Electronic Spectroscopy of Phthalocyanine and Porphyrin Derivatives in Superfluid Helium Nanodroplets
Abstract
:1. Introduction
2. Phthalocyanine at 0.37 Kelvin inside a Helium Droplet
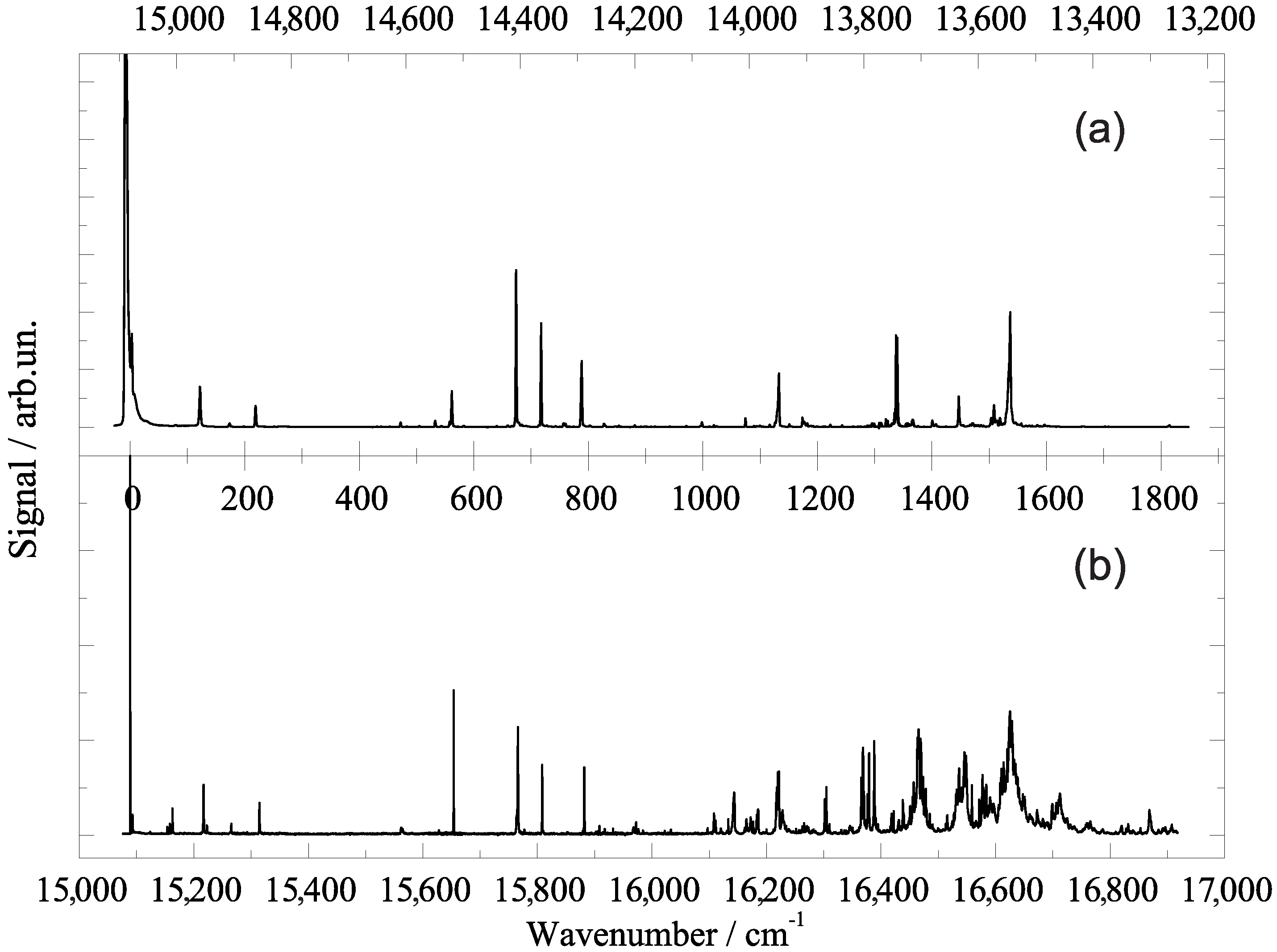
2.1. Vibronic Frequencies
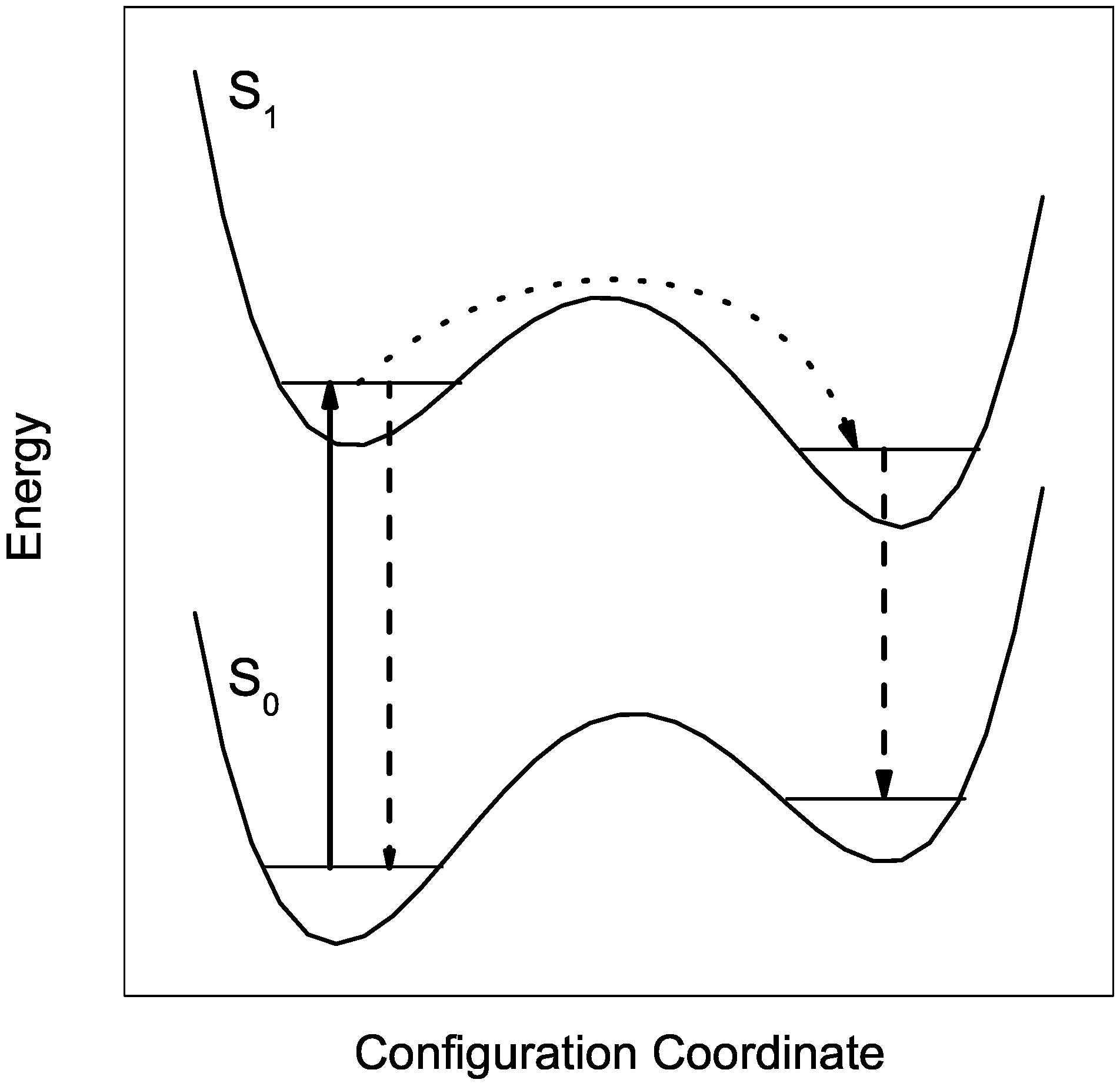
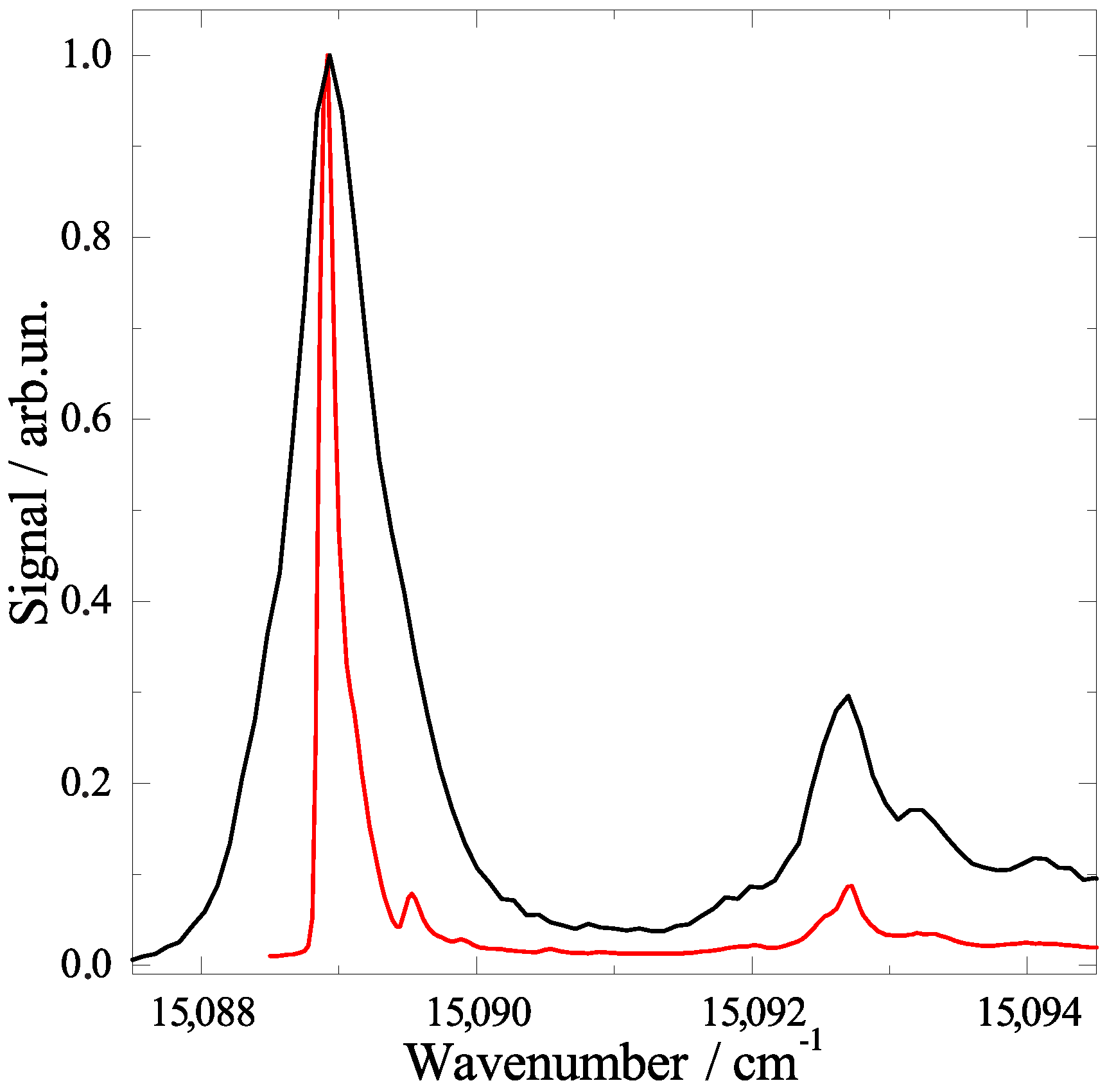
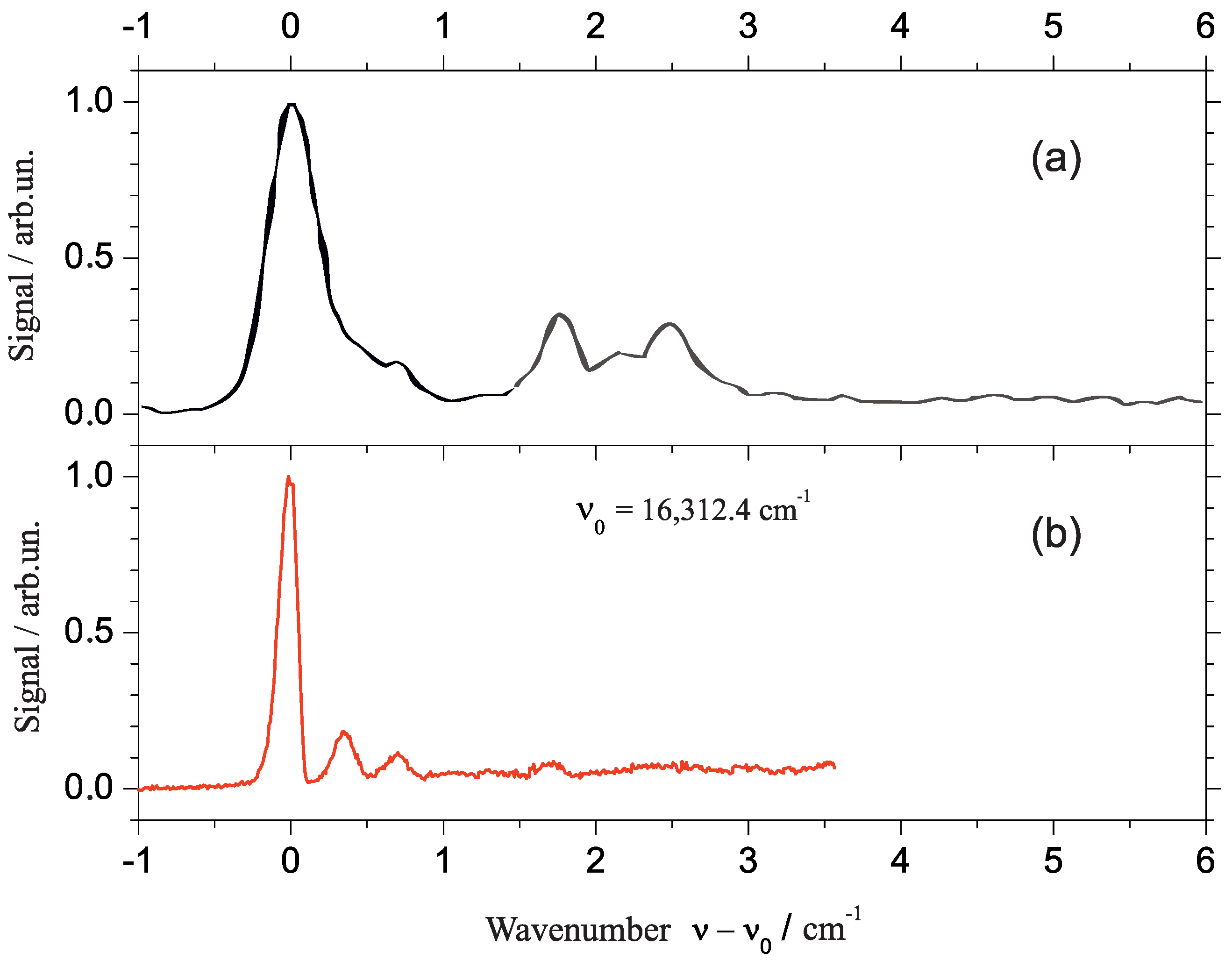
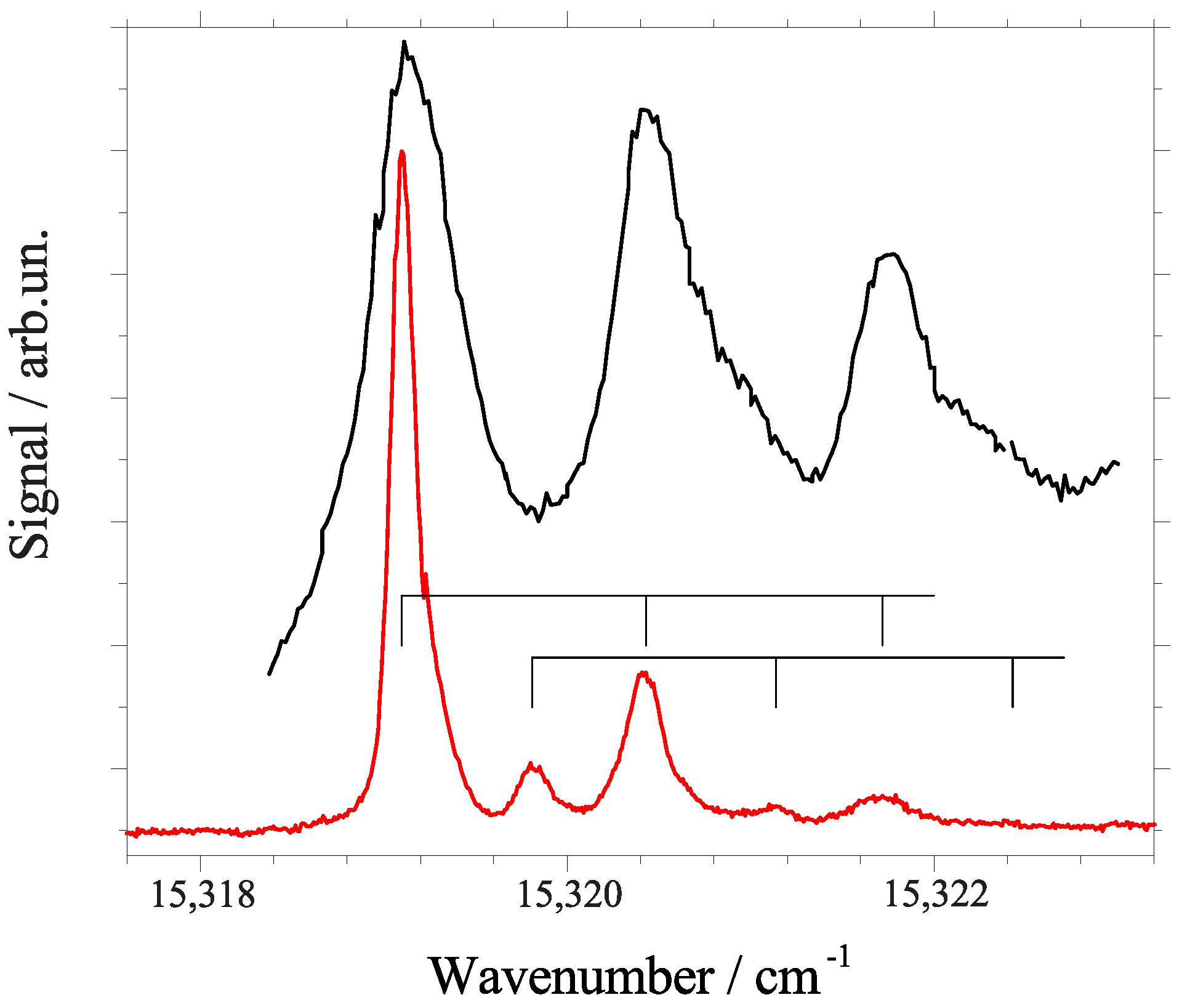
2.2. Line Shape Analysis
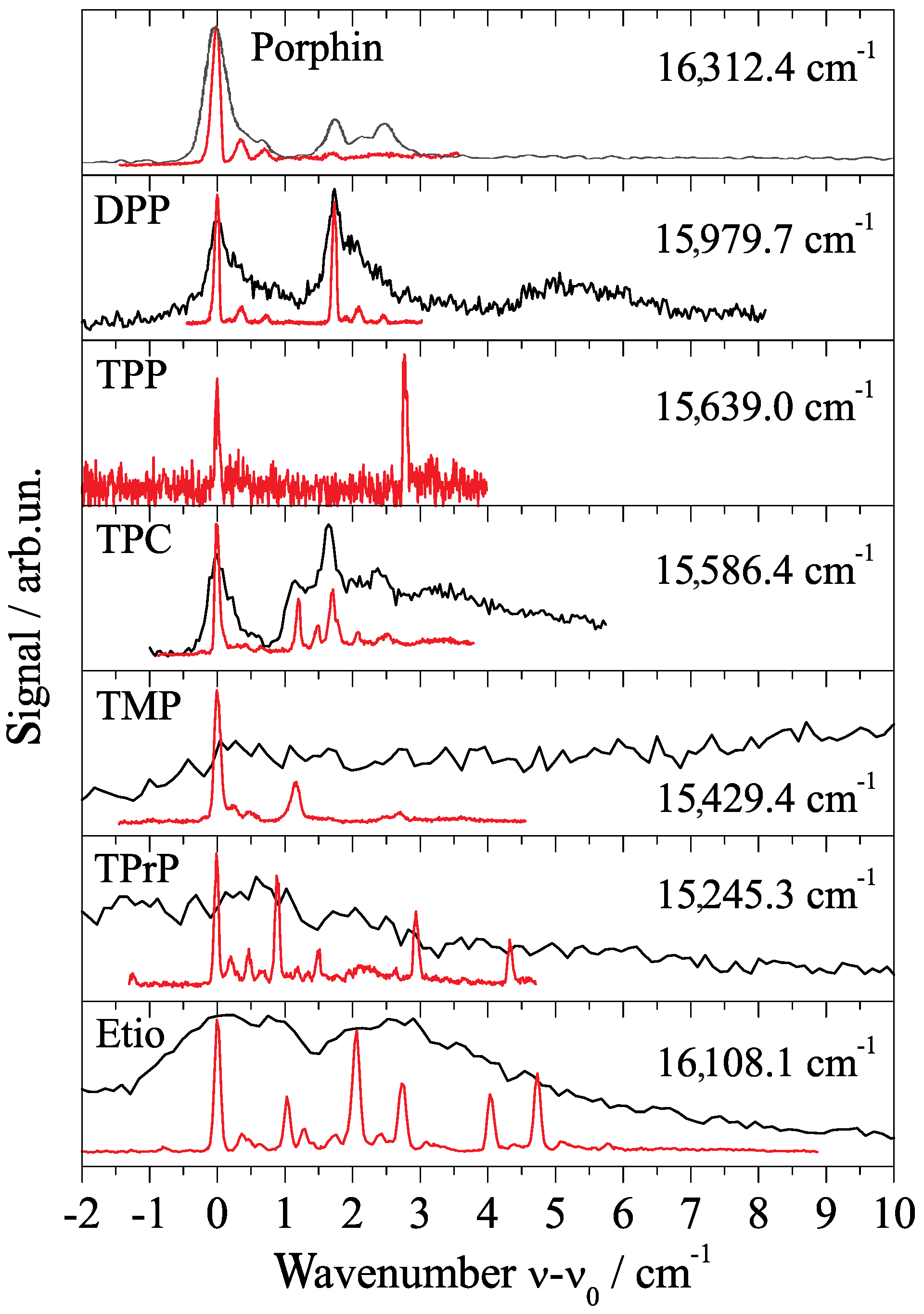
3. Porphyrin Derivatives at 0.37 Kelvin inside Helium Droplets
4. Phthalocyanine Derivatives and Tetraphenyl-Chlorin at 0.37 Kelvin in Superfluid Helium Droplets
5. Summary
Acknowledgments
Conflicts of Interest
References
- Toennies, J.P.; Vilesov, A.F. Spectroscopy of atoms and molecules in liquid helium. Annu. Rev. Phys. Chem. 1998, 49, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Toennies, J.P.; Vilesov, A.F. Suprafluide Heliumtröpfchen: Außergewöhnlich kalte Nanomatrices für Moleküle und molekulare Komplexe. Angew. Chem. 2004, 116, 2674–2702. [Google Scholar] [CrossRef]
- Toennies, J.P.; Vilesov, A.F. Matrix techniques: Superfluid helium droplets: A uniquely cold nanomatrix for molecules and molecular complexes. Angew. Chem. Int. Ed. 2004, 43, 2622–2648. [Google Scholar] [CrossRef]
- Stienkemeier, F.; Vilesov, A.F. Electronic spectroscopy in He droplets. J. Chem. Phys. 2001, 115, 10119–10137. [Google Scholar] [CrossRef]
- Stienkemeier, F.; Lehmann, K.K. Spectroscopy and dynamics in helium nanodroplets. J. Phys. B At. Mol. Opt. Phys. 2006, 39, R127–R166. [Google Scholar] [CrossRef]
- Callegari, C.; Ernst, W.E. Helium Droplets as Nanocryostats for Molecular Spectroscopy—From the Vacuum Ultraviolet to the Microwave Regime. In Handbook of High-resolution Spectroscopy; Quack, M., Merkt, F., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; Volume 3, pp. 1551–1594. [Google Scholar]
- Harms, J.; Toennies, J.P.; Dalfovo, F. Density of superfluid helium droplets. Phys. Rev. B 1998, 58, 3341–3350. [Google Scholar] [CrossRef]
- Hartmann, M.; Miller, R.E.; Toennies, J.P.; Vilesov, A. Rotationally resolved Spectroscopy of SF6 in liquid helium clusters: A molecular probe of cluster temperature. Phys. Rev. Lett. 1995, 75, 1566–1569. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.Y.; Douberly, G.E.; Falconer, T.M.; Lewis, W.K.; Lindsay, C.M.; Merritt, J.M.; Stiles, P.L.; Miller, R.E. Infrared spectroscopy of helium nanodroplets: Novel methods for physics and chemistry. Int. Rev. Phys. Chem. 2006, 25, 15–75. [Google Scholar] [CrossRef]
- Slipchenko, M.N.; Kuma, S.; Momose, T.; Vilesov, A.F. Intense pulsed helium droplet beams. Rev. Sci. Instrum. 2002, 73, 3600–3605. [Google Scholar] [CrossRef]
- Yang, S.; Brereton, S.M.; Ellis, A.M. Controlled growth of helium nanodroplets from a pulsed source. Rev. Sci. Instrum. 2005, 76, 104102. [Google Scholar] [CrossRef]
- Yang, S.; Ellis, A.M. Selecting the size of helium nanodroplets using time-resolved probing of a pulsed helium droplet beam. Rev. Sci. Instrum. 2007, 79, 016106. [Google Scholar] [CrossRef] [PubMed]
- Pentlehner, D.; Riechers, R.; Dick, B.; Slenczka, A.; Even, U.; Lavie, N.; Brown, R.; Luria, K. Rapidly pulsed helium droplet source. Rev. Sci. Instrum. 2009, 80, 043302. [Google Scholar] [CrossRef] [PubMed]
- Grebenev, S.; Toennies, J.P.; Vilesov, A.F. Superfluidity Within a Small Helium-4 Cluster: The Microscopic Andronikashvili Experiment. Science 1998, 279, 2083–2086. [Google Scholar] [CrossRef] [PubMed]
- Roden, J.; Eisfeld, A.; Dvorák, M.; Bünermann, O.; Stienkemeier, F. Vibronic line shapes of PTCDA oligomers in helium nanodroplets. J. Chem. Phys. 2011, 134, 054907. [Google Scholar]
- Pentlehner, D.; Riechers, R.; Vdovin, A.; Ptzl, G.M.; Slenczka, A. Electronic Spectroscopy of Molecules in Superfluid Helium Nanodroplets: An Excellent Sensor for Intramolecular Charge Redistribution. J. Phys. Chem. A 2011, 115, 7034–7043. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, N.; Toennies, J.P.; Vilesov, A.F.; Stienkemeier, F. Anomalous fine structures of the band of tetracene in large He droplets and their dependence on droplet size. Mol. Phys. 2012, 110, 1767–1780. [Google Scholar] [CrossRef]
- Hartmann, M.; Lindinger, A.; Toennies, J.P.; Vilesov, A.F. The phonon wings in the (S1–S0) spectra of tetracene, pentacene, porphin and phthalocyanine in liquid helium droplets. Phys. Chem. Chem. Phys. 2002, 4, 4839–4844. [Google Scholar] [CrossRef]
- Lindinger, A.; Lugovoj, E.; Toennies, J.P.; Vilesov, A.F. Splitting of the Zero Phonon Lines of Indole, 3-Methyl Indole, Tryptamine and N-Acetyl, Tryptophan Amide in Helium Droplets. Z. Phys. Chem. 2001, 215, 401–416. [Google Scholar] [CrossRef]
- Whitley, H.D.; Huang, P.; Kwon, Y.; Whaley, K.B. Multiple solvation configurations around phthalocyanine in helium droplets. J. Chem. Phys. 2005, 123, 054307. [Google Scholar] [CrossRef] [PubMed]
- Lehnig, R.; Slipchenko, M.; Kuma, S.; Momose, T.; Sartakov, B.; Vilesov, A. Fine structure of the S1–S0 band origins of phthalocyanine molecules in helium droplets. J. Chem. Phys. 2004, 121, 9396–9405. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Lindinger, A.; Toennies, J.P.; Vilesov, A.F. Laser-induced fluorescence spectroscopy of van der Waals complexes of tetracene-ArN (N≤5) and pentacene-Ar within ultracold liquid He droplets. Chem. Phys. 1998, 139, 139–149. [Google Scholar] [CrossRef]
- Hartmann, H.; Lindinger, A.; Toennies, J.P.; Vilesov, A.F. Hole-Burning Studies of the Splitting in the Ground and Excited Vibronic States of Tetracene in Helium Droplets. J. Phys. Chem. A 2001, 105, 6369–6377. [Google Scholar] [CrossRef]
- Lindinger, A.; Toennies, J.P.; Vilesov, A.F. Pump-probe study of the reconstruction of helium surrounding a tetracene molecule inside a helium droplet. Phys. Chem. Chem. Phys. 2001, 3, 2581–2587. [Google Scholar] [CrossRef]
- Pörtner, N.; Vilesov, A.F.; Havenith, M. Spontaneous alignment of tetracene molecules in 4He droplets. Phys. Chem. Lett. 2003, 368, 458–464. [Google Scholar] [CrossRef]
- Whitley, H.D.; DuBois, J.L.; Whaley, K.B. Spectral shifts and helium configurations in 4HeN-tetracene clusters. J. Chem. Phys 2009, 131, 124514. [Google Scholar] [CrossRef] [PubMed]
- Whitley, H.D.; DuBois, J.L.; Whaley, K.B. Theoretical Analysis of the Anomalous Spectral Splitting of Tetracene in 4He Droplets. J. Phys. Chem. A 2011, 115, 7220–7233. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M. Hochauflösende Spektroskopie von Molekülen in 4Helium- und 3Helium-Clustern; Bericht 10; Max-Planck-Institut für Strömungsforschung: Göttingen, Germany, 1997; ISSN 0436-1199. [Google Scholar]
- Lehnig, R. Anregungs- und Emissionsspektroekopie von Organischen Molekülen in 4He-Tröpfchen. Ph.D. Thesis, Universität Regensburg, Regensburg, Germany, 2004. [Google Scholar]
- Lehnig, R.; Slenczka, A. Spectroscopic investigation of the solvation of organic molecules in superfluid helium droplets. J. Chem. Phys. 2005, 122, 244317. [Google Scholar] [CrossRef] [PubMed]
- Benedek, G.; Hizhnyakov, V. Fermi sea excitations in the optical spectrum of a dopes 3He droplet. Chem. Phys. Lett. 2012, 548, 17–22. [Google Scholar] [CrossRef]
- Hartmann, M.; Mielke, F.; Toennies, J.P.; Vilesov, A.F. Direct Spectroscopic Observation of Elementary Excitations in Superfluid He Droplets. Phys. Rev. Lett. 1996, 76, 4560–4563. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, N.; Toennies, J.P.; Vilesov, A.F. The observation of large changes in the rotational constants of glyoxal in superfluid helium droplets upon electronic excitation. J. Chem. Phys. 2002, 117, 6054–6060. [Google Scholar] [CrossRef]
- Pörtner, N.; Toennies, J.P.; Vilesov, A.F.; Benedek, G.; Hizhnyakov, V. Anomalously sharp phonon excitation in 3He droplets. Eur. Phys. Lett. 2009, 88, 26007. [Google Scholar] [CrossRef]
- Lehnig, R.; Slenczka, A. Emission spectra of free base phthalocyanine in superfluid helium droplets. J. Chem. Phys. 2003, 118, 8256–8260. [Google Scholar] [CrossRef]
- Lehnig, R.; Slenczka, A. Microsolvation of Phthalocyanines in Superfluid Helium Droplets. ChemPhysChem 2004, 5, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Lehnig, R.; Slenczka, A. Quantum solvation of phthalocyanine in superfluid helium droplets. J. Chem. Phys. 2004, 120, 5064–5066. [Google Scholar] [CrossRef] [PubMed]
- Dick, B.; Slenczka, A. Inhomogeneous line shape theory of electronic transitions for molecules embedded in superfluid helium droplets. J. Chem. Phys. 2001, 115, 10206–10213. [Google Scholar] [CrossRef]
- Slenczka, A.; Dick, B.; Hartmann, M.; Toennies, J.P. Inhomogeneous broadening of the zero phonon line of phthalocyanine in superfluid helium droplets. J. Chem. Phys. 2001, 115, 10199–10205. [Google Scholar] [CrossRef]
- Fitch, P.S.H.; Wharton, L.; Levy, D.H. The fluorescence excitation spectrum of free base phthalocyanine cooled in a supersonic expansion. J. Chem. Phys. 1978, 69, 3424–3426. [Google Scholar] [CrossRef]
- Fitch, P.S.H.; Wharton, L.; Levy, D.H. The fluorescence spectrum of free base phthalocyanine cooled in a supersonic free jet. J. Chem. Phys. 1979, 70, 2018–2019. [Google Scholar] [CrossRef]
- Fitch, P.S.H.; Haynam, C.A.; Levy, D.H. The fluorescence excitation spectrum of free base phthalocyanine cooled in a supersonic free jet. J. Chem. Phys. 1980, 73, 1064–1072. [Google Scholar] [CrossRef]
- Fitch, P.S.H.; Haynam, C.A.; Levy, D.H. Intramolecular vibrational relaxation in jet-cooled phthalocyanine. J. Chem. Phys. 1981, 74, 6612–6620. [Google Scholar] [CrossRef]
- Nauta, K.; Miller, R.E. Metastable vibrationally excited HF (v=1) in helium nanodroplets. J. Chem. Phys. 2000, 113, 9466–9469. [Google Scholar] [CrossRef]
- Lindsay, C.M.; Lewis, W.K.; Miller, R.E. Confirmation of the metastability of HF (v=1) in helium nanodroplets. J. Chem. Phys. 2004, 121, 6095–6096. [Google Scholar] [CrossRef] [PubMed]
- Uzer, T.; Miller, W.H. Theories of intramolecular vibrational energy transfer. Phys. Rep. 1991, 199, 73–146. [Google Scholar] [CrossRef]
- Bondybey, V.E. Relaxation and vibrational energy redistribution processes in polyatomic molecules. Annu. Rev. Phys. Chem. 1984, 35, 591–612. [Google Scholar] [CrossRef]
- Lehnig, R.; Sebree, J.A.; Slenczka, A. Structure and Dynamics of Phthalocyanine-Argonn (n = 1–4) Complexes Studied in Helium Nanodroplets. J. Phys. Chem. A 2007, 111, 7576–7584. [Google Scholar] [CrossRef] [PubMed]
- Riechers, R.; Pentlehner, D.; Slenczka, A. Microsolvation in superfluid helium droplets studied by the electronic spectra of six porphyrin derivatives and one chlorine compound. J. Chem. Phys. 2013, 138, 244303. [Google Scholar] [CrossRef] [PubMed]
- Premke, T.; Wirths, E.M.; Pentlehner, D.; Riechers, R.; Lehnig, R.; Vdovin, A.; Slenczka, A. Microsolvation of molecules insuperfluid heliumnanodroplets revealed by means of electronic spectroscopy. Front. Chem. 2015, 2, 51. [Google Scholar] [CrossRef]
- Pei, L.; Zhang, J.; Kong, W. Electronic polarization spectroscopy of metal phthalocyanine chloride compounds in superfluid helium droplets. J. Chem. Phys. 2007, 127, 174308. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Zhang, J.; Kong, W.; Xu, D.; Guo, H. Polarization spectroscopy of aluminum phthalocyanine hydroxide embedded in superfluid helium droplets. Chem. Phys. Lett. 2008, 462, 173–177. [Google Scholar] [CrossRef]
- Zhang, J.; He, Y.; Freund, W.M.; Kong, W. Electron Diffraction of Superfluid Helium Droplets. J. Phys. Chem. Lett. 2014, 5, 1801–1805. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slenczka, A. Electronic Spectroscopy of Phthalocyanine and Porphyrin Derivatives in Superfluid Helium Nanodroplets. Molecules 2017, 22, 1244. https://doi.org/10.3390/molecules22081244
Slenczka A. Electronic Spectroscopy of Phthalocyanine and Porphyrin Derivatives in Superfluid Helium Nanodroplets. Molecules. 2017; 22(8):1244. https://doi.org/10.3390/molecules22081244
Chicago/Turabian StyleSlenczka, Alkwin. 2017. "Electronic Spectroscopy of Phthalocyanine and Porphyrin Derivatives in Superfluid Helium Nanodroplets" Molecules 22, no. 8: 1244. https://doi.org/10.3390/molecules22081244




