Specialized Metabolites of the Lichen Vulpicida pinastri Act as Photoprotective Agents
Abstract
:1. Introduction
2. Results and discussion
2.1. Detection and Isolation of the Major Lichen Compounds
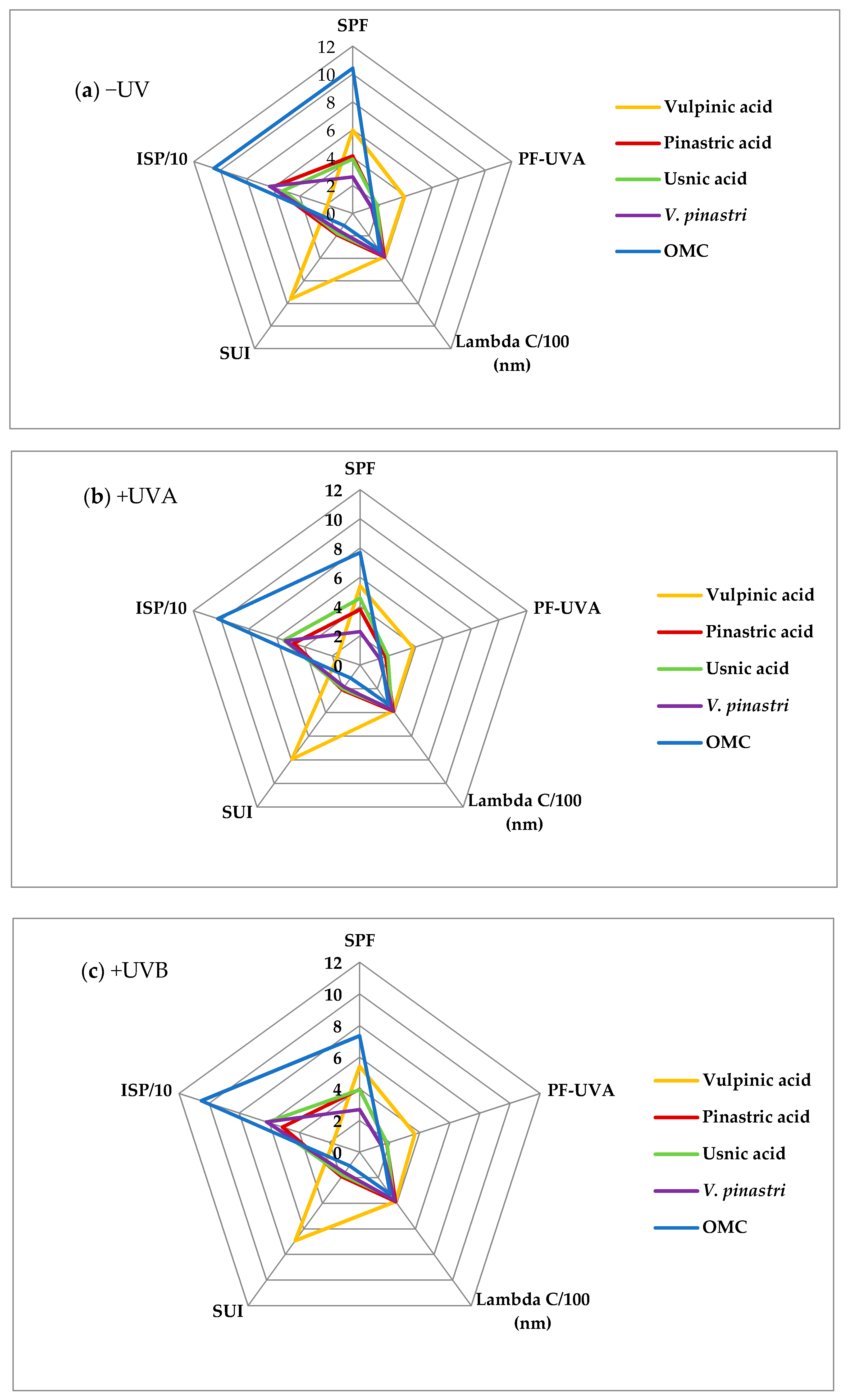
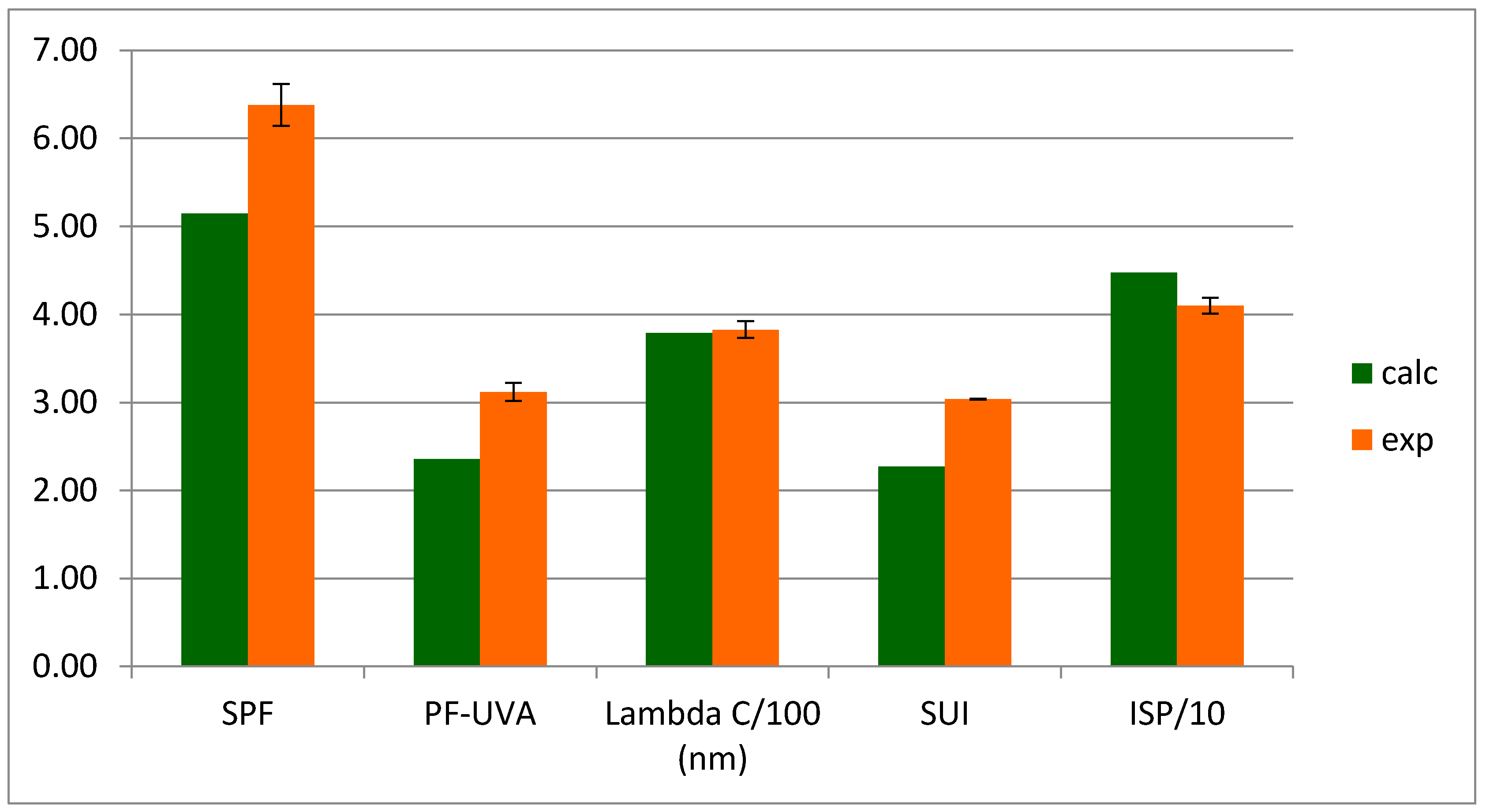
2.2. Lichen Compounds and Extract as UV-Blockers
2.3. Photostability of the Compounds
2.4. Antioxidant and Phototoxic Activities of Lichen Compounds and Extract
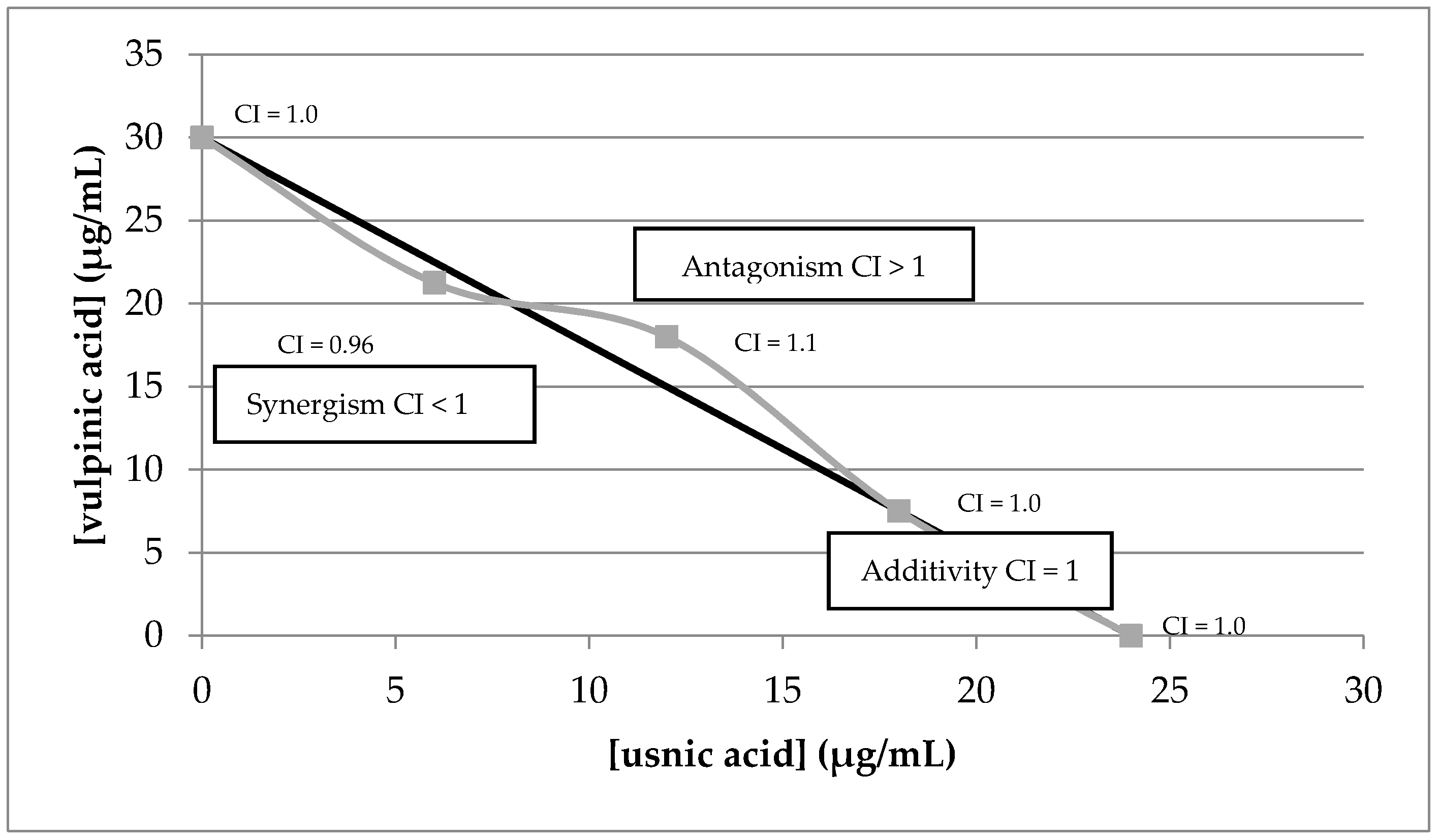
2.5. Synergistic Activity of Lichen Compounds and Extract
2.5.1. UV-Blockers
2.5.2. Antioxidant Activity
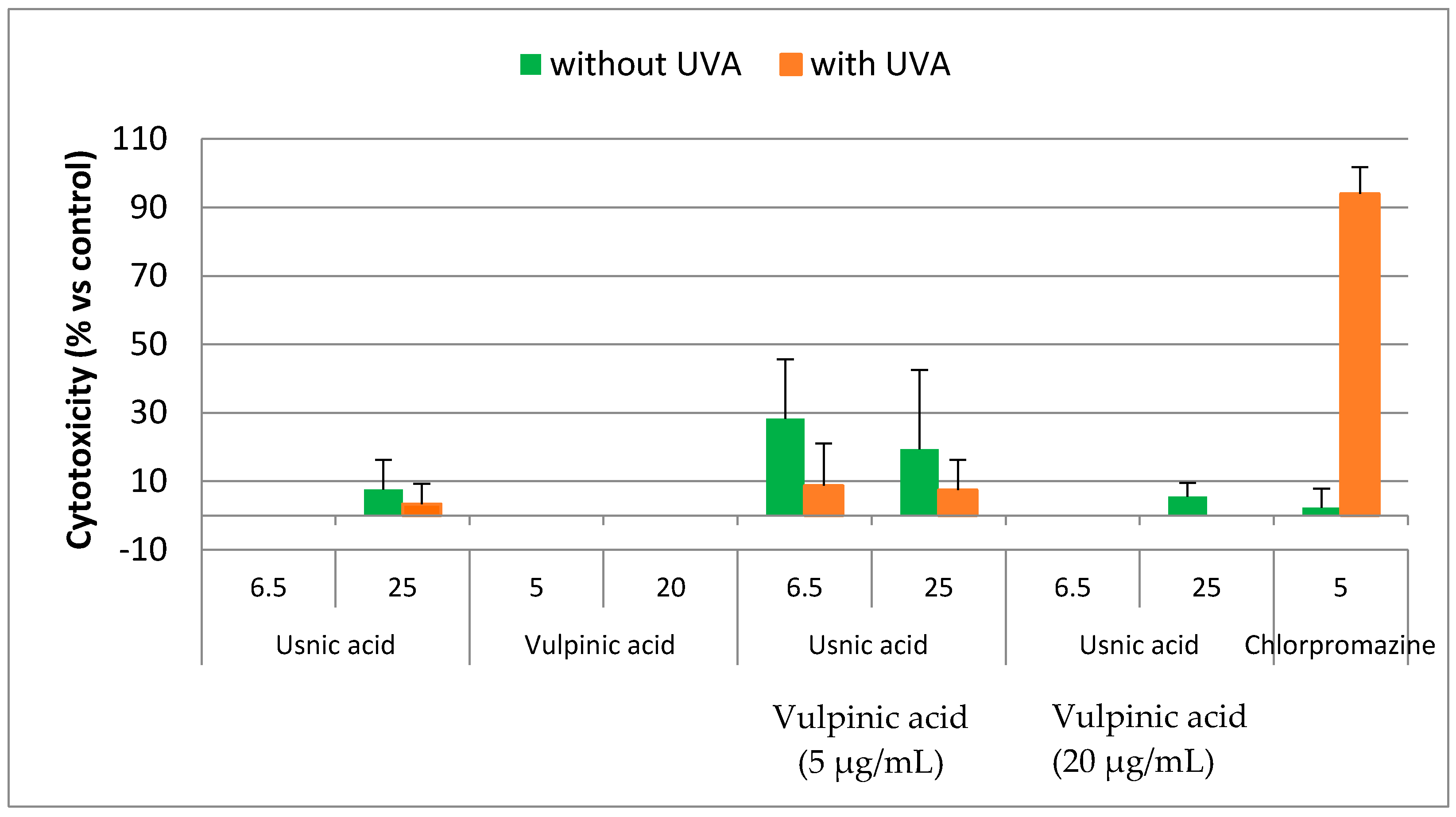
2.5.3. Photocytotoxicity of Usnic Acid and Pinastric Acid
3. Materials and Methods
3.1. General
3.2. Lichen Material
3.3. Vulpicida pinastri Extraction and Isolation of Its Major Compounds 1, 2, 3
3.4. Electronic Circular Dichroism
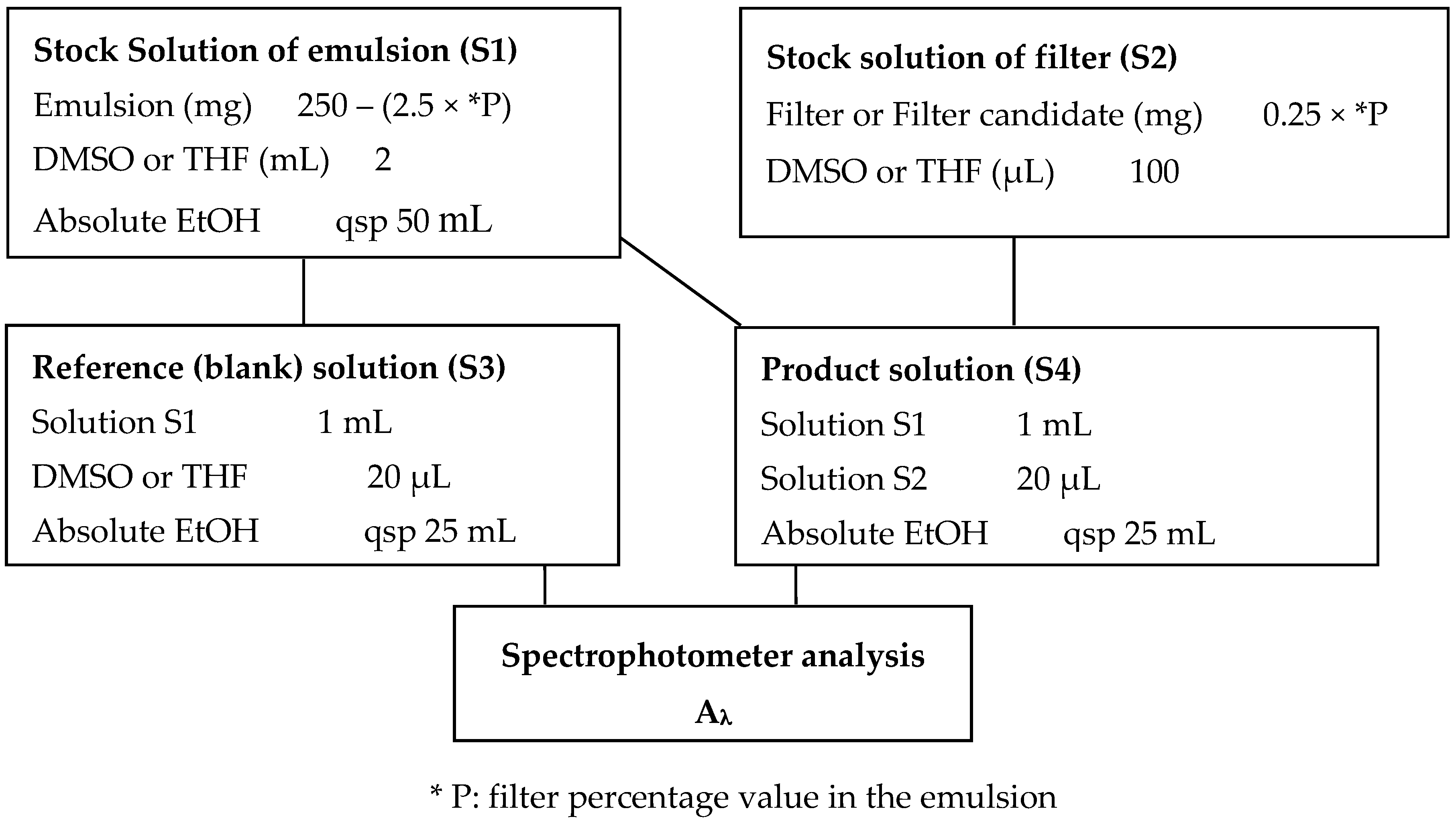
3.5. In Vitro Sun Protection Indexes
3.5.1. In Vitro Screening Method for Sun Protection Indexes Evaluation
3.5.2. Stability of the Tested Compound after UVA or UVB Irradiation
3.5.3. Synergistic Study
3.6. Antioxidant Testing
3.6.1. Single Compounds
3.6.2. Compounds in Combination
3.7. Phototoxicity
3.7.1. Single Compounds
3.7.2. Compounds in Combination
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lucas, R.M.; Repacholi, M.H.; McMichael, A.J. Is the current public health message on UV exposure correct? Bull. World Health Organ. 2006, 84, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.B.; Brewer, J.D.; Lohse, C.M.; Bringe, K.E.; Pruitt, C.N.; Gibson, L.E. Increasing incidence of melanoma among young adults: An epidemiological study in Olmsted County, Minnesota. Mayo Clin. Proc. 2012, 87, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Chauvet, C.; Paparis, E.; Coiffard, L. UV filters, ingredients with a recognized anti-inflammatory effect. PLoS ONE 2012, 7, e46187. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.; Pirot, F.; Bertholle, V.; Roussel, L.; Falson, F.; Padois, K. Commonly used UV filter toxicity on biological functions: Review of last decade studies. Int. J. Cosmet. Sci. 2013, 35, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens cause coral bleaching by promoting viral infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Downs, C.A.; Kramarsky-Winter, E.; Fauth, J.E.; Segal, R.; Bronstein, O.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; Pennington, P.; Kushmaro, A. Toxicological effects of the sunscreen UV filter, benzophenone-2, on planulae and in vitro cells of the coral, Stylophora pistillata. Ecotoxicol. 2014, 23, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.R.; Guimarães, A.G.; Quintans, J.S.; Araújo, A.A.; Nunes, P.S.; Quintans-Júnior, L.J. Natural compounds for solar photoprotection: A patent review. Expert Opin. Ther. Pat. 2015, 25, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Korać, R.R.; Khambholja, K.M. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn. Rev. 2011, 5, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Schürch, C.; Zülli, F. Mycosporine-like amino acids from red algae protect against premature skin-aging. Euro Cosmet. 2006, 9, 1–4. [Google Scholar]
- Torres, A.; Hochberg, M.; Pergament, I.; Smoum, R.; Niddam, V.; Dembitsky, V.M.; Temina, M.; Dor, I.; Lev, O.; Srebnik, M. A new UV-B absorbing mycosporine with photo protective activity from the lichenized ascomycete Collema cristatum. Eur. J. Biochem. 2004, 271, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Edwards, H.; Russell, N.; Seaward, M. Calcium oxalate in lichen biodeterioration studied using FT-Raman spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1997, 53, 99–105. [Google Scholar] [CrossRef]
- Nguyen, K.-H.; Chollet-Krugler, M.; Gouault, N.; Tomasi, S. UV-protectant metabolites from lichens and their symbiotic partners. Nat. Prod. Rep. 2013, 30, 1490–1508. [Google Scholar] [CrossRef] [PubMed]
- Balskus, E.P.; Walsh, C.T. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 2010, 329, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Hauck, M.; Jürgens, S.-R.; Willenbruch, K.; Huneck, S.; Leuschner, C. Dissociation and metal-binding characteristics of yellow lichen substances suggest a relationship with site preferences of lichens. Ann. Bot. 2009, 103, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Le Pogam, P.; Legouin, B.; Le Lamer, A.-C.; Boustie, J.; Rondeau, D. Analysis of the cyanolichen Lichina pygmaea metabolites using in situ DART-MS: From detection to thermochemistry of mycosporine serinol. J. Mass Spectrom. 2015, 50, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Le Pogam, P.; Le Lamer, A.-C.; Legouin, B.; Boustie, J.; Rondeau, D. In situ DART-MS as a versatile and rapid dereplication tool in lichenology: Chemical fingerprinting of Ophioparma ventosa. Phytochem. Anal. 2016, 27, 354–363. [Google Scholar] [CrossRef] [PubMed]
- ISO 24442:2011. Cosmetics-Sun Protection Test Method—Determination of Sunscreen UVA Photoprotection In Vivo; International Organization for Standardization (ISO): Geneva, Switzerland, 2011. [Google Scholar]
- ISO 24443:2012. Cosmetics-Sun Protection Test Method—Determination of Sunscreen UVA Photoprotection In Vitro; International Organization for Standardization (ISO): Geneva, Switzerland, 2012. [Google Scholar]
- Moyal, D.; Alard, V.; Bertin, C.; Boyer, F.; Brown, M.W.; Kolbe, L.; Matts, P.; Pissavini, M. The revised COLIPA in vitro UVA method. Int. J. Cosmet. Sci. 2013, 35, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.; Hausner, G.; Piercey-Normore, M.D. Bioactivity of secondary metabolites and thallus extracts from lichen fungi. Mycoscience 2011, 52, 413–418. [Google Scholar] [CrossRef]
- Letcher, R.M. Chemistry of lichen constituents—VII: Mass spectra of some pulvic acid derivatives. Org. Mass Spectrom. 1968, 1, 805–817. [Google Scholar] [CrossRef]
- Van der Sar, S.A.; Blunt, J.W.; Cole, A.L.; Din, L.B.; Munro, M.H. Dichlorinated pulvinic acid derivative from a Malaysian Scleroderma sp. J. Nat. Prod. 2005, 68, 1799–1801. [Google Scholar] [CrossRef] [PubMed]
- Letcher, R.M.; Eggers, S.H. Chemistry of lichen constituents. Part IV. Tetrahedron Lett. 1967, 8, 3541–3546. [Google Scholar] [CrossRef]
- Stocker-Wörgötter, E. Metabolic diversity of lichen-forming ascomycetous fungi: Culturing, polyketide and shikimate metabolite production, and PKS genes. Nat. Prod. Rep. 2008, 25, 188–200. [Google Scholar] [CrossRef] [PubMed]
- König, G.M.; Wright, A.D. 1H and 13C-NMR and biological activity investigations of four lichen-derived compounds. Phytochem. Anal. 1999, 10, 279–284. [Google Scholar] [CrossRef]
- Dias, D.; White, J.M.; Urban, S. Pinastric acid revisited: A complete NMR and X-ray structure assignment. Nat. Prod. Res. 2007, 21, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Huneck, S.; Yoshimura, I. Identification of Lichen Substances; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Kinoshita, Y.; Yamamoto, Y.; Yoshimura, I. Distribution of optical isomers of usnic and isousnic acids analyzed by high performance liquid chromatography. J. Hattori Bot. Lab. 1997, 83, 173–178. [Google Scholar]
- Smeds, A.I.; Kytöviita, M.-M. Determination of usnic and perlatolic acids and identification of olivetoric acids in Northern reindeer lichen (Cladonia stellaris) extracts. Lichenologist 2010, 42, 739–749. [Google Scholar] [CrossRef]
- Diffey, B. Spectral uniformity: A new index of broad spectrum (UVA) protection. Int. J. Cosmet. Sci. 2009, 31, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Diffey, B.L.; Brown, M.W. The Ideal Spectral Profile of Topical Sunscreens. Photochem. Photobiol. 2012, 88, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Pissavini, M.; Diffey, B.; Doucet, O. The perplexing dilemma of measuring sun protection factors. Int. J. Cosmet. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Faure, A.; Fortin, J.; Paparis, E.; Coiffard, L.J. Study of the photostability of 18 sunscreens in creams by measuring the SPF in vitro. J. Pharm. Biomed. Anal. 2007, 44, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; El-Boury, S.; Paparis, E.; Sébille-Rivain, V.; Coiffard, L.J. In vitro UV-A protection factor (PF-UVA) of organic and inorganic sunscreens. Pharm. Dev. Technol. 2009, 14, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Lohézic-Le Dévéhat, F.; Legouin, B.; Couteau, C.; Boustie, J.; Coiffard, L. Lichenic extracts and metabolites as UV filters. J. Photochem. Photobiol. B 2013, 120, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Kristmundsdóttir, T.; Jónsdóttir, E.; Ögmundsdóttir, H.M.; Ingólfsdóttir, K. Solubilization of poorly soluble lichen metabolites for biological testing on cell lines. Eur. J. Pharm. Sci. 2005, 24, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Rancan, F.; Rosan, S.; Boehm, K.; Fernández, E.; Hidalgo, M.E.; Quihot, W.; Rubio, C.; Boehm, F.; Piazena, H.; Oltmanns, U. Protection against UVB irradiation by natural filters extracted from lichens. J. Photochem. Photobiol. B 2002, 68, 133–139. [Google Scholar] [CrossRef]
- Boehm, F.; Clarke, K.; Edge, R.; Fernandez, E.; Navaratnam, S.; Quilhot, W.; Rancan, F.; Truscott, T.G. Lichens–Photophysical studies of potential new sunscreens. J. Photochem. Photobiol. B 2009, 95, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Photostability of sunscreens. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 91–110. [Google Scholar] [CrossRef]
- Puglia, C.; Damiani, E.; Offerta, A.; Rizza, L.; Tirendi, G.G.; Tarico, M.S.; Curreri, S.; Bonina, F.; Perrotta, R.E. Evaluation of nanostructured lipid carriers (NLC) and nanoemulsions as carriers for UV-filters: Characterization, in vitro penetration and photostability studies. Eur. J. Pharm. Sci. 2014, 51, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Fernández, E.; Quilhot, W.; Rubio, C.; Hidalgo, M.E.; Diaz, R.; Ojeda, J. Effects of UV radiation on usnic acid in Xanthoparmelia microspora (Müll. Arg. Hale). Photochem. Photobiol. 2006, 82, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, E. Skin protection against UV light by dietary antioxidants. Food Funct. 2014, 5, 1994–2003. [Google Scholar] [CrossRef] [PubMed]
- Lohézic-Le Dévéhat, F.; Thüs, H.; Abasq, M.-L.; Delmail, D.; Boustie, J. Oxidative stress regulation in lichens and its relevance for survival in coastal habitats. Recent Adv. Bot. Res. 2014, 71, 467–503. [Google Scholar]
- Ahmed, Z.; Langer, P. Synthesis of natural pulvinic acids based on a [3 + 2] cyclization–Suzuki cross-coupling strategy. Tetrahedron 2005, 61, 2055–2063. [Google Scholar] [CrossRef]
- Nadal, B.; Sophie, A.-L.; Pin, S.; Renault, J.-P.; Cressier, D.; Rima, G.; Le Roux, A.; Meunier, S.; Wagner, A.; Lion, C. Synthesis and antioxidant properties of pulvinic acids analogues. Bioorg. Med. Chem. 2010, 18, 7931–7939. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, A.; Kuzmanovski, I.; Habrant, D.; Meunier, S.; Bischoff, P.; Nadal, B.; Thetiot-Laurent, S.A.-L.; Le Gall, T.; Wagner, A.; Novic, M. Design and synthesis of new antioxidants predicted by the model developed on a set of pulvinic acid derivatives. J. Chem. Inf. Model. 2011, 51, 3050–3059. [Google Scholar] [CrossRef] [PubMed]
- Martinčič, R.; Mravljak, J.; Švajger, U.; Perdih, A.; Anderluh, M.; Novič, M. In Silico discovery of novel potent antioxidants on the basis of pulvinic axid and coumarine derivatives and their experimental evaluation. PLoS ONE 2015, 10, e0140602. [Google Scholar] [CrossRef] [PubMed]
- Habrant, D.; Poigny, S.; Ségur-Derai, M.; Brunel, Y.; Heurtaux, B.; Le Gall, T.; Strehle, A.; Saladin, R.; Meunier, S.; Mioskowski, C. Evaluation of antioxidant properties of monoaromatic derivatives of pulvinic acids. J. Med. Chem. 2009, 52, 2454–2464. [Google Scholar] [CrossRef] [PubMed]
- Varol, M.; Türk, A.; Candan, M.; Tay, T.; Koparal, A.T. Photoprotective activity of vulpinic and gyrophoric acids toward Ultraviolet B-induced damage in human keratinocytes. Phytother. Res. 2016, 30, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.C. Absence of Psoralen—Type Phototoxicity from Usnic Acid, Some Lichens, and Lichen Substances. J. Investig. Dermatol. 1966, 47, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Badea, G.; Lăcătuşu, I.; Badea, N.; Ott, C.; Meghea, A. Use of various vegetable oils in designing photoprotective nanostructured formulations for UV protection and antioxidant activity. Ind. Crops Prod. 2015, 67, 18–24. [Google Scholar] [CrossRef]
- Martins, F.J.; Caneschi, C.A.; Vieira, J.L.; Barbosa, W.; Raposo, N.R. Antioxidant activity and potential photoprotective from amazon native flora extracts. J. Photochem. Photobiol. B 2016, 161, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Santesson, J. Chemical studies on lichens. Thin layer chromatography of lichen substances. Acta Chem. Scand. 1967, 21, 1162–1172. [Google Scholar] [CrossRef]
- Culberson, C.F. Improved conditions and new data for identification of lichen products by standardized thin-layer chromatographic method. J. Chromatogr. A 1972, 72, 113–125. [Google Scholar] [CrossRef]
- European Union. Reglement (ce) n° 1223/2009 du parlement europeen et du conseil du 30 nov 2009 relatif aux produits cosmetiques. J. Off. L’union Eur. 2009, L342/59–L342/209. [Google Scholar]
- Matsukawa, R.; Dubinsky, Z.; Kishimoto, E.; Masaki, K.; Masuda, Y.; Takeuchi, T.; Chihara, M.; Yamamoto, Y.; Niki, E.; Karube, I. A comparison of screening methods for antioxidant activity in seaweeds. J. Appl. Phycol. 1997, 9, 29–35. [Google Scholar] [CrossRef]
- Lohézic-Le Dévéhat, F.; Tomasi, S.; Elix, J.A.; Bernard, A.; Rouaud, I.; Uriac, P.; Boustie, J. Stictic acid derivatives from the lichen Usnea articulata and their antioxidant activities. J. Nat. Prod. 2007, 70, 1218–1220. [Google Scholar] [CrossRef] [PubMed]
- Ismed, F.; Lohézic-Le Dévéhat, F.; Delalande, O.; Sinbandhit, S.; Bakhtiar, A.; Boustie, J. Lobarin from the Sumatran lichen, Stereocaulon halei. Fitoterpia 2012, 83, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Breitinger, H.-G. Drug Synergy–Mechanisms and Methods of Analysis. In Toxicity Drug Testing; Bill Acree Edition: Rijeka, Croatia, 2012; pp. 143–166. ISBN 978-953-51-0004-1. [Google Scholar]
- Parasramka, M.A.; Gupta, S.V. Synergistic effect of garcinol and curcumin on antiproliferative and apoptotic activity in pancreatic cancer cells. J. Oncol. 2012, 23, 709739–709747. [Google Scholar] [CrossRef] [PubMed]
- Organization for Economic Co-operation and Development (OECD). Guideline for Testing Chemicals 432—In Vitro 3T3 NRU Phototoxicity Test; OECD: Paris, France, 2004; pp. 1–15. [Google Scholar]
Sample Availability: Samples of the compounds are not available. |





| Compound | Measured Mass | Proposed Formula | Calculated Mass (Error in ppm) |
|---|---|---|---|
| Vulpinic acid | 321.07581 | C19H13O5 | 321.07630 (−1.53) |
| Pinastric acid | 351.08616 | C20H15O6 | 351.08686 (−2.01) |
| Usnic acid | 343.08103 | C18H15O7 | 343.08233 (−3.24) |
| Compound | Antioxidant Activities | Phototoxic Activities on HaCaT Cells | |||
|---|---|---|---|---|---|
| DPPH Assay IC50 ± SD (µg/mL) | NBT Assay IC50 ± SD (µg/mL) | IC50 ± SD (µg/mL) | Photo-Irritancy Factor (PIF) | ||
| Without Irradiation | With Irradiation | ||||
| Vulpinic acid | 55.0 ± 8.0 | 30.0 ± 3.0 | >200.0 | >200.0 | *1.0 c |
| Pinastric acid | 80.0 ± 8.0 | 70.0±23.0 | >200.0 | >200.0 | *1.0 c |
| Usnic acid | >500.0 | 24.0 ± 9 | 33.0 ± 2.0 | 47.0 ± 10.0 | 0.7 d |
| V. pinastri extract | 75.6 ± 0.6 | 65.0 ± 5.0 | >200.0 | 58.0 ± 2.0 | >3.4 e |
| Ascorbic acid a | 12.5 ± 0.5 | 3.0 ± 0.4 | - | - | - |
| Chlorpromazine b | - | - | 13.2 ± 1.7 | 2.4 ± 0.3 | 5.5 d |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legouin, B.; Lohézic-Le Dévéhat, F.; Ferron, S.; Rouaud, I.; Le Pogam, P.; Cornevin, L.; Bertrand, M.; Boustie, J. Specialized Metabolites of the Lichen Vulpicida pinastri Act as Photoprotective Agents. Molecules 2017, 22, 1162. https://doi.org/10.3390/molecules22071162
Legouin B, Lohézic-Le Dévéhat F, Ferron S, Rouaud I, Le Pogam P, Cornevin L, Bertrand M, Boustie J. Specialized Metabolites of the Lichen Vulpicida pinastri Act as Photoprotective Agents. Molecules. 2017; 22(7):1162. https://doi.org/10.3390/molecules22071162
Chicago/Turabian StyleLegouin, Béatrice, Françoise Lohézic-Le Dévéhat, Solenn Ferron, Isabelle Rouaud, Pierre Le Pogam, Laurence Cornevin, Michel Bertrand, and Joël Boustie. 2017. "Specialized Metabolites of the Lichen Vulpicida pinastri Act as Photoprotective Agents" Molecules 22, no. 7: 1162. https://doi.org/10.3390/molecules22071162





