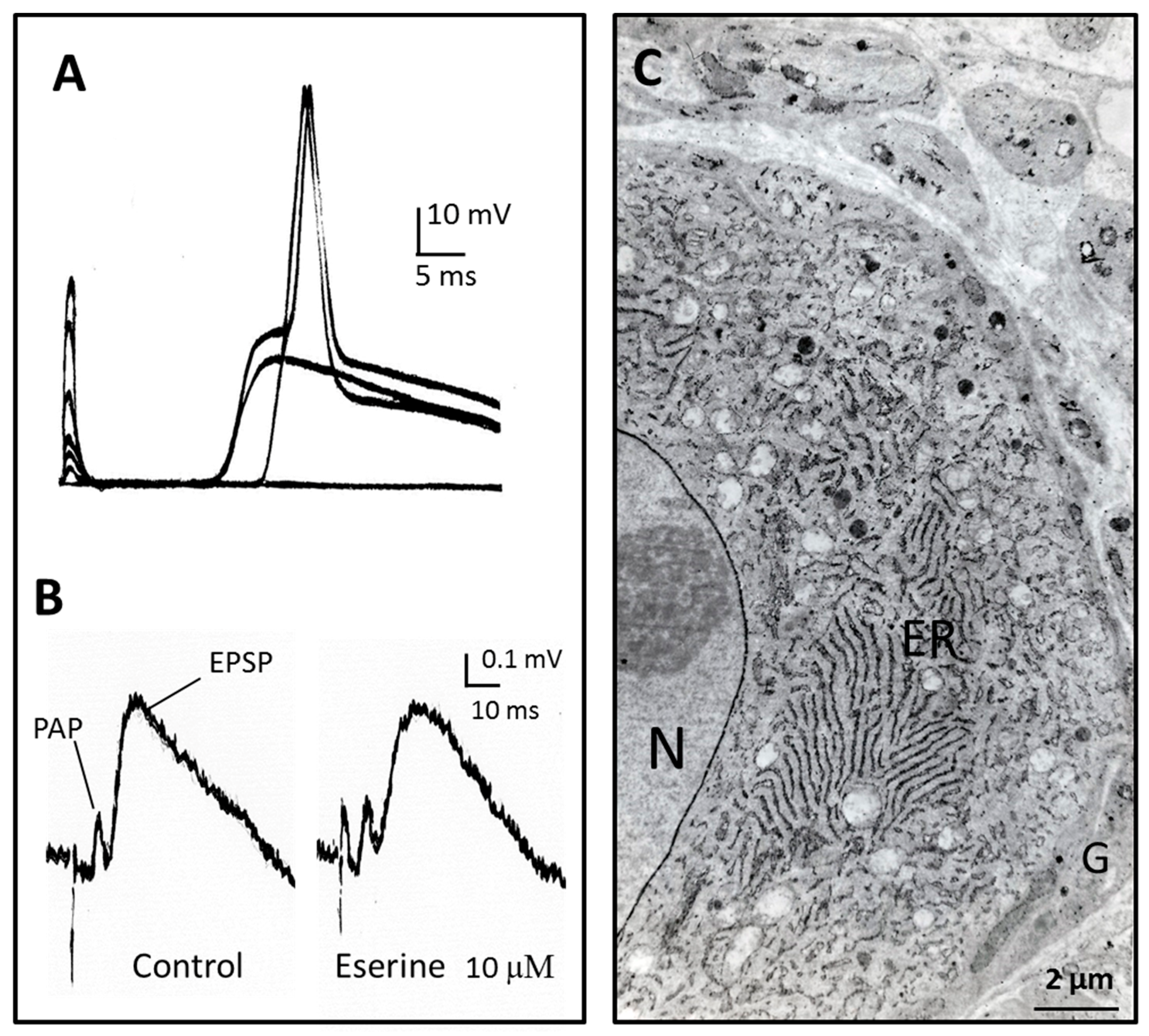
2.3. Two Presynaptic Mechanisms Which Curtail the Duration of Individual Impulses in Ultra-Fast Cholinergic Synapses
2.4. Other Rapid Protagonists of the Neuro-Muscular Junction and the Nerve-Electroplaque Junction
3. Cholinergic Transmission in Neuro-Neuronal Synapses
4. The Great Diversity of Slow Cholinergic Processes in the Central and Autonomic Nervous Systems
4.1. Postganglionic Parasympathetic Nervous System
4.2. Central Nervous System
4.2.1. Relative Paucity of “True” Cholinergic Synapses in the Central Nervous System
4.2.2. Many Nicotinic and Muscarinic Acetylcholine Receptors in the Brain Are Extrasynaptic
4.2.3. Acetylcholinesterase Forms in the Central Nervous System
4.2.4. Diffuse or “Volume” Transmission Seems to Be the Major Mode of Acetylcholine Action in the Central Nervous System
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | acetylcholine |
| AChE | acetylcholinesterase |
| mAChRs and nAChRs | muscarinic and nicotinic ACh receptors, respectively |
| AmPy | aminopyridine |
| ANS and CNS | autonomic and central nervous system, respectively |
| ChAT | choline acetyltransferase |
| EPP | endplate potential or electroplaque potential |
| EPSP | excitatory postsynaptic potential |
| MEPPs | miniature endplate or miniature electroplaque potentials |
| NEJ | nerve-electroplaque junctions of electric organs |
| NMJ | neuromuscular junction |
| PRiMA | proline-rich membrane anchor |
References
- Loewi, O. Ueber humorale Uebertragbarkeit der Herznervenwirkung. Pflugers Arch. 1921, 189, 239–242. (In German) [Google Scholar] [CrossRef]
- Bacq, Z.M. Chemical Transmission of Nerve Impulses. A Historical Sketch; Pergamon Press: Oxford, UK; New York, NY, USA; Toronto, ON, Canada, 1975. [Google Scholar]
- Brown, D.A. Acetylcholine. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S120–S126. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Misawa, H.; Moriwaki, Y.; Fujii, Y.X.; Fujii, T.; Horiuchi, Y.; Yamada, T.; Imanaka, T.; Kamekura, M. Ubiquitous expression of acetylcholine and its biological functions in life forms without nervous systems. Life Sci. 2007, 80, 2206–2209. [Google Scholar] [CrossRef] [PubMed]
- Wessler, I.; Kirkpatrick, C.J. Acetylcholine beyond neurons: The non-neuronal cholinergic system in humans. Br. J. Pharmacol. 2008, 154, 1558–1571. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Takada-Takatori, Y.; Horiguchi, K.; Kawashima, K. Mediatophore regulates acetylcholine release from T cells. J. Neuroimmunol. 2012, 244, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, C.J.; Bittinger, F.; Nozadze, K.; Wessler, I. Expression and function of the non-neuronal cholinergic system in endothelial cells. Life Sci. 2003, 72, 2111–2116. [Google Scholar] [CrossRef]
- Maeda, S.; Jun, J.G.; Kuwahara-Otani, S.; Tanaka, K.; Hayakawa, T.; Seki, M. Non-neuronal expression of choline acetyltransferase in the rat kidney. Life Sci. 2011, 89, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Diaz, R.; Dando, R.; Jacques-Silva, M.C.; Fachado, A.; Molina, J.; Abdulreda, M.H.; Ricordi, C.; Roper, S.D.; Berggren, P.O.; Caicedo, A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat. Med. 2011, 17, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Katz, B. Looking back at the neuromuscular junction. In Neuromuscular Junction; Selling, L.C., Libelius, R., Thesleff, S., Eds.; Elsevier Science Publisher: Amstredam, The Netherlands, 1989; pp. 3–9. [Google Scholar]
- Massoulie, J. The origin of the molecular diversity and functional anchoring of cholinesterases. Neurosignals 2002, 11, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Gautron, J. Localisation des cholinestérases au niveau de la jonction nerf-électroplaque de l’organe électrique de la Torpille marbrée. C. R. Acad. Sci. Paris 1970, 271, 714–717. (In French) [Google Scholar]
- Witzemann, V.; Boustead, C. Changes in acetylcholinesterase molecular forms during the embryonic development of Torpedo marmorata. J. Neurochem. 1982, 39, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Gisiger, V.; Stephens, H.R. Localization of the pool of G4 acetylcholinesterase characterizing fast muscles and its alteration in murine muscular dystrophy. J. Neurosci. Res. 1988, 19, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Bernard, V.; Girard, E.; Hrabovska, A.; Camp, S.; Taylor, P.; Plaud, B.; Krejci, E. Distinct localization of collagen Q and PRiMA forms of acetylcholinesterase at the neuromuscular junction. Mol. Cell. Neurosci. 2011, 46, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Descarries, L.; Gisiger, V.; Steriade, M. Diffuse transmission by acetylcholine in the CNS. Prog. Neurobiol. 1997, 53, 603–625. [Google Scholar] [CrossRef]
- Massoulie, J.; Perrier, N.; Noureddine, H.; Liang, D.; Bon, S. Old and new questions about cholinesterases. Chem. Biol. Interact. 2008, 175, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Dobbertin, A.; Hrabovska, A.; Dembele, K.; Camp, S.; Taylor, P.; Krejci, E.; Bernard, V. Targeting of acetylcholinesterase in neurons in vivo: A dual processing function for the proline-rich membrane anchor subunit and the attachment domain on the catalytic subunit. J. Neurosci. 2009, 29, 4519–4530. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Parikh, V.; Howe, W.M. Phasic acetylcholine release and the volume transmission hypothesis: Time to move on. Nat. Rev. Neurosci. 2009, 10, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Coppola, J.J.; Ward, N.J.; Jadi, M.P.; Disney, A.A. Modulatory compartments in cortex and local regulation of cholinergic tone. J. Physiol. Paris 2016, 110, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Couteaux, R.; Pécot-Dechavassine, M. Vésicules synaptiques et poches au niveau des “zones actives” de la jonction neuromusculaire. C. R. Acad. Sci. Paris 1970, 271, 2346–2349. (In French) [Google Scholar]
- Katz, B. The Release of Neural Transmitter Substances; University Press: Liverpool, UK, 1969. [Google Scholar]
- Kuno, M.; Turkanis, S.A.; Weakly, J.N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J. Physiol. 1971, 213, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Couteaux, R.; Axi, J. Recherches histochimiques sur la distribution des activités cholinestérasiques au niveau de la synapse myoneurale. Arch. Anat. Micr. 1952, 41, 352–392. (In French) [Google Scholar]
- Couteaux, R. Localization of cholinesterase at neuromuscular junctions. Int. Rev. Cytol. 1955, 4, 355–375. [Google Scholar]
- Massoulie, J.; Pezzementi, L.; Bon, S.; Krejci, E.; Vallette, F.M. Molecular and cellular biology of cholinesterases. Prog. Neurobiol. 1993, 41, 31–91. [Google Scholar] [CrossRef]
- Campanari, M.L.; Garcia-Ayllon, M.S.; Ciura, S.; Saez-Valero, J.; Kabashi, E. Neuromuscular junction impairment in amyotrophic lateral sclerosis: Reassessing the role of acetylcholinesterase. Front. Mol. Neurosci. 2016, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vigny, M.; Bon, S.; Massoulie, J.; Gisiger, V. The subunit structure of mammalian acetylcholinesterase: Catalytic subunits, dissociating effect of proteolysis and disulphide reduction on the polymeric forms. J. Neurochem. 1979, 33, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Vigny, M.; Bon, S.; Massoulie, J.; Leterrier, F. Active-site catalytic efficiency of acetylcholinesterase molecular forms in Electrophorus, Torpedo, Rat and Chicken. Eur. J. Biochem. 1978, 85, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Girod, R.; Corrèges, P.; Jacquet, J.; Dunant, Y. Space and time characteristics of transmitter release at the nerve-electroplaque junction of Torpedo. J. Physiol. 1993, 471, 129–157. [Google Scholar] [CrossRef] [PubMed]
- Van der Kloot, W.; Molgo, J.; Cameron, R.; Colasante, C. Vesicle size and transmitter release at the frog neuromuscular junction when quantal acetylcholine content is increased or decreased. J. Physiol. 2002, 541, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Skorinkin, A.I.; Shaihutdinova, A.R.; Vyskocil, F. Model of concentration changes across the synaptic cleft during a single quantum release. Gen. Physiol. Biophys. 2008, 27, 19–24. [Google Scholar] [PubMed]
- Feng, T.P.; Shen, S.C. Studies on the neuro-muscular junction. III The contracture in eserinized muscle produced by nerve stimulation. Chin. J. Physiol. 1937, 11, 51–70. [Google Scholar]
- Eccles, J.C.; Katz, B.; Kuffler, S.W. Effect of eserine on neuromuscular transmission. J. Neurophysiol. 1942, 5, 211–230. [Google Scholar]
- Fatt, P.; Katz, B. Spontaneous subthreshold activity at motor nerve endings. J. Physiol. 1952, 117, 109–128. [Google Scholar] [PubMed]
- Perrier, A.L.; Massoulie, J.; Krejci, E. PRiMA: The membrane anchor of acetylcholinesterase in the brain. Neuron 2002, 33, 275–285. [Google Scholar] [CrossRef]
- Carter, J.L.; Brimijoin, S. Effects of acute and chronic denervation on the release of acetylcholinesterase and its molecular forms in rat diaphragms. J. Neurochem. 1981, 36, 1018–1025. [Google Scholar] [CrossRef]
- Gisiger, V.; Belisle, M.; Gardiner, P.F. Acetylcholinesterase adaptation to voluntary wheel running is proportional to the volume of activity in fast, but not slow, rat hindlimb muscles. Eur. J. Neurosci. 1994, 6, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Jasmin, B.J.; Gisiger, V. Regulation by exercise of the pool of G4 acetylcholinesterase characterizing fast muscles: Opposite effect of running training in antagonist muscles. J. Neurosci. 1990, 10, 1444–1454. [Google Scholar] [PubMed]
- Rossi, S.G.; Vazquez, A.E.; Rotundo, R.L. Local control of acetylcholinesterase gene expression in multinucleated skeletal muscle fibers: Individual nuclei respond to signals from the overlying plasma membrane. J. Neurosci. 2000, 20, 919–928. [Google Scholar] [PubMed]
- Wathey, J.C.; Nass, M.M.; Lester, H.A. Numerical reconstruction of the quantal event at nicotinic synapses. Biophys. J. 1979, 27, 145–164. [Google Scholar] [CrossRef]
- Kuffler, S.W.; Yoshikami, D. The number of transmitter molecules in a quantum: An estimate from iontophoretic application of acetylcholine at the neuromuscular synapse. J. Physiol. 1975, 251, 465–482. [Google Scholar] [CrossRef] [PubMed]
- Dunant, Y.; Muller, D. Quantal release of acetylcholine evoked by focal depolarisation at the Torpedo nerve-electroplaque junction. J. Physiol. 1986, 379, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Kriebel, M.E.; Gross, C.E. Multimodal distribution of frog miniature endplate potentials in adult, denervated and tadpole leg muscle. J. Gen. Physiol. 1974, 64, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Feldberg, W.; Fessard, A.; Nachmansohn, D. The cholinergic nature of the nervous supply to the electric organ of the Torpedo (Torpedo marmorata). J. Physiol. 1940, 97, 3. [Google Scholar]
- Dunant, Y.; Eder, L.; Servetiadis-Hirt, L. Acetylcholine release evoked by single or a few nerve impulses in the electric organ of Torpedo. J. Physiol. 1980, 298, 185–203. [Google Scholar] [CrossRef] [PubMed]
- Dunant, Y.; Cordeiro, J.M. Presynaptic K+ Channels, vesicular Ca2+/H+ antiport-synaptotagmin, and acetylcholinesterase, three mechanisms cutting short the cholinergic signal at neuromuscular and nerve-electroplaque junctions. J. Mol. Neurosci. 2014, 53, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Szerb, J.C.; Somogyi, G.T. Depression of acetylcholine release from cerebral cortical slices by cholinesterase inhibition and by oxotremorine. Nature 1973, 241, 121–122. [Google Scholar] [CrossRef]
- Dunant, Y.; Walker, A.I. Cholinergic inhibition of acetylcholine release in the electric organ of Torpedo. Eur. J. Pharmacol. 1982, 78, 201–212. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of the membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef] [PubMed]
- Katz, B.; Miledi, R.B. Tetrodotoxin-resistant electric activity in presynaptic terminals. J. Physiol. 1969, 203, 459–487. [Google Scholar] [CrossRef] [PubMed]
- Dunant, Y. Some properties of the presynaptic nerve terminals in a mammalian sympathetic ganglion. J. Physiol. 1972, 221, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Brigant, J.L.; Mallart, A. Presynaptic currents in mouse motor endings. J. Physiol. 1982, 333, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Van der Kloot, W.; Molgo, J. Quantal acetylcholine release at the vertebrate neuromuscular junction. Physiol. Rev. 1994, 74, 899–991. [Google Scholar] [PubMed]
- Katz, B.; Miledi, R.B. Estimates of quantal content during “chemical potentiation” of transmitter release. Proc. R. Soc. Lond. B 1979, 205, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Molgo, J.; Thesleff, S. 4-aminoquinoline-induced “giant” endplate potentials at mammalian neuromuscular junctions. Proc. R. Soc. Lond. B 1982, 214, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Corthay, J.; Dunant, Y.; Loctin, F. Acetylcholine changes underlying transmission of a single nerve impulse in the presence of 4-aminopyridine in Torpedo. J. Physiol. 1982, 325, 461–479. [Google Scholar] [CrossRef] [PubMed]
- Muller, D. Potentiation by 4-aminopyridine of quantal acetylcholine release at the Torpedo nerve electroplaque junction. J. Physiol. 1986, 379, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Bancila, V.; Nikonenko, I.; Dunant, Y.; Bloc, A. Zinc inhibits glutamate release via activation of pre-synaptic K channels and reduces ischaemic damage in rat hippocampus. J. Neurochem. 2004, 90, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Lemeignan, M.; Millart, H.; Lamiable, D.; Molgo, J.; Lechat, P. Evaluation of 4-aminopyridine and 3,4-diaminopyridine penetrability into cerebrospinal fluid in anesthetized rats. Brain Res. 1984, 304, 166–169. [Google Scholar] [CrossRef]
- Israël, M.; Manaranche, R.; Marsal, J.; Meunier, F.M.; Morel, N.; Frachon, P.; Lesbats, B. ATP-dependent calcium uptake by cholinergic synaptic vesicles isolated from Torpedo electric organ. J. Membr. Biol. 1980, 54, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Castonguay, A.; Robitaille, R. Differential regulation of transmitter release by presynaptic and glial Ca2+ internal stores at the neuromuscular synapse. J. Neurosci. 2001, 21, 1911–1922. [Google Scholar] [PubMed]
- Rizzuto, R.; Pozzan, T. Microdomains of intracellular Ca2+: Molecular determinants and functional consequences. Physiol. Rev. 2006, 86, 369–408. [Google Scholar] [CrossRef] [PubMed]
- Desai-Shah, M.; Cooper, R.L. Different mechanisms of Ca2+ regulation that influence synaptic transmission: Comparison between crayfish and Drosophila neuromuscular junctions. Synapse 2009, 63, 1100–1121. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Taschenberger, H.; Neher, E. Dynamics of volume-averaged intracellular Ca2+ in a rat CNS nerve terminal during single and repetitive voltage-clamp depolarizations. J. Physiol. 2017, 595, 3219–3236. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.P.; Meireles, S.M.; Gravato, C.; Vale, M.G. Ca2+-H+-Antiport activity in synaptic vesicles isolated from sheep brain cortex. Neurosci. Lett. 1998, 247, 87–90. [Google Scholar] [CrossRef]
- Gonçalves, P.P.; Meireles, S.M.; Neves, P.; Vale, M.G. Distinction between Ca2+ pump and Ca2+/H+ antiport activities in synaptic vesicles of sheep brain cortex. Neurochem. Int. 2000, 37, 387–396. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Goncalves, P.P.; Dunant, Y. Synaptic vesicles control the time course of neurotransmitter secretion via a Ca2+/H+ antiport. J. Physiol. 2011, 589, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, J.M.; Boda, B.; Goncalves, P.P.; Dunant, Y. Synaptotagmin 1 is required for vesicular Ca2+/H+-antiport activity. J. Neurochem. 2013, 126, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Sakmann, B.; Methfessel, C.; Mishina, M.; Takahashi, T.; Takai, T.; Kurasaki, M.; Fukuda, K.; Numa, S. Role of acetylcholine receptor subunits in gating of the channel. Nature 1985, 318, 538–543. [Google Scholar] [CrossRef]
- Katz, B.; Thesleff, S. A study of the “desensitization” produced by acetylcholine at the motor endplate. J. Physiol. 1957, 138, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Israël, M.; Morel, N.; Lesbats, B.; Birman, S.; Manaranche, R. Purification of a presynaptic membrane protein that mediates a calcium-dependent translocation of acetylcholine. Proc. Natl. Acad. Sci. USA 1986, 83, 9226–9230. [Google Scholar] [CrossRef] [PubMed]
- Dunant, Y.; Israël, M. Neurotransmitter release in rapid synapses. Biochimie 2000, 82, 289–302. [Google Scholar] [CrossRef]
- Dunant, Y.; Cordeiro, J.M.; Goncalves, P.P. Exocytosis, mediatophore, and vesicular Ca2+/H+ antiport in rapid neurotransmission. Ann. N. Y. Acad. Sci. 2009, 1152, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Israël, M.; Meunier, F.M.; Morel, N.; Lesbats, B. Calcium-induced desensitization of acetylcholine release from synaptosomes or proteoliposomes equiped with mediatophore, a presynaptic membrane protein. J. Neurochem. 1987, 49, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Gisiger, V.; Gautron, J.; Dunant, Y. Differences in acetylcholinesterase of neuro-muscular and sympathetic ganglia. Experientia 1977, 33, 804. [Google Scholar]
- Eccles, J.C. The nature of synaptic transmission in a sympathetic ganglion. J. Physiol. 1944, 103, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Eccles, J.C.; Fatt, P.; Koketsu, K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J. Physiol. 1954, 126, 524–562. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.; Arroyo, S.; Berns, D.; Hestrin, S. Mechanisms generating dual-component nicotinic EPSCs in cortical interneurons. J. Neurosci. 2012, 32, 17287–17296. [Google Scholar] [CrossRef] [PubMed]
- Lamotte d’Incamps, B.; Krejci, E.; Ascher, P. Mechanisms shaping the slow nicotinic synaptic current at the motoneuron-renshaw cell synapse. J. Neurosci. 2012, 32, 8413–8423. [Google Scholar] [CrossRef] [PubMed]
- Farar, V.; Mohr, F.; Legrand, M.; Lamotte, D.B.; Cendelin, J.; Leroy, J.; Abitbol, M.; Bernard, V.; Baud, F.; Fournet, V.; et al. Near-complete adaptation of the PRiMA knockout to the lack of central acetylcholinesterase. J. Neurochem. 2012, 122, 1065–1080. [Google Scholar] [CrossRef] [PubMed]
- Hay, Y.A.; Lambolez, B.; Tricoire, L. Nicotinic Transmission onto Layer 6 Cortical Neurons Relies on Synaptic Activation of Non-α7 Receptors. Cereb. Cortex 2016, 26, 2549–2562. [Google Scholar] [CrossRef] [PubMed]
- Gisiger, V.; Vigny, M. A specific form of acetylcholinesterase is secreted by rat sympathetic ganglia. FEBS Lett. 1977, 84, 253–256. [Google Scholar] [CrossRef]
- Gisiger, V.; Vigny, M.; Gautron, J.; Rieger, F. Acetylcholinesterase of rat sympathetic ganglion: Molecular forms, localization and effects of denervation. J. Neurochem. 1978, 30, 501–516. [Google Scholar] [CrossRef] [PubMed]
- Thoenen, H.; Mueller, R.A.; Axelrod, J. Trans-synaptic induction of adrenal tyrosine hydroxylase. J. Pharmacol. Exp. Ther. 1969, 169, 249–254. [Google Scholar] [PubMed]
- Gisiger, V. Triggering of RNA synthesis by acetylcholine stimulation of the postsynaptic membrane in a mammalian sympathetic ganglion. Brain Res. 1971, 33, 139–146. [Google Scholar] [CrossRef]
- Emmelin, N.G.; MacIntosh, F.C. The release of acetylcholine from perfused sympathetic ganglia and skeletal muscles. J. Physiol. 1956, 131, 477–496. [Google Scholar] [CrossRef] [PubMed]
- Couteaux, R.; Nachmansohn, D. Changes of cholinesterase at endplate of voluntary muscle following section of sciatic nerve. Proc. Soc. Exp. Biol. Med. 1940, 43, 177–181. [Google Scholar] [CrossRef]
- Lamotte d’Incamps, B.; Ascher, P. Four excitatory postsynaptic ionotropic receptors coactivated at the motoneuron-Renshaw cell synapse. J. Neurosci. 2008, 28, 14121–14131. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.R.; Bloom, F.E.; Roth, R.H. The Biochemical Basis of Neuropharmacology, 7th ed.; Oxford University Press: New York, NY, USA, 1996. [Google Scholar]
- Hefft, S.; Hulo, S.; Bertrand, D.; Muller, D. Synaptic transmission at nicotinic acetylcholine receptors in rat hippocampal organotypic cultures and slices. J. Physiol. 1999, 515, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Turrini, P.; Casu, M.A.; Wong, T.P.; De, K.Y.; Ribeiro-da-Silva, A.; Cuello, A.C. Cholinergic nerve terminals establish classical synapses in the rat cerebral cortex: Synaptic pattern and age-related atrophy. Neuroscience 2001, 105, 277–285. [Google Scholar] [CrossRef]
- Takacs, V.T.; Freund, T.F.; Nyiri, G. Neuroligin 2 is expressed in synapses established by cholinergic cells in the mouse brain. PLoS ONE 2013, 8, e72450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wonnacott, S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997, 20, 92–98. [Google Scholar] [CrossRef]
- Dani, J.A.; Bertrand, D. Nicotinic Acetylcholine Receptors and Nicotinic Cholinergic Mechanisms of the Central Nervous System. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 699–729. [Google Scholar] [CrossRef] [PubMed]
- Lendvai, B.; Vizi, E.S. Nonsynaptic chemical transmission through nicotinic acetylcholine receptors. Physiol. Rev. 2008, 88, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Krnjevic, K.; Pumain, R.; Renaud, L. The mechanism of excitation by acetylcholine in the cerebral cortex. J. Physiol. 1971, 215, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Grybko, M.; Vijayaraghavan, S. Action potential-independent and nicotinic receptor-mediated concerted release of multiple quanta at hippocampal CA3-mossy fiber synapses. J. Neurosci. 2008, 28, 2563–2575. [Google Scholar] [CrossRef] [PubMed]
- Bancila, V.; Cordeiro, J.M.; Bloc, A.; Dunant, Y. Nicotine-induced and depolarisation-induced glutamate release from hippocampus mossy fibre synaptosomes: Two distinct mechanisms. J. Neurochem. 2009, 110, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Massoulié, J. Molecular forms and anchoring of acetylcholinesterase. In Cholinesterases and Cholinesterase Inhibitors; Giacobini, E., Ed.; Martin Dunitz Ltd.: London, UK, 2000; pp. 81–101. [Google Scholar]
- Greenfield, S.A.; Smith, A.D. The influence of electrical stimulation of certain brain regions on the concentration of acetylcholinesterase in rabbit cerebrospinal fluid. Brain Res. 1979, 177, 445–459. [Google Scholar] [CrossRef]
- Peters, C.; Bayer, M.J.; Bühler, S.; Andersen, J.S.; Mann, M.; Mayer, A. Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature 2001, 409, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Higashida, H.; Yokoyama, S.; Tsuji, C.; Muramatsu, S.I. Neurotransmitter release: Vacuolar ATPase Vo sector c-subunits in possible gene or cell therapies for Parkinson’s, Alzheimer’s, and psychiatric diseases. J. Physiol. Sci. 2017, 67, 11–17. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunant, Y.; Gisiger, V. Ultrafast and Slow Cholinergic Transmission. Different Involvement of Acetylcholinesterase Molecular Forms. Molecules 2017, 22, 1300. https://doi.org/10.3390/molecules22081300
Dunant Y, Gisiger V. Ultrafast and Slow Cholinergic Transmission. Different Involvement of Acetylcholinesterase Molecular Forms. Molecules. 2017; 22(8):1300. https://doi.org/10.3390/molecules22081300
Chicago/Turabian StyleDunant, Yves, and Victor Gisiger. 2017. "Ultrafast and Slow Cholinergic Transmission. Different Involvement of Acetylcholinesterase Molecular Forms" Molecules 22, no. 8: 1300. https://doi.org/10.3390/molecules22081300



