Cholinesterase and Prolyl Oligopeptidase Inhibitory Activities of Alkaloids from Argemone platyceras (Papaveraceae)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Choice of Plant Material
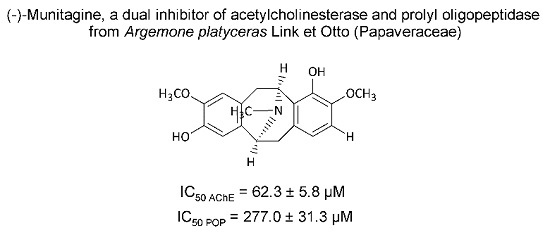
2.2. Isolated Alkaloids
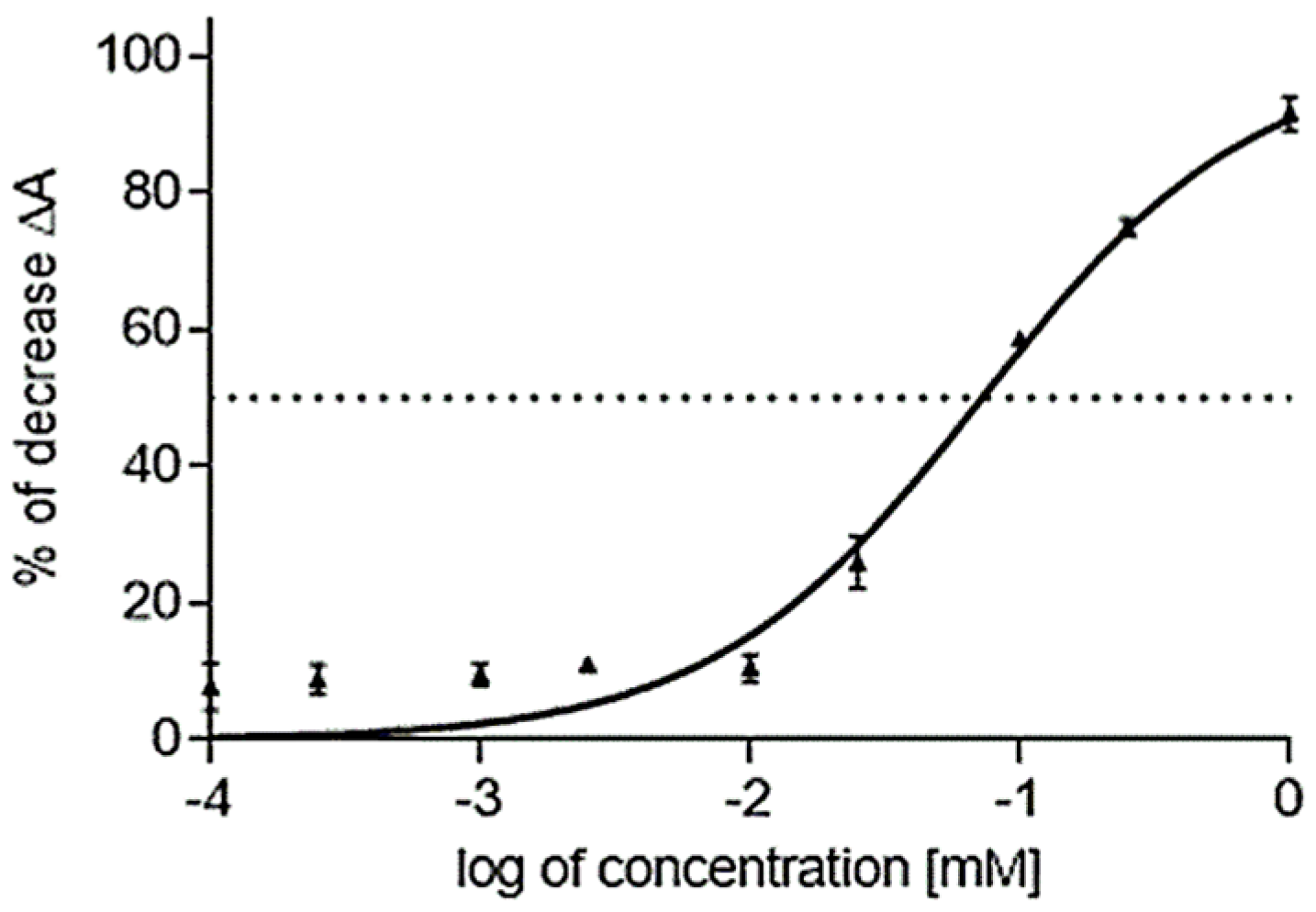
2.3. Cholinesterase Inhibitory Activity of Isolated Alkaloids
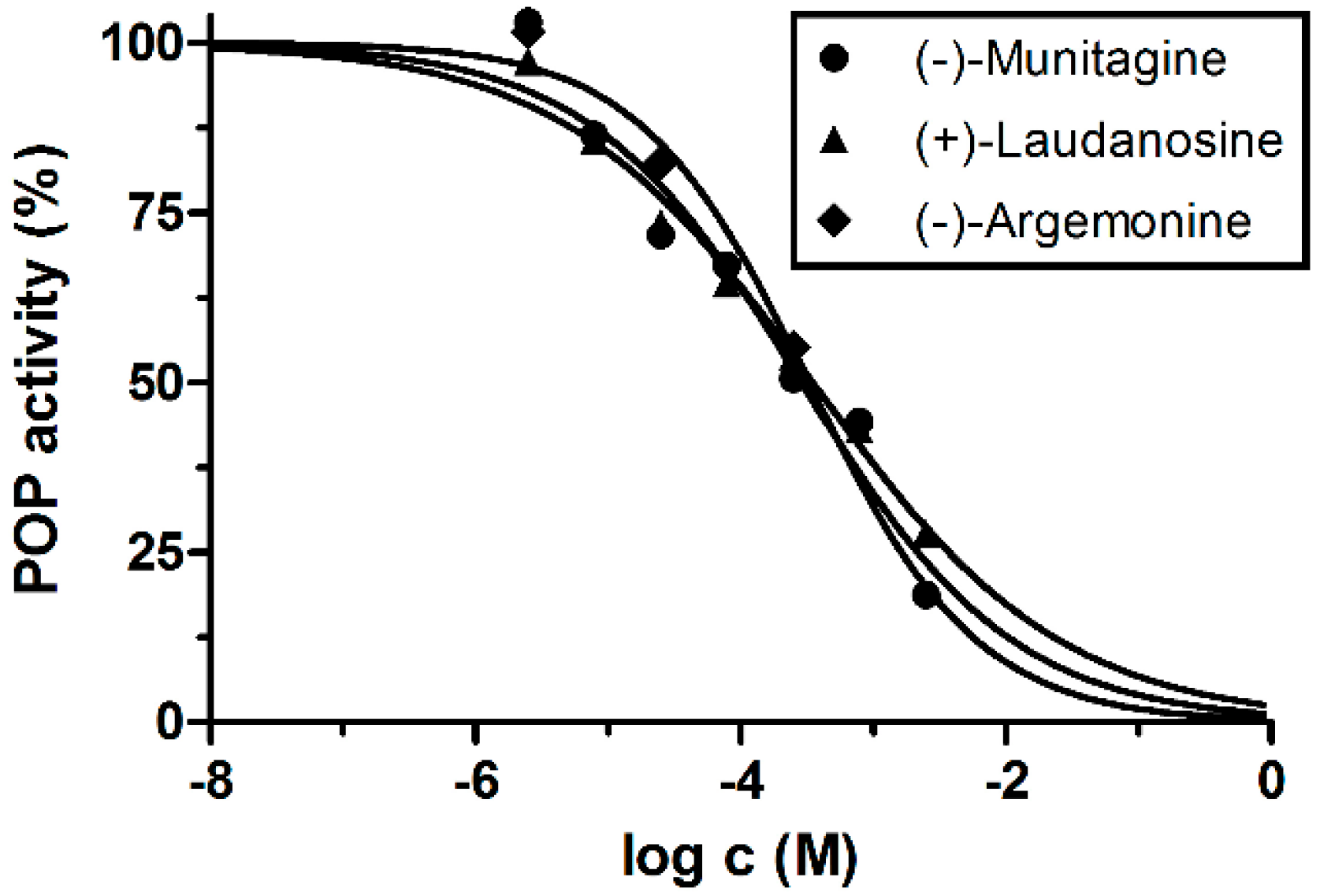
2.4. Prolyl Oligopeptidase Inhibitory Activity of Isolated Alkaloids
3. Experimental
3.1. Plant Material
3.2. General Experimental Procedures
3.3. Extraction and Isolation of Alkaloids
3.4. Characterization Data
3.5. Biological Assays
3.5.1. Materials
3.5.2. Preparation of Enzymes for AChE, BChE Assays
3.5.3. Cholinesterases Assays
3.5.4. Prolyl Oligopeptidase Assay
3.5.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lleó, A. Current therapeutic options for Alzheimer’s disease. Curr. Genom. 2007, 8, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y. Potential therapeutic agents against Alzheimer’s disease from natural sources. Arch. Pharm. Res. 2010, 33, 1589–1609. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.; Malik, A.; Qureshi, M.S.; Manan, A.; Pushparaj, P.N.; Asif, M.; Qazi, M.H.; Qazi, A.M.; Kamal, M.A.; Gan, S.H.; et al. Recent updates in the treatment of neurodegenerative disorders using natural compounds. Evid. Based Complement. Alternat. Med. 2014, 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, A.; Ballard, C.; Bullock, R.; Darreh-Shori, T.; Somogyi, M. A Review of Butyrylcholinesterase as a Therapeutic Target in the Treatment of Alzheimer’s disease. Prim. Care Companion CNS Disord. 2013, 15. [Google Scholar] [CrossRef]
- Standridge, J.B. Pharmacotherapeutic approaches to the treatment of Alzheimer’s disease. Clin. Ther. 2004, 26, 615–630. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Yu, Q.; Zhu, X.; Holloway, H.W.; Perry, T.A.; Lee, B.; Ingram, D.K.; Lahiri, D.K. A New therapeutic target in Alzheimer’s disease treatment: Attention to butyrylcholinesterase. Curr. Med. Res. Opin. 2001, 17, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.K.; Atack, J.R.; Perry, R.H.; Hardy, J.A.; Dodd, P.R.; Edwardson, J.A.; Blessed, G.; Tomlinson, B.E.; Fairbairn, A.F. Intralaminar neurochemical distributions in human midtemporal cortex: Comparison between Alzheimer’s disease and the normal. J. Neurochem. 1984, 42, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.K.; Tomlinson, B.E.; Blessed, G.; Bergmann, K.; Gibson, P.H.; Perry, R.H. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. BMJ 1978, 2, 1457–1459. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007, 14, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Sener, B.; Choudhary, M.I.; Khalid, A. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. J. Ethnopharmacol. 2004, 91, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Adsersen, A.; Gauguin, B.; Gudiksen, L.; Jäger, A.K. Screening of plants used in Danish folk medicine to treat memory dysfunction for acetylcholinesterase activity. J. Ethnopharmacol. 2006, 104, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Berkov, S.; Bastida, J.; Nikolova, M.; Viladomat, F.; Codina, C. Rapid TLC/GC-MS identification of acetylcholinesterase inhibitors in alkaloid extracts. Phytochem. Anal. 2008, 19, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Hayes, M.; Carney, B. Angiotensin-I-converting enzyme and prolyl endopeptidase inhibitory peptides from natural sources with a focus on marine processing by-products. Food Chem. 2011, 129, 235–244. [Google Scholar] [CrossRef]
- López, A.; Tarragó, T.; Giralt, E. Low molecular weight inhibitors of prolyl oligopeptidase: A review of compounds patented from 2003 to 2010. Expert Opin. Ther. Pat. 2011, 21, 1023–1044. [Google Scholar]
- Männistö, P.T.; García-Horsman, J.A. Mechanism of Action of prolyl oligopeptidase (PREP) in degenerative brain diseases: Has peptidase activity only a modulatory role on the interactions of PREP with proteins? Front. Aging Neurosci. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Brandt, I.; Scharpé, S.; Lambeir, A.M. Suggested functions for prolyl oligopeptidase: A puzzling paradox. Clin. Chim. Acta 2007, 377, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Morain, P.; Lestage, P.; de Nanteuil, G.; Jochemsen, R.; Robin, J.L.; Guez, D.; Boyer, P.A. S 17092: A prolyl endopeptidase inhibitor as a potential therapeutic drug for memory impairment. Preclinical and clinical studies. CNS Drug Rev. 2002, 8, 31–52. [Google Scholar] [CrossRef] [PubMed]
- Mellow, A.H.; Aronson, S.M.; Giordani, B.; Berent, S. A peptide enhancement strategy in Alzheimer’s disease; Pilot study with TRH_physostigmine infusions. Biol. Psychiatry 1993, 34, 271–273. [Google Scholar] [CrossRef]
- Mellow, A.H.; Sunderland, T.S.; Cohen, R.M.; Lawlor, B.A.; Hill, J.L.; Newhouse, P.A.; Cohen, M.R.; Murphy, D.L. Acute effects of high-dose thyrotrophin-releasing hormone infusions in Alzheimer’s disease. Psychopharmacol. 1989, 98, 403–407. [Google Scholar] [CrossRef]
- Tezuka, Y.; Fan, W.; Kasimu, R.; Kadota, S. Screening of crude drug extracts for prolyl endopeptidase inhibitory activity. Phytomedicine 1999, 6, 197–203. [Google Scholar] [CrossRef]
- Ishiura, S.; Tsukahara, T.; Tabira, T.; Shimizu, T.; Arahata, K.; Sugita, H. Identification of a putative amyloid A4-generating enzyme as a prolyl endopeptidase. FEBS Lett. 1990, 260, 131–134. [Google Scholar] [CrossRef]
- Lawandi, J.; Gerber-Lemaire, S.; Juillerat-Jeanneret, L.; Moitessier, N. Inhibitors of prolyl oligopeptidases for the therapy of human diseases: Defining diseases and inhibitors. J. Med. Chem. 2010, 53, 3423–3438. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, K.S.M.; van Groen, T.; Tanila, H.; Venäläinen, J.; Männistö, P.T.; Alafuzoff, I. Brain prolyl oligopeptidase activity is associated with neuronal damage rather than β-amyloid accumulation. Neuroreport 2001, 12, 3309–3312. [Google Scholar] [CrossRef] [PubMed]
- Morain, P.; Robin, J.L.; Nanteuil, G.D.; Jochemsen, R.; Heidet, V.; Guez, D. Pharmacodynamic and pharmacokinetic profile of S 17092, a new orally active prolyl endopeptidase inhibitor, in elderly healthy volunteers. A phase I study. Br. J. Clin. Pharmacol. 2000, 50, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Morain, P.; Boeijinga, P.H.; Demazières, A.; de Nanteuil, G.; Luthringer, R. Psychotropic Profile of S 17092, a prolyl endopeptidase inhibitor, Using quantitative EEG in young healthy volunteers. Neuropsychobiology 2007, 55, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E. Current concepts on selected plant secondary metabolites with promising inhibitory effects against enzymes linked to Alzheimer’s disease. Curr. Med. Chem. 2012, 19, 2252–2261. [Google Scholar] [CrossRef] [PubMed]
- Cahlíková, L.; Hulová, L.; Hrabinová, M.; Chlebek, J.; Hošťálková, A.; Adamcová, M.; Šafratová, M.; Jun, D.; Opletal, L.; Ločárek, M.; et al. Isoquinoline alkaloids as prolyl oligopeptidase inhibitors. Fitoterapia 2015, 103, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Chlebek, J.; Novák, Z.; Kassemová, D.; Šafratová, M.; Kostelník, J.; Malý, L.; Ločárek, M.; Opletal, L.; Hošťálková, A.; Hrabinová, M.; et al. Isoquinoline alkaloids from Fumaria officinalis L. and their biological activities related to Alzheimer’s disease. Chem. Biodivers. 2016, 13, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Cahlíková, L.; Hrabinová, M.; Kulhánková, A.; Benešová, N.; Chlebek, J.; Jun, D.; Novák, Z.; Macáková, K.; Kuneš, J.; Kuča, K.; et al. Alkaloids from Chlidanthus fragrans and their acetylcholinesterase, butyrylcholinesterase and prolyl oligopeptidase. Nat. Prod. Commun. 2013, 8, 1541–1544. [Google Scholar] [PubMed]
- Schwarzbach, A.E.; Kadereit, J.W. Phylogeny of prickly poppies, Argemone (Papaveraceae), and the evolution of morphological and alkaloid characters based on ITS nrDNA sequence variation. Plant Syst. Evol. 1999, 218, 257–279. [Google Scholar] [CrossRef]
- Fernandez, J.; Reyes, R.; Ponce, H.; Oropeza, M.; Vancalsteren, M.R.; Jankowski, C.; Campos, M.G. Isoquercitrin from Argemone platyceras inhibits carbachol and leukotriene D4-induced contraction in guinea-pig airways. Eur. J. Pharmacol. 2005, 522, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Boit, H.G.; Flentje, H. Alkaloide aus Argemone platyceras. Naturwissenschaften 1960, 47, 323. [Google Scholar]
- Slavík, J.; Slavíková, L. Alkaloide der Mohngewächse (Papaveraceae) XII. Über die Alkaloide aus Argemone platyceras Link et Otto. Coll. Czech. Chem. Commun. 1963, 28, 1728–1737. [Google Scholar] [CrossRef]
- Slavík, J.; Slavíková, L.; Haisová, K. Alkaloids of the Papaveraceae. L. On the quaternary alkaloids from Argemone platyceras Link et Otto. Coll. Czech. Chem. Commun. 1973, 38, 2513–2517. [Google Scholar] [CrossRef]
- Israilov, I.A.; Yunusov, M.S. Alkaloids of four species of Argemone. Chem. Nat. Comp. 1986, 22, 189–192. [Google Scholar] [CrossRef]
- Chelombitko, V.A.; Nazarova, L.E. Alkaloids from several Argemone species. Pharm. Chem. J. 1988, 22, 580–585. [Google Scholar]
- Slavík, J.; Slavíková, L. Quaternary alkaloids from the roots of Argemone platyceras Link et Otto. Coll. Czech. Chem. Commun. 1976, 41, 285–289. [Google Scholar] [CrossRef]
- Cahlíková, L.; Kučera, R.; Hošťálková, A.; Klimeš, J.; Opletal, L. Identification of pavinane alkaloids in the genera Argemone and Eschscholzia by GC-MS. Nat. Prod. Commun. 2012, 7, 1279–1281. [Google Scholar] [PubMed]
- Khorana, N.; Markmee, S.; Ingkaninan, K.; Ruchirawat, S.; Kitbunnadaj, R.; Pullagurla, M.R. Evaluation of new lead for acetylcholinesterase inhibition. Med. Chem. Res. 2009, 18, 231–241. [Google Scholar] [CrossRef]
- Castaing, N.; Benali, L.; Ducint, D.; Molimard, M.; Gromb, S.; Titier, K. Suicide with cisatracurium and thiopental: Forensic and analytical aspects. J. Anal. Toxicol. 2011, 35, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.H.A.M.; Wijkens, P.; Kruk, C.; Biessels, H.W.A.; Menichini, F.; Theuns, H.G. Assignments of 1H and 13C-NMR resonances of some isoquinoline alkaloids. Phytochemistry 1990, 29, 3331–3339. [Google Scholar] [CrossRef]
- Takahashi, H.; Iguchi, M.; Onda, M. Utilization of protopine and related alkaloids. XVII. Spectroscopic studies on the ten-membered ring conformations of protopine and α-allocryptopine. Chem. Pharm. Bull. 1985, 33, 4775–4782. [Google Scholar] [CrossRef]
- Leyva-Peralta, M.A.; Robles-Zepeda, R.E.; Garibay-Escobar, A.; Ruiz-Bustos, E.; Alvarez-Berber, L.P.; Gálvez-Ruiz, J.C. In vitro anti-proliferative activity of Argemone gracilenta and identification of some active components. BMC Complement. Alternat. Med. 2015, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Shamma, M.; Moniot, J.L. A novel isoquinoline alkaloid group. The aporphine-pavine dimers. J. Am. Chem. Soc. 1974, 96, 3338–3340. [Google Scholar] [CrossRef]
- Lee, S.S.; Doskotch, R.W. Four dimeric aporphine-containing alkaloids from Thalictrum fauriei. J. Nat. Prod. 1999, 62, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Stermitz, F.R.; Seiber, J.N. Alkaloids of the Papaveraceae. IV. Argemone hispida and A. munita subspecies routundata. J. Org. Chem. 1966, 31, 2925–2933. [Google Scholar]
- Stermitz, F.R.; Seiber, J.N. Alkaloids of Papaveraceae. III. Synthesis and structure of norargemonine. Tetrahedron Lett. 1966, 11, 1177–1183. [Google Scholar] [CrossRef]
- Benn, M.H.; Mitchell, R.E. Alkaloids of Argemone grandiflora. Phytochemistry 1972, 11, 461–464. [Google Scholar] [CrossRef]
- Dang, T.T.T.; Facchini, P.J. Characterization of three O-methyltransferases involved in noscapine biosynthesis in opium poppy. Plant Physiol. 2012, 159, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Vacek, J.; Walterová, D.; Vrublová, E.; Šimánek, V. The chemical and biological properties of protopine and allocryptopine. Heterocycles 2010, 81, 1773–1789. [Google Scholar] [CrossRef]
- Gözler, B.; Lantz, M.S.; Shamma, M. The pavine and isopavine alkaloids. J. Nat. Prod. 1983, 46, 293–309. [Google Scholar] [CrossRef]
- Ehret, M.J.; Chamberlin, K.W. Current practices in the treatment of Alzheimer disease: Where is the evidence after the phase III Trials? Clin. Ther. 2015, 37, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.S.; Mangialasche, F.; Andreasen, N.; Feldman, H.; Giacobini, E.; Jones, R.; Mantua, V.; Mecocci, P.; Pani, L.; Winblad, B.; et al. Clinical trials and late-stage drug development for Alzheimer’s disease: An appraisal from 1984 to 2014. J. Intern. Med. 2014, 275, 251–283. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E. Cholinesterase inhibitors: New roles and therapeutic alternatives. Pharmacol. Res. 2004, 50, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, N.; Pai, M.; Senanarong, V.; Looi, I.; Ampil, E.; Park, K.W.; Karanam, A.K.; Christopher, S. Rivastigmine: The advantages of dual inhibition of acetylcholinesterase and butyrylcholinesterase and its role in subcortical vascular dementia and Parkinson’s disease dementia. Clin. Interv. Aging 2017, 12, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Cahlíková, L.; Macáková, K.; Kuneš, J.; Kurfürst, M.; Opletal, M.; Cvačka, J.; Chlebek, J.; Blunden, G. Acetylcholinesterase and butyrylcholinesterase inhibitory compounds from Eschscholzia californica (Papaveraceae). Nat. Prod. Commun. 2010, 5, 1035–1038. [Google Scholar] [PubMed]
- Sener, B.; Orhan, I. Discovery of drug candidates from some Turkish plants and conservation of biodiversity. Pure Appl. Chem. 2005, 77, 53–64. [Google Scholar] [CrossRef]
- Hošt’álková, A.; Opletal, L.; Kuneš, J.; Novák, Z.; Hrabinová, M.; Chlebek, J.; Čegan, L.; Cahlíková, L. Alkaloids from Peumus boldus and their acetylcholinesterase, butyrylcholinesterase and prolyl oligopeptidase inhibition activity. Nat. Prod. Commun. 2015, 10, 577–580. [Google Scholar] [PubMed]
- Chlebek, J.; Macáková, K.; Cahlíková, L.; Kurfürst, M.; Kuneš, J.; Opletal, L. Acetylcholinesterase and butyrylcholinesterase inhibitory compounds from Corydalis cava (Fumariaceae). Nat. Prod. Commun. 2011, 6, 607–610. [Google Scholar] [PubMed]
- Markmee, S.; Ruchirawat, S.; Prachyawarakorn, V.; Ingkaninan, K.; Khorana, N. Isoquinoline derivatives as potential acetyl-cholinesterase inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 2170–2172. [Google Scholar] [CrossRef] [PubMed]
- Sener, B.; Orhan, I. Molecular diversity in the bioactive compounds from Turkish plants–evaluation of acetylcholinesterase inhibitory activity of Fumaria species. J. Chem. Soc. Pak. 2004, 26, 313–315. [Google Scholar]
- Brunhofer, G.; Karlsson, D.; Batista-Gonzalez, A.; Shinde, P.C.; Mohan, C.G.; Vuorela, P. Exploration of natural compounds as sources of new bifunctional scaffolds targeting cholinesterases and beta amyloid aggregation: The case of chelerythrine. Bioorg. Med. Chem. 2012, 20, 6669–6679. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.J.; Shen, L. Berberine: A potential multipotent natural product to combat Alzheimer’s disease. Molecules 2011, 9, 6732–6740. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Min, B.S.; Yokozawa, T.; Lee, J.H.; Kim, Y.S.; Choi, J.S. Anti-Alzheimer and antioxidant activities of Coptidis rhizoma alkaloids. Biol. Pharm. Bull. 2009, 32, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Kukula-Koch, W.; Mroczek, T. Application of hydrostatic CCC-TLC-HPLC-ESI-TOF-MS for the bioguided fractionation of anticholinesterase alkaloids from Argemone mexicana L. roots. Anal. Bioanal. Chem. 2015, 407, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Lin, Q.; Luo, G.S.; Dai, Y.Y. Solubility of berberine chloride in various solvents. J. Chem. Eng. Data 2006, 51, 642–644. [Google Scholar] [CrossRef]
- NCBI. PubChem. Pubchem Compound. Available online: https://www.ncbi.nlm.nih.gov/pccompound (accessed on 15 May 2017).
- Steck, T.L.; Kant, J.A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974, 31, 172–180. [Google Scholar] [PubMed]
- Vaněčková, N.; Hošťálková, A.; Šafratová, M.; Kuneš, J.; Hulcová, D.; Hrabinová, M.; Doskočil, I.; Štěpanková, Š.; Opletal, L.; Nováková, L.; et al. Isolation of Amaryllidaceae alkaloids from Nerine bowdenii W. Watson and their biological activities. RSC Adv. 2016, 6, 80114. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds, except 2 and 4, are not available from the authors. |
| Alkaloid | Plant Part [Literature] |
|---|---|
| (–)-platycerine | aerial parts [33,34,35], roots [33] |
| (–)-munitagine | aerial parts [35], aerial parts + roots [36] * |
| (–)-argemonine | aerial parts [33,34,35], aerial parts + roots [36] * |
| protopine | aerial parts [32,33,34,35], roots [33] |
| (+)-reticuline | aerial parts [35], aerial parts + roots [36] * |
| (–)-norargemonine | aerial parts [32,33,34], roots [33] |
| allocryptopine | aerial parts [32,34,35], roots [33] |
| chelerythrine | aerial parts [34] |
| coptisine | aerial parts [33,34], roots [33,37] |
| berberine | aerial parts [33,34,35], roots [33,37] |
| (–)-O-methylplatycerine | aerial parts [35] |
| corysamine | aerial parts [34], roots [37] |
| sanguinarine | aerial parts [33,34,35], roots [33] |
| (–)-scoulerine | aerial parts + roots [36] * |
| (–)-platycerine methohydroxide | aerial parts [34], roots [37] |
| (–)-argemonine methohydroxide | aerial parts [34] |
| (–)-α-stylopine methohydroxide | aerial parts [34,35], roots [37] |
| (+)-magnoflorine | aerial parts [35], roots [37] |
| (–)-α-canadine methohydroxide | roots [37] |
| (–)-cyclanoline | roots [37] |
| armepavine | aerial parts [38] ** |
| escholtzine | aerial parts [38] ** |
| isonorargemonine | aerial parts [38] ** |
| bisnorargemonine | aerial parts [38] ** |
| cryptocavine | aerial parts [38] ** |
| cryptopalmatine | aerial parts [38] ** |
| Compounds | AChE (IC50, µM) a | BChE (IC50, µM) a | POP (IC50, µM) a |
|---|---|---|---|
| 1 | >1000 | >1000 | 341.0 ± 37.5 |
| 2 | 230.0 ± 21.0 | 208.9 ± 17.7 | >1000 |
| 3 | >1000 | 885.5 ± 119.5 | 337.0 ± 83.1 |
| 4 | 250.0 ± 25.0 | 530.0 ± 28.2 | >1000 |
| 5 | 223.7 ± 19.6 | >1000 | 687.0 ± 74.0 b |
| 6 | 62.3 ± 5.8 | 837.4 ± 23.0 | 277.0 ± 31.3 |
| 7 | 205.2 ± 11.7 | >1000 | n.d c |
| Galanthamine * | 1.71 ± 0.07 | 42.30 ± 1.3 | n.d. |
| Huperzine A | 0.033 ± 0.001 | >1000 | n.d. |
| Serine * | 0.063 ± 0.001 | 0.13 ± 0.004 | n.d. |
| Berberine * | n.d. | n.d. | 142.0 ± 21.5 |
| Z-Pro-prolinal * | n.d | n.d | 3.27 ± 0.02 × 10−3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siatka, T.; Adamcová, M.; Opletal, L.; Cahlíková, L.; Jun, D.; Hrabinová, M.; Kuneš, J.; Chlebek, J. Cholinesterase and Prolyl Oligopeptidase Inhibitory Activities of Alkaloids from Argemone platyceras (Papaveraceae). Molecules 2017, 22, 1181. https://doi.org/10.3390/molecules22071181
Siatka T, Adamcová M, Opletal L, Cahlíková L, Jun D, Hrabinová M, Kuneš J, Chlebek J. Cholinesterase and Prolyl Oligopeptidase Inhibitory Activities of Alkaloids from Argemone platyceras (Papaveraceae). Molecules. 2017; 22(7):1181. https://doi.org/10.3390/molecules22071181
Chicago/Turabian StyleSiatka, Tomáš, Markéta Adamcová, Lubomír Opletal, Lucie Cahlíková, Daniel Jun, Martina Hrabinová, Jiří Kuneš, and Jakub Chlebek. 2017. "Cholinesterase and Prolyl Oligopeptidase Inhibitory Activities of Alkaloids from Argemone platyceras (Papaveraceae)" Molecules 22, no. 7: 1181. https://doi.org/10.3390/molecules22071181