Structure Elucidation and Botanical Characterization of Diterpenes from a Specific Type of Bee Glue
Abstract
:1. Introduction
2. Results
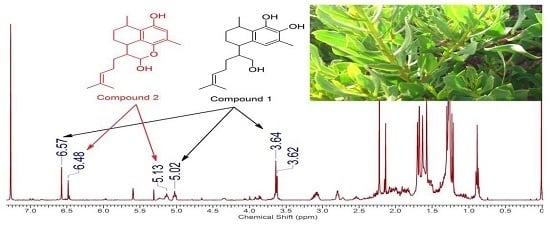
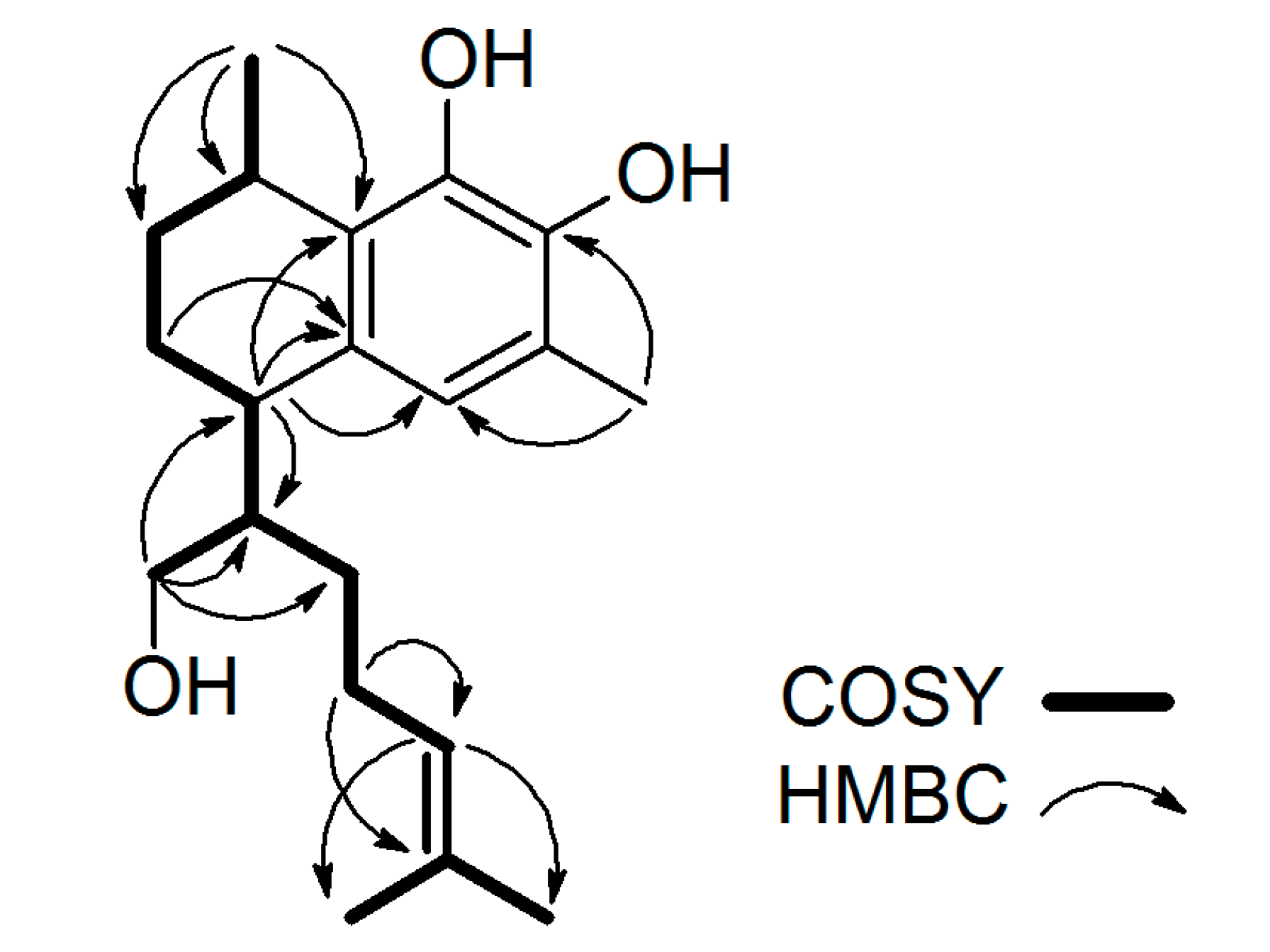
2.1. Structure Elucidation of Serrulatanes from Myoporum Bee Glue
2.2. Derivatization of Isolated Serrulatanes
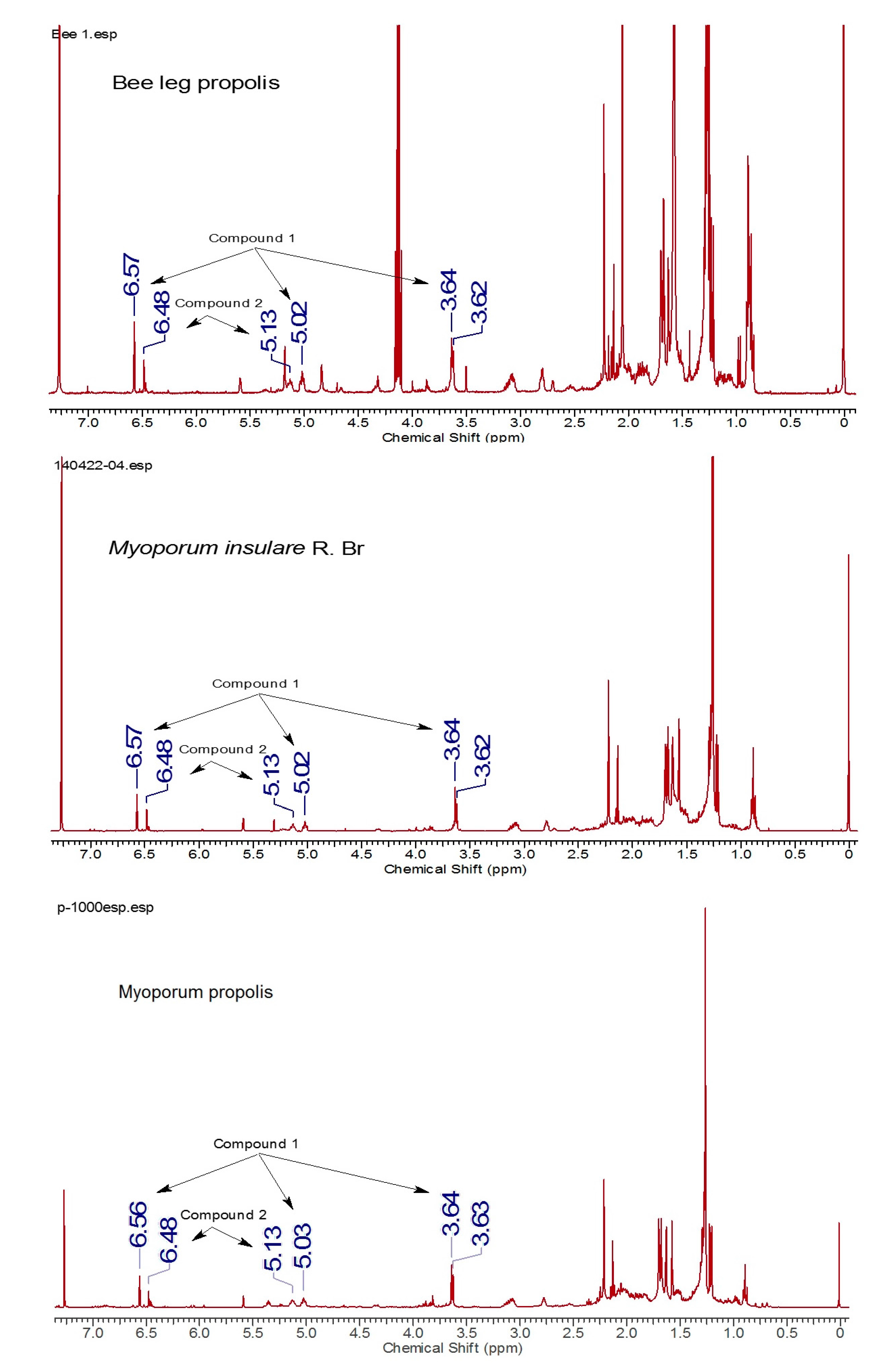
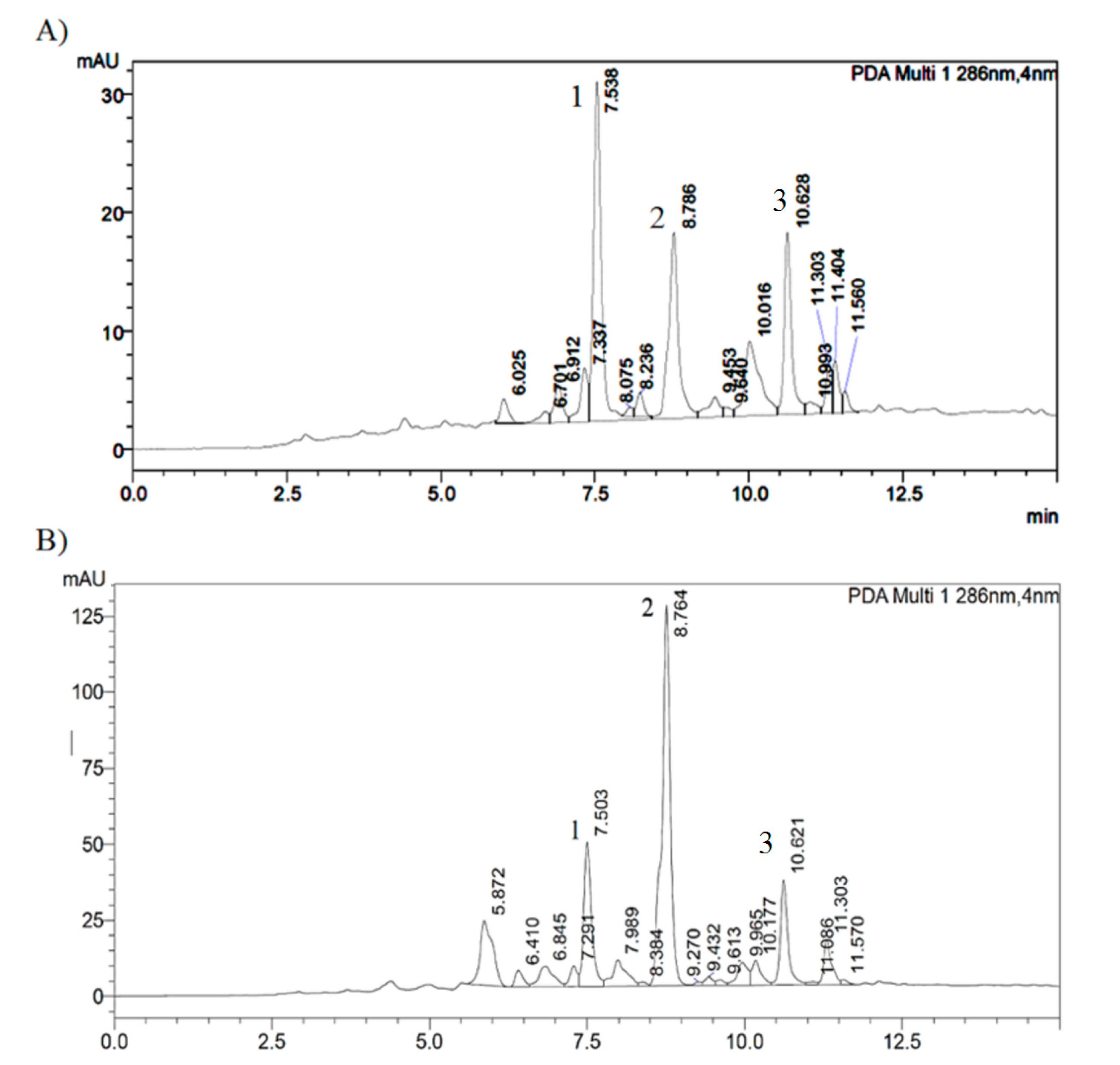
2.3. Identification of Botanical Origin
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Materials
4.3. Extraction and Isolation of Serrulatane Diterpenes from Propolis
4.4. Acetylation of Serrulatane Diterpenes
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.A.; Ferreira, T.S.; Nesi, R.T.; Lanzetti, M.; Pires, K.M.; Silva, A.M.; Borges, R.M.; Silva, A.J.; Valenca, S.S.; Porto, L.C. Antioxidant action of propolis on mouse lungs exposed to short-term cigarette smoke. Bioorgan. Med. Chem. 2013, 21, 7570–7577. [Google Scholar] [CrossRef] [PubMed]
- Athikomkulchai, S.; Awale, S.; Ruangrungsi, N.; Ruchirawat, S.; Kadota, S. Chemical constituents of Thai propolis. Fitoterapia 2013, 88, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.L. Biological activity of bee propolis in health and disease. Asian Pac. J. Cancer Preve. 2006, 7, 22–31. [Google Scholar]
- Castaldo, S.; Capasso, F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73, S1–S6. [Google Scholar] [CrossRef]
- Choudhari, M.K.; Punekar, S.A.; Ranade, R.V.; Paknikar, K.M. Antimicrobial activity of stingless bee (Trigona sp.) propolis used in the folk medicine of Western Maharashtra, India. J. Ethnopharmacol. 2012, 141, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical aspects of propolis research in modern times. Evid. Based Complement. Altern. Med. 2013, 2013, 964149–964160. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, M.G.; Franchin, M.; de Carvalho Galvao, L.C.; de Ruiz, A.L.; de Carvalho, J.E.; Ikegaki, M.; de Alencar, S.M.; Koo, H.; Rosalen, P.L. Antimicrobial and antiproliferative activities of stingless bee Melipona scutellaris geopropolis. BMC Complement. Altern. Med. 2013, 13, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Trusheva, B.; Popova, M.; Bankova, V.; Simova, S.; Marcucci, M.C.; Miorin, P.L.; da Rocha Pasin, F.; Tsvetkova, I. Bioactive constituents of brazilian red propolis. Evid. Based Complement. Altern. Med. 2006, 3, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, M.C. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- Bankova, V.S.; Castro, S.L.D.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Aminimoghadamfarouj, N.; Nematollahi, A. Propolis Diterpenes as a Remarkable Bio-Source for Drug Discovery Development: A Review. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Tippett, L.M.; Massywestropp, R.A. Serrulatane Diterpenes from Eremophila-Duttonii. Phytochemistry 1993, 33, 417–421. [Google Scholar] [CrossRef]
- O’Hora, P.S.; Incerti-Pradillos, C.A.; Kabeshov, M.A.; Shipilovskikh, S.A.; Rubtsov, A.E.; Elsegood, M.R.J.; Malkov, A.V. Catalytic Asymmetric Crotylation of Aldehydes: Application in Total Synthesis of (−)-Elisabethadione. Chemistry 2015, 21, 4551–4555. [Google Scholar] [CrossRef] [PubMed]
- Abell, A.D.; Massy-Westropp, R.A. Eremophilane and Serrulatane Terpenoids from Eremophila rotundifolia. Aust. J. Chem. 1985, 38, 1263–1269. [Google Scholar] [CrossRef]
- Duke, C.C.; Tran, V.H.; Duke, R.K.; Abu-Mellal, A.; Plunkett, G.T.; King, D.I.; Hamid, K.; Wilson, K.L.; Barrett, R.L.; Bruhl, J.J. A sedge plant as the source of Kangaroo Island propolis rich in prenylated p-coumarate ester and stilbenes. Phytochemistry 2017, 134, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.H.; Duke, R.K.; Abu-Mellal, A.; Duke, C.C. Propolis with high flavonoid content collected by honey bees from Acacia paradoxa. Phytochemistry 2012, 81, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Duke, C.; Duke, R.; Tran, V.H.; Abu-Mellal, A.; King, D. Kangaroo island propolis types and their distribution. In Proceedings of the 42th APIMONDIA International Apicultural Congress, Ukrain, Istanbul, Turkey, 29 September–4 October 2017. [Google Scholar]
- Abell, A.; Horn, E.; Jones, G.; Snow, M.; Massywestropp, R.; Riccio, R. The Structure of a Stable Serrulatane Diterpenoid Acetal From Eremophila-Rotundifolia. Aust. J. Chem. 1985, 38, 1837–1845. [Google Scholar] [CrossRef]
- Forster, P.G.; Ghisalberti, E.L.; Jefferies, P.R.; Poletti, V.M.; Whiteside, N.J. Serrulatane diterpenes from Eremophila spp. Phytochemistry 1986, 25, 1377–1383. [Google Scholar] [CrossRef]
- Syah, Y.M.; Ghisalberti, E.L. Serrulatane diterpenes from a new Eremophila species. Phytochemistry 1997, 45, 1479–1482. [Google Scholar] [CrossRef]
- Molina-Salinas, G.M.; Rivas-Galindo, V.M.; Said-Fernandez, S.; Lankin, D.C.; Munoz, M.A.; Joseph-Nathan, P.; Pauli, G.F.; Waksman, N. Stereochemical analysis of leubethanol, an anti-TB-active serrulatane, from Leucophyllum frutescens. J. Nat. Prod. 2011, 74, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.R.; Raston, C.L.; Skelton, B.W.; White, A.H. Structural studies of some serrulatane diterpenes. J. Chem. Soc. Perkin Trans. 1981. [Google Scholar] [CrossRef]
- Ndi, C.P.; Semple, S.J.; Griesser, H.J. Instability of Antibacterial Serrulatane Compounds from the Australian Plant Species Eremophila duttonii. Aust. J. Chem. 2012, 65, 20–27. [Google Scholar] [CrossRef]
- Ndi, C.P.; Semple, S.J.; Griesser, H.J.; Pyke, S.M.; Barton, M.D. Antimicrobial compounds from the Australian desert plant Eremophila neglecta. J. Nat. Prod. 2007, 70, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.C.; Kavanagh, A.M.; Ramu, S.; Blaskovich, M.A.; Cooper, M.A.; Davis, R.A. Antibacterial serrulatane diterpenes from the Australian native plant Eremophila microtheca. Phytochemistry 2013, 93, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Harrington, D.; Kohen, J.L.; Vemulpad, S.; Jamie, J.F. Bactericidal and cyclooxygenase inhibitory diterpenes from Eremophila sturtii. Phytochemistry 2006, 67, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Croft, K.D.; Ghisalberti, E.L.; Jefferies, P.R.; Raston, C.L.; White, A.H.; Hall, S.R. Chemistry of Eremophila spp. 6. Stereochemistry and Crystal-Structure of Dihydroxyserrulatic Acid. Tetrahedron 1977, 33, 1475–1480. [Google Scholar] [CrossRef]
- Chinnock, R.J. Eremophila and Allied Genera: A Monograph of the Plant Family Myoporaceae; Rosenberg Publishing: Kenthurst, Australia, 2007. [Google Scholar]
- Overton, D.S.; Overton, B.M. Discover Kangaroo Island’s Native Plants; Environmental Realist: Kingscote, Australia, 2012. [Google Scholar]
- Ball, D.M.; Carruthers, S. Kangaroo Island Vegetation Mapping; Information and Data Analysis Branch, Planning SA, Department for Transport, Urban Planning and the Arts: Adelaide, Australia, 1998. [Google Scholar]
- Jackson, I.; Parks, S.A.N.; Service, W. The Flora of Kangaroo Island: From the Sketch Books of Ida Jackson; National Parks and Wildlife Service, Department of Environment and Planning: Adelaide, Australia, 1988. [Google Scholar]
- Righi, A.A.; Negri, G.; Salatino, A. Comparative chemistry of propolis from eight brazilian localities. Evid. Based Complement. Altern. Med. 2013, 2013, 267878–267892. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Araujo, M.J.; Mattar, N.S.; Reis, A.S.; Serra, I.C.; Fialho, E.M.; Assuncao, A.K.; Dutra, R.P.; Nogueira, A.M.; Liberio, S.A.; Guerra, R.N.; et al. Pharmacognostic and acute toxicological evaluation of Scaptotrigona aff. postica propolis extract in pre-clinical assays. Nat. Prod. Res. 2011, 25, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.P.; Graikou, K.; Chinou, I.; Bankova, V.S. GC-MS Profiling of Diterpene Compounds in Mediterranean Propolis from Greece. J. Agric. Food Chem. 2010, 58, 3167–3176. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Trusheva, B.; Antonova, D.; Cutajar, S.; Mifsud, D.; Farrugia, C.; Tsvetkova, I.; Najdenski, H.; Bankova, V. The specific chemical profile of Mediterranean propolis from Malta. Food Chem. 2011, 126, 1431–1435. [Google Scholar] [CrossRef]
- Popova, M.; Bankova, V.; Tsvetkova, I.; Naydenski, C.; Silva, M.V. The first glycosides isolated from propolis: Diterpene rhamnosides. Z. Naturforsch. C 2001, 56, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Jackson, H.; Kendal, L.P. The oxidation of catechol and homocatechol by tyrosinase in the presence of amino-acids. Biochem. J. 1949, 44, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Busch, J.M. Enzymic browning in potatoes: A simple assay for a polyphenol oxidase catalysed reaction. Biochem. Educ. 1999, 27, 171–173. [Google Scholar] [CrossRef]
- Davis, R.A.; Carroll, A.R.; Pierens, G.K.; Quinn, R.J. New lamellarin alkaloids from the australian ascidian, didemnum chartaceum. J. Nat. Prod. 1999, 62, 419–424. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| #H | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 3.07 pd (6.8, 1.6) | 3.11 sextet (7.6) | 2.82 m | 2.92 m | 2.96 sextet (7.4) |
| 2 | A: 1.51 m B: 1.99 m | A: 2.25 m B: 1.42 m | A: 2.15 m B: 1.23 m | A: 1.87 m B: 1.50 m | A: 2.19 m B: 1.42 m |
| 3 | A: 1.66 m B: 1.86 m | A: 2.08 m B: 1.05 m | A: 2.13 m B: 1.11 m | A: 1.87 m B:1.60 m | A: 2.12 m B: 1.10 m |
| 4 | 2.76 td (5.6, 2.6) | 2.54 td (11.4, 3.6) | 2.14 m | 2.88 m | 2.54 td (11.6, 3.8) |
| 5 | 6.56 s | - | - | 6.92 s | - |
| 6 | - | - | - | - | - |
| 7 | - | 6.48 s | - | - | 6.70 s |
| 8 | - | - | - | - | - |
| 9 | - | - | - | - | - |
| 10 | - | - | - | - | - |
| 11 | 1.83 m | 1.52 m | 1.74 m | 2.12 m | 1.65 m |
| 12 | A: 1.36 m B: 1.25 m | A: 1.69 m B: 1.27 m | A: 1.19 m B: 1.76 m | A: 1.33 m B: 1.23 m | A: 1.73 m B: 1.30 m |
| 13 | A: 1.86 m B: 1.90 m | A: 2.20 m B: 2.09 m | A: 2.09 m B: 1.97 m | A: 1.99 m B: 1.87 m | A: 2.19 m B: 2.02 m |
| 14 | 5.04 bt (7.0) | 5.14 bt (7.0) | 5.07 bt (6.8) | 4.97 bt (7.1) | 5.08 bt (6.5) |
| 15 | - | - | - | - | - |
| 16 | 1.68 s | 1.70 s | 1.71 s | 1.66 s | 1.69 s |
| 17 | 1. 58 s | 1. 63 s | 1.63 s | 1. 54 s | 1.62 s |
| 18 | 3.63 d (6.2) | 5.59 bs | A: 3.86 t (10.8) B: 4.49 dd (10.8, 3.6) | 4.11 d (6.3) | 6.53 d (1.7) |
| 19 | 2.2 s | 2.13 s | 1.81 s | 2.14 s | 2.14 s |
| 20 | 1.21 d (7.0) | 1.28 d (6.9) | 1.13 d (6.9) | 1.15 d (7.0) | 1.23 d (6.8) |
| 21 | - | - | - | - | - |
| 22 | - | - | - | 2.06 s | 2.09 s |
| 23 | - | - | - | - | - |
| 24 | - | - | - | 2.29 s | 2.30 s |
| 25 | - | - | - | - | - |
| 26 | - | - | - | 2.31 s | - |
| #C | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 26.9 | 27.8 | 28.7 | 27.6 | 28.3 |
| 2 | 26.5 | 31.7 | 30.8 | 26.9 | 31.4 |
| 3 | 20.5 | 26.6 | 25.3 | 19.2 | 26.2 |
| 4 | 37.9 | 31.9 | 40.1 | 36.9 | 32.3 |
| 5 | 122.0 | 141.7 | 162.8 | 128.1 | 145.6 |
| 6 | 121.3 | 122.9 | 114.4 | 128.3 | 123.9 |
| 7 | 139.7 | 115.9 | 179.4 | 139.3 | 122.5 |
| 8 | 141.6 | 146.8 | 181.9 | 140.6 | 142.4 |
| 9 | 127.2 | 125.2 | 139.4 | 134.1 | 130.9 |
| 10 | 130.7 | 124.3 | 149.7 | 137.1 | 123.8 |
| 11 | 45.4 | 40.5 | 37.1 | 41.9 | 38.9 |
| 12 | 29.8 | 28.9 | 29.1 | 28.3 | 28.3 |
| 13 | 26.2 | 25.4 | 24.8 | 26.1 | 25.1 |
| 14 | 124.4 | 124.1 | 123.1 | 123.9 | 123.5 |
| 15 | 131.7 | 131.9 | 132.9 | 132.0 | 132.6 |
| 16 | 25.7 | 25.7 | 25.7 | 25.7 | 25.7 |
| 17 | 17.7 | 17.7 | 17.8 | 17.6 | 17.7 |
| 18 | 64.4 | 91.9 | 71.8 | 65.4 | 90.2 |
| 19 | 15.5 | 15.9 | 7.6 | 16.1 | 15.9 |
| 20 | 21.1 | 22.9 | 21.7 | 21.4 | 22.8 |
| 21 | - | - | - | 171.2 | 170.2 |
| 22 | - | - | - | 20.9 | 21.1 |
| 23 | - | - | - | 168.1 | 170.0 |
| 24 | - | - | - | 20.4 | 21.2 |
| 25 | - | - | - | 168.3 | - |
| 26 | - | - | - | 20.5 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aminimoghadamfarouj, N.; Nematollahi, A. Structure Elucidation and Botanical Characterization of Diterpenes from a Specific Type of Bee Glue. Molecules 2017, 22, 1185. https://doi.org/10.3390/molecules22071185
Aminimoghadamfarouj N, Nematollahi A. Structure Elucidation and Botanical Characterization of Diterpenes from a Specific Type of Bee Glue. Molecules. 2017; 22(7):1185. https://doi.org/10.3390/molecules22071185
Chicago/Turabian StyleAminimoghadamfarouj, Noushin, and Alireza Nematollahi. 2017. "Structure Elucidation and Botanical Characterization of Diterpenes from a Specific Type of Bee Glue" Molecules 22, no. 7: 1185. https://doi.org/10.3390/molecules22071185