The Inhibitory Effect of Tartary Buckwheat Extracts on Adipogenesis and Inflammatory Response
Abstract
:1. Introduction
2. Results and Discussion
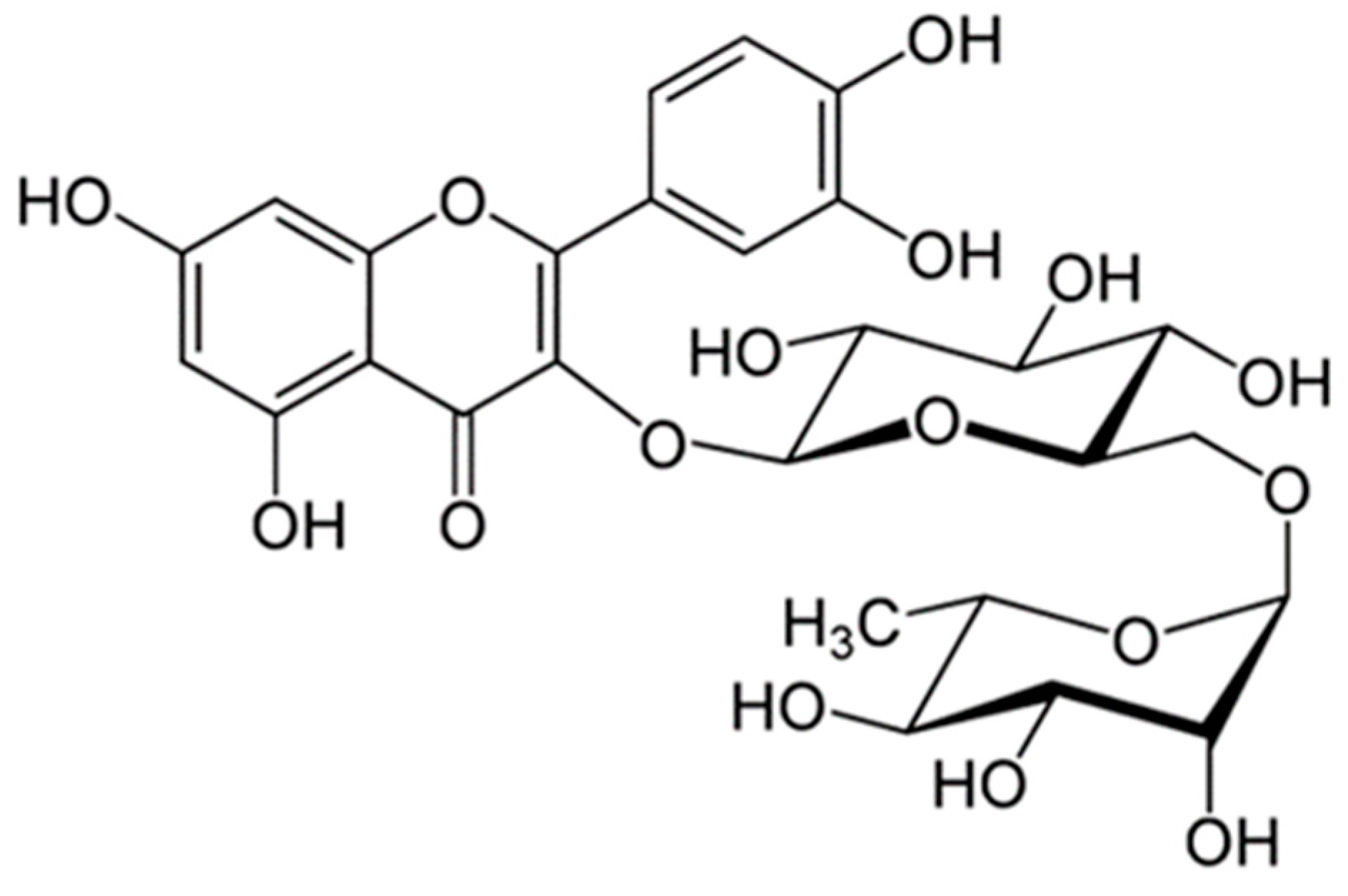
2.1. Rutin Contents of TBE
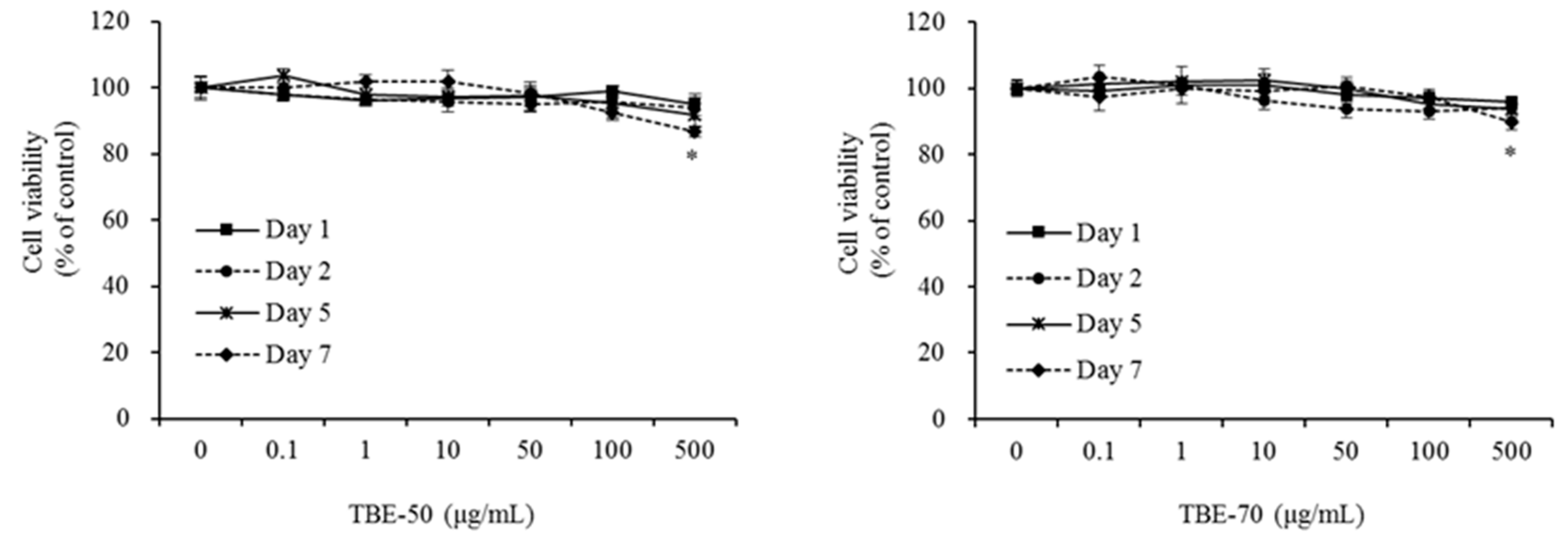
2.2. Effect of TBEs on 3T3-L1 Cell Viability
2.3. Effects of TBEs on Lipid Accumulation and TG Content during Adipocyte Differentiation
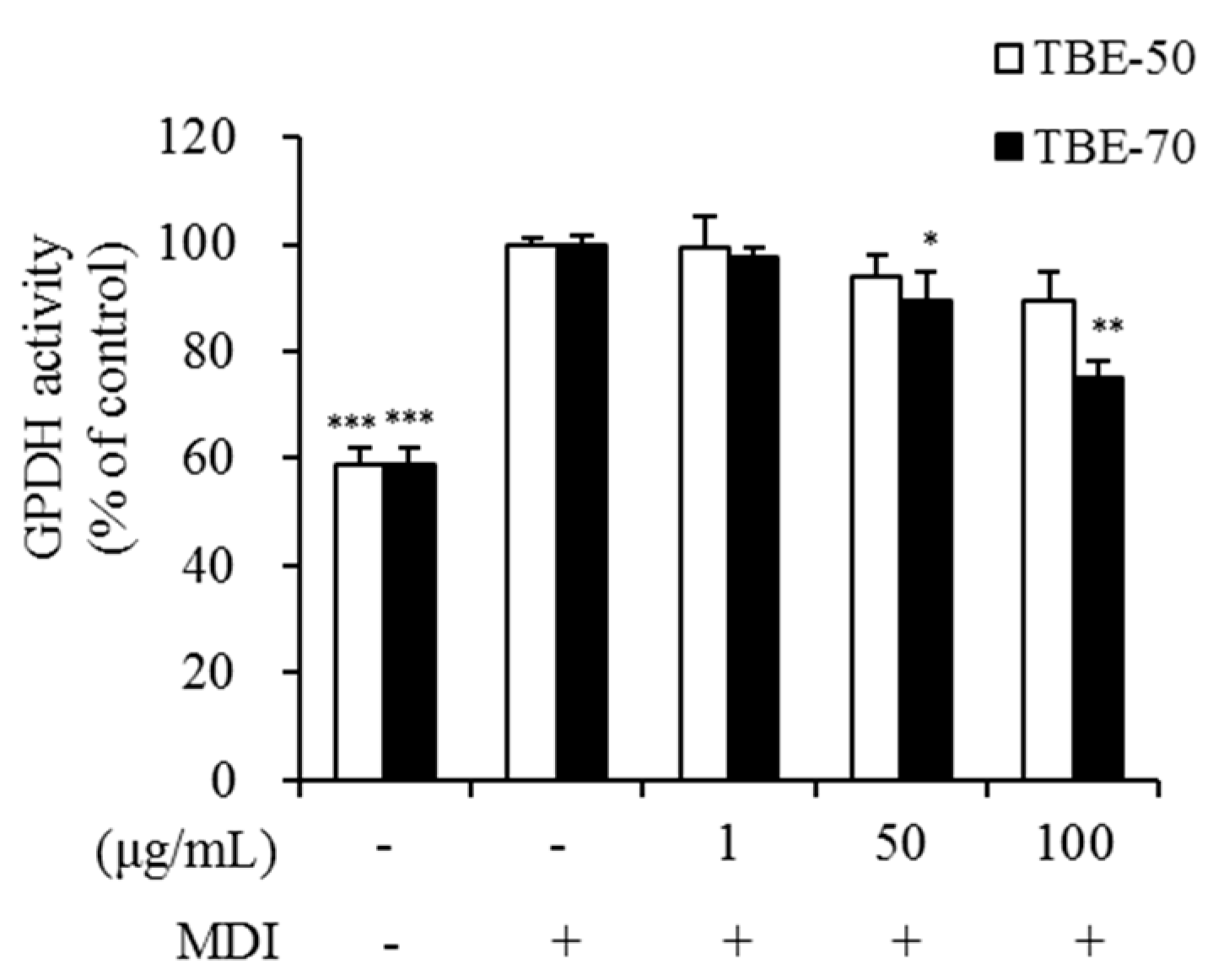
2.4. Effect of TBEs on GPDH Activity in Adipocytes
2.5. Effect of TBEs on mRNA Expression of Genes Involved in Fatty Acid Synthesis in Adipocytes
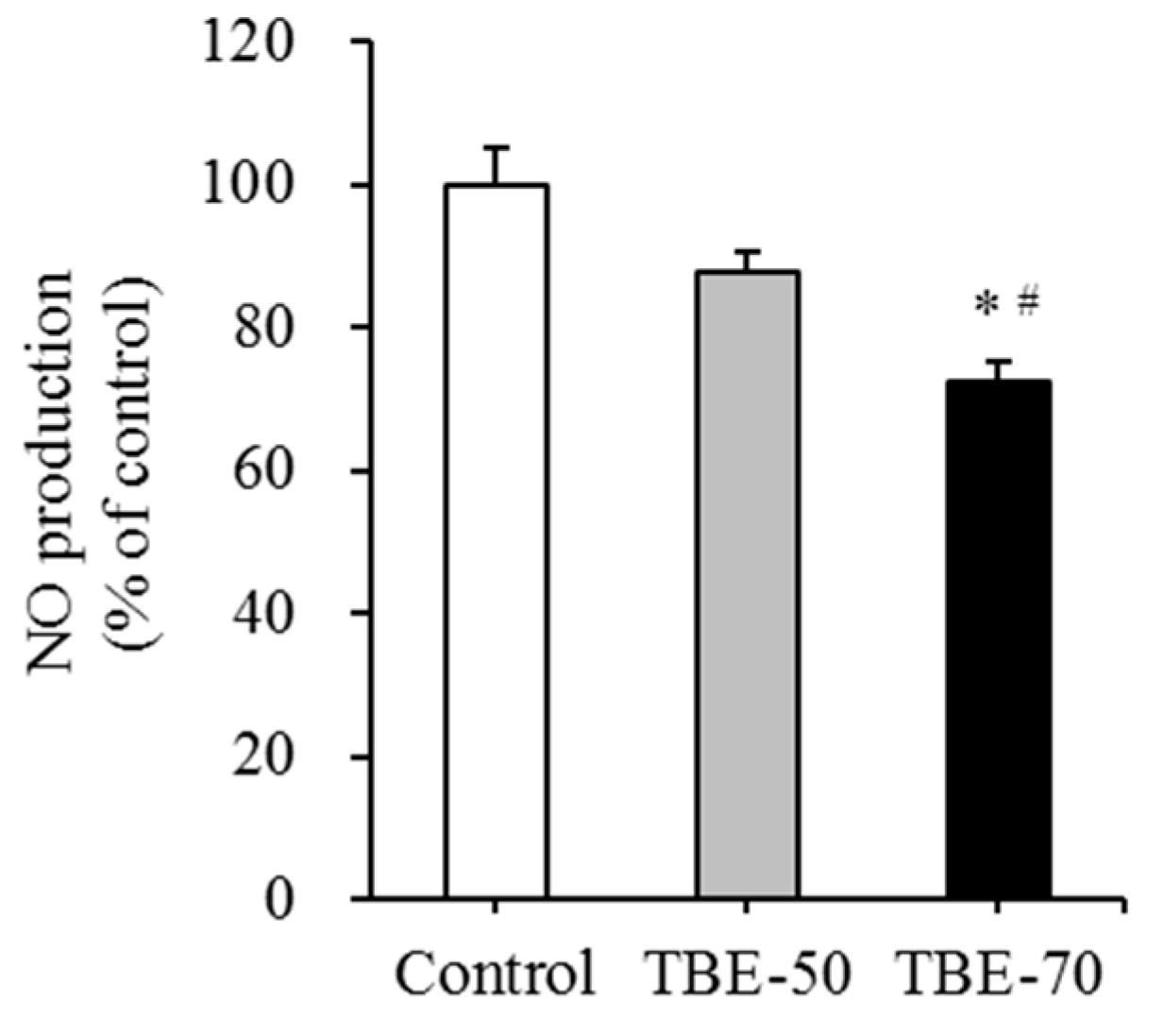
2.6. Effect of TBEs on mRNA Expression of Inflammatory Mediators and NO Production in Adipocytes
3. Materials and Methods
3.1. Materials
3.2. Preparation of Tartary Buckwheat Extracts
3.3. HPLC Analysis
3.4. Cell Culture
3.5. Cell Viability Assay
3.6. Oil-Red O Staining
3.7. TG Assay
3.8. GPDH Activity
3.9. Real-Time qPCR
3.10. NO Production
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Frühbeck, G.; Gómez-Ambrosi, J.; Muruzábal, F.J.; Burrell, M.A. The adipocyte: A model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am. J. Physiol. Endocr. Metab. 2001, 280, E827–E847. [Google Scholar] [PubMed]
- Tang, Q.Q.; Jiang, M.S.; Lane, M.D. Possible role of SpI as repressor of the C/EBPa gene in the adipocyte differentiation program. Mol. Cell Biol. 1999, 19, 4855–4865. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.O.; Peters, J.C. Environmental contributions to the obesity epidemic. Science 1998, 280, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Alessi, M.C.; Lijnen, H.R.; Bastelica, D.; Juhan-Vague, I. Adipose tissue and atherothrombosis. Pathophysiol. Haemost. Thromb. 2003, 33, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Flier, J.S. Adipose tissue as an endocrine organ. Trends Endocr. Metab. 2000, 11, 327–332. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Wijngaard, H.H.; Arendt, E.K. Buckwheat. Cereal Chem. 2006, 83, 391–401. [Google Scholar] [CrossRef]
- Eggum, B.O.; Kreft, I.; Javornik, B. Chemical composition and protein quality of buckwheat (Fagopyrum esculentum Moench). Plant Foods Hum. Nutr. 1980, 30, 175–179. [Google Scholar] [CrossRef]
- Choi, B.H. Status of buckwheat genetic resources in East Asia-1991. Korean J. Breed. Sci. 1992, 24, 293–301. [Google Scholar]
- Campbell, C.G. Major and minor production areas. In Buckwheat: Fagopyrum esculentum Moench; Bioversity International: Manitoba, MB, Canada, 1997; Volume 19, p. 52. [Google Scholar]
- Fabjan, N.; Rode, J.; Košir, I.J.; Wang, Z.; Zhang, Z.; Kreft, I. Tartary buckwheat (Fagopyrum tataricum Gaertn.) as a source of dietary rutin and quercitrin. J. Agric. Food Chem. 2003, 51, 6452–6455. [Google Scholar] [CrossRef] [PubMed]
- Minghe, Z.; Fukang, Q. Tartary flavonoids characteristics and its applications. In Proceedings of the 7th International Symposium on Buckwheat, Winnipeg, MB, Canada, August 1998; pp. 40–45. [Google Scholar]
- Nishimura, M.; Ohkawara, T.; Sato, Y.; Satoh, H.; Suzuki, T.; Ishiguro, K.; Nodac, T.; Morishitac, T.; Nishihiraa, J. Effectiveness of rutin-rich Tartary buckwheat (Fagopyrum tataricum Gaertn.) ‘Manten-Kirari’ in body weight reduction related to its antioxidant properties: A randomised, double-blind, placebo-controlled study. J. Funct. Foods 2016, 26, 460–469. [Google Scholar] [CrossRef]
- Hu, Y.; Hou, Z.; Liu, D.; Yang, X. Tartary buckwheat flavonoids protect hepatic cells against high glucose-induced oxidative stress and insulin resistance via MAPK signaling pathways. Food Funct. 2016, 7, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Choi, J.Y.; Lee, J.M.; Lee, S.; Cho, E.J. Tartary buckwheat on nitric oxide-induced inflammation in RAW264. 7 macrophage cells. Food Funct. 2015, 6, 2664–2670. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Chen, Y.S.; Yang, J.H.; Chiang, B.H. Antioxidant activity of tartary (Fagopyrum tataricum (L.) Gaertn.) and common (Fagopyrum esculentum Moench) buckwheat sprouts. J. Agric. Food Chem. 2008, 56, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhu, K.; Zhang, H.; Yao, H. Purification and characterization of the antitumor protein from Chinese tartary buckwheat (Fagopyrum tataricum Gaertn.) water-soluble extracts. J. Agric. Food Chem. 2007, 55, 6958–6961. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Shan, F.; Bian, J.; Chen, F.; Wang, M.; Ren, G. d-chiro-inositol-enriched tartary buckwheat bran extract lowers the blood glucose level in KK-Ay mice. J. Agric. Food Chem. 2008, 56, 10027–10031. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.R.; Cho, B.J.; Lee, H.K.; Kim, D.J.; Rhee, S.K.; Hong, H.D.; Kim, K.T.; Cho, C.W.; Choi, H.S.; Lee, B.Y.; et al. Antioxidant and anti-adipogenic effects of ethanolic extracts from tartary and common buckwheats. Korean J. Food Pres. 2012, 19, 123–130. [Google Scholar] [CrossRef]
- Kurisawa, M.; Chung, J.E.; Uyama, H.; Kobayashi, S. Enzymatic synthesis and antioxidant properties of poly (rutin). Biomacromolecules 2003, 4, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Sedej, I.; Sakač, M.; Mandić, A.; Mišan, A.; Tumbas, V.; Čanadanović-Brunet, J. Buckwheat (Fagopyrum esculentum Moench) grain and fractions: Antioxidant compounds and activities. J. Food Sci. 2012, 77, C954–C959. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Kwon, S.M.; Park, J.I.; Chan, K.J.; Park, C.H. Phenolic compounds in common and tartary buchweat. Korean J. Crop Sci. 2005, 50, 175–180. [Google Scholar]
- Morishita, T.; Yamaguchi, H.; Degi, K. The contribution of polyphenols to antioxidative activity in common buckwheat and Tartary buckwheat grain. Plant Prod. Sci. 2007, 10, 99–104. [Google Scholar] [CrossRef]
- Choi, I.; Park, Y.; Choi, H.; Lee, E.H. Anti-adipogenic activity of rutin in 3T3-L1 cells and mice fed with high-fat diet. Biofactors 2006, 26, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Wu, C.H.; Huang, S.L.; Yen, G.C. Phenolic compounds rutin and o-coumaric acid ameliorate obesity induced by high-fat diet in rats. J. Agric. Food Chem. 2009, 57, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Ma, Y.; Liu, D. Rutin suppresses palmitic acids-triggered inflammation in macrophages and blocks high fat diet-induced obesity and fatty liver in mice. Pharm. Res. 2013, 30, 2940–2950. [Google Scholar] [CrossRef] [PubMed]
- Nafees, S.; Rashid, S.; Ali, N.; Hasan, S.K.; Sultana, S. Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: Role of NFκB/MAPK pathway. Chem. Biol. Interact. 2015, 231, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Yuandani; Jantan, I.; Husain, K. 4,5,4′-Trihydroxychalcone, 8,8′-(ethene-1,2-diyl)-dinaphtalene-1,4,5-triol and rutin from Gynura segetum inhibit phagocytosis, lymphocyte proliferation, cytokine release and nitric oxide production from phagocytic cells. BMC Complement. Altern. Med. 2017, 17, 211. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, B.A.; Adineh, M.; Hajiali, F.; Nassiri-Asl, M. Treatment with Rutin-A Therapeutic Strategy for Neutrophil-Mediated Inflammatory and Autoimmune Diseases: Anti-inflammatory Effects of Rutin on Neutrophils. J. Pharmacopunct. 2017, 20, 52–56. [Google Scholar] [CrossRef]
- Abreu, F.F.; Souza, A.C.; Teixeira, S.A.; Soares, A.G.; Teixeira, D.F.; Soares, R.C.; Santana, M.T.; Lauton Santos, S.; Costa, S.K.; Muscará, M.N.; et al. Elucidating the role of oxidative stress in the therapeutic effect of rutin on experimental acute pancreatitis. Free Radic. Res. 2016, 50, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Saklani, R.; Gupta, S.K.; Mohanty, I.R.; Kumar, B.; Srivastava, S.; Mathur, R. Cardioprotective effects of rutin via alteration in TNF-α, CRP, and BNP levels coupled with antioxidant effect in STZ-induced diabetic rats. Mol. Cell. Biochem. 2016, 420, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Dong, Y.; Huo, R.; Wen, K.; Zhang, Y.; Liang, G. Rutin inhibits neuroinflammation and provides neuroprotection in an experimental rat model of subarachnoid hemorrhage, possibly through suppressing the RAGE-NF-κB inflammatory signaling pathway. Neurochem. Res. 2016, 41, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Ðurendić-Brenese, M.; Popović, T.; Pilija, V.; Arsić, A.; Milić, M.; Kojić, D.; Jojić, N.; Milić, N. Hypolipidemic and antioxidant effects of buckwheat leaf and flower mixture in hyperlipidemic rats. Bosnina J. Basic Med. Sci. 2013, 13, 100–108. [Google Scholar]
- Hsu, C.L.; Yen, G.C. Effects of flavonoids and pehnolic acids on the inhibition of adipogenesis in 3T3-L1 adipocytes. J. Agric. Food Chem. 2007, 55, 8404–8410. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.J.; Kim, K.J.; Seo, Y.J.; Choi, J.; Lee, B.Y. Modulation of HO-1 by ferulic acid attenuates adipocyte differentiation in 3T3-L1 cells. Molecules 2017, 22, E745. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.J.; Lee, Y.J.; Hwang, J.H.; Kim, K.J.; Lee, B.Y. The inhibitory effects of quercetin on obesity and obesity-induced inflammation by regulation of MAPK signaling. J. Nutr. Biochem. 2015, 26, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Eseberri, I.; Miranda, J.; Lasa, A.; Churruca, I.; Portillo, M.P. Doses of quercetin in the range of serum concentrations exert delipidating effects in 3T3-L1 preadipocytes by acting on different stages of adipogenesis, but not in mature adipocytes. Oxid. Med. Cell. Longev. 2015, 2015, 480943. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.R.; Park, Y.K.; Cha, Y.S. Quercetin-rich onion peel extract suppresses adipogenesis by downregulating adipogenic transcription factors and gene expression in 3T3-L1 adipocytes. J. Sci. Food Agric. 2014, 94, 2655–2660. [Google Scholar] [CrossRef] [PubMed]
- Spiegelman, B.M.; Frank, M.; Green, H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J. Biol. Chem. 1983, 258, 10083–10089. [Google Scholar] [PubMed]
- Ntambi, J.M.; Young-Cheul, K. Adipocyte differentiation and gene expression. J. Nutr. 2000, 130, 3122S–3126S. [Google Scholar] [PubMed]
- Kang, S.W.; Kang, S.I.; Shin, H.S.; Yoon, S.A.; Kim, J.H.; Ko, H.C.; Kim, S.J. Sasa quelpaertensis Nakai extract and its constituent p-coumaric acid inhibit adipogenesis in 3T3-L1 cells through activation of the AMPK pathway. Food Chem. Toxicol. 2013, 59, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Prusty, D.; Park, B.H.; Davis, K.E.; Farmer, S.R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor γ (PPARγ) and C/EBPα gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2002, 277, 46226–46232. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.M.; Trainor, D.; Biswas, C.; LaCerte, C.; Berger, J.P.; Kelly, L.J. A high-capacity assay for PPARγ ligand regulation of endogenous aP2 expression in 3T3-L1 cells. Anal. Biochem. 2004, 330, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Duplus, E.; Forest, C. Is there a single mechanism for fatty acid regulation of gene transcription? Biochem. Pharmacol. 2002, 64, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Thampy, K.G.; Koshy, A.G. Purification, characterization, and ontogeny of acetyl-CoA carboxylase isozyme of chick embryo brain. J. Lipid Res. 1991, 32, 1667–1673. [Google Scholar] [PubMed]
- Ntambi, J.M.; Miyazaki, M. Recent insights into stearoyl-CoA desaturase-1. Curr. Opin. Lipidol. 2003, 14, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.P.; Kaneda, T.; Morita, H. Plant natural products as an anti-lipid droplets accumulation agent. J. Nat. Med. 2014, 68, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Seog, H.; Park, Y.; Kim, Y.; Choi, H. Suppressive effects of germinated buckwheat on development of fatty liver in mice fed with high-fat diet. Phytomedicine 2007, 14, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Filho, M.A.; Ueno, M.; Carvalheira, J.B.; Velloso, L.A.; Saad, M.J. Targeted disruption of iNOS prevents LPS induced S nitrosation of IRbeta/IRS 1 and Akt and insulin resistance in muscle of mice. Am. J. Physiol. Endocr. Metab. 2006, 291, E476–E482. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Lin, Y.J.; Ho, C.T.; Yen, G.C. The inhibitory effect of pterostilbene on inflammatory responses during the interaction of 3T3 L1 adipocytes and RAW 264.7 macrophages. J. Agric. Food Chem. 2013, 61, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.I.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Watanabe, W.; Oomagari, H.; Tsuruta, E.; Shida, M.; Kurokawa, M. Citrus flavonoid naringenin inhibits TLR2 expression in adipocytes. J. Nutr. Biochem. 2013, 24, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.Y.; Park, W.B. Production of cytokine and NO by RAW 264.7 macrophages and PBMC in vitro incubation with flavonoids. Arch. Pharm. Res. 2005, 28, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Hirai, S.; Kim, Y.I.; Goto, T.; Kang, M.S.; Yoshimura, M.; Obata, A.; Yu, R.; Kawada, T. Inhibitory effect of naringenin chalcone on inflammatory changes in the interaction between adipocytes and macrophages. Life Sci. 2007, 81, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Shimizu, N.; Kunii, K.; Martyn, J.A.; Ueki, K.; Kaneki, M. A role for iNOS in fasting hyperglycemia and impaired insulin signaling in the liver of obese diabetic mice. Diabetes 2005, 54, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ha, S.J.; Lee, H.J.; Kim, M.J.; Kim, J.H.; Kim, Y.T.; Song, K.M.; Kim, Y.J.; Kim, H.K.; Jung, S.K. Protective effect of Tremella fuciformis Berk extract on LPS-induced acute inflammation via inhibition of the NF-κB and MAPK pathways. Food Funct. 2016, 7, 3263–3272. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.R.; Seo, C.S.; Lee, N.R.; Shin, H.K.; Jeong, S.J. Phytochemical analysis on quantification and the inhibitory effects on inflammatory responses from the fruit of xanthii fructus. Pharmacogn. Mag. 2015, 11, S585–S591. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.R.; Jeong, S.J.; Lee, N.R.; Shin, H.K.; Seo, C.S. Simultaneous determination and anti-inflammatory effects of four phenolic compounds in Dendrobii Herba. Nat. Prod. Res. 2017, 10:1–10:4. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.J.; Koh, D.J.; Kim, S.H.; Park, S.J.; Ryu, J.H.; Kim, D.G.; Lee, J.Y.; Lee, K.T. Anti-inflammatory effects of sinapic acid through the suppression of inducible nitric oxide synthase, cyclooxygase-2, and proinflammatory cytokines expressions via nuclear factor-kappaB inactivation. J. Agric. Food Chem. 2008, 56, 10265–10272. [Google Scholar] [CrossRef] [PubMed]
- Endale, M.; Park, S.C.; Kim, S.; Kim, S.H.; Yang, Y.; Cho, J.Y.; Rhee, M.H. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology 2013, 218, 1452–1467. [Google Scholar] [CrossRef] [PubMed]
- Agbo, M.O.; Nworu, C.S.; Okoye, F.B.; Osadebe, P.O. Isolation and structure elucidaton of polyphenols from Loranthus micranthus Linn. parasitic on Hevea brasiliensis with antiinflammatory property. EXCLI. J. 2014, 13, 859–868. [Google Scholar] [PubMed]
- Lee, M.S.; Kim, C.T.; Kim, I.H.; Kim, Y. Effects of Capsaicin on Lipid Catabolism in 3T3-L1 Adipocytes. Phytother. Res. 2011, 25, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Aliaga, M.J.; Matsumura, F. Endrin inhibits adipocyte differentiation by selectively altering expression pattern of CCAAT/enhancer binding protein-α in 3T3-L1 cells. Mol. Pharmacol. 1999, 56, 91–101. [Google Scholar] [PubMed]
- Lee, M.S.; Jung, S.; Oh, S.; Shin, Y.; Kim, C.T.; Kim, I.H.; Kim, Y. Effect of high hydrostatic pressure extract of fresh ginseng on adipogenesis in 3T3-L1 adipocytes. J. Sci. Food Agric. 2015, 95, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 1999, 132, 365–386. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |



| Genes | Control | TBE-50 | TBE-70 |
|---|---|---|---|
| PPAR-γ | 1.00 ± 0.06 | 0.85 ± 0.13 | 0.61 ± 0.06 * |
| CEBP-α | 1.00 ± 0.07 | 0.77 ± 0.03 * | 0.48 ± 0.04 **,# |
| aP2 | 1.00 ± 0.02 | 0.86 ± 0.10 | 0.69 ± 0.01 * |
| ACC | 1.00 ± 0.12 | 0.88 ± 0.09 | 0.56 ± 0.03 *,# |
| FAS | 1.00 ± 0.12 | 0.88 ± 0.09 | 0.56 ± 0.03 *,# |
| SCD-1 | 1.00 ± 0.03 | 0.97 ± 0.10 | 0.65 ± 0.04 *,# |
| Genes | Control | TBE-50 | TBE-70 |
|---|---|---|---|
| TNF-α | 1.00 ± 0.03 | 0.71 ± 0.06 | 0.47 ± 0.11 ** |
| IL-6 | 1.00 ± 0.09 | 0.76 ± 0.08 | 0.51 ± 0.04 **,# |
| MCP-1 | 1.00 ± 0.06 | 0.80 ± 0.06 | 0.64 ± 0.06 * |
| iNOS | 1.00 ± 0.10 | 0.64 ± 0.04 * | 0.40 ± 0.03 **,# |
| Name | GeneBank No. | Primer Sequence (5′-3′) |
|---|---|---|
| ACC | AY451393 | F: CAAGTGCTCAAGTTTGGCGC |
| R: CAAGAACCACCCCGAAGCTC | ||
| aP2 | NM_024406 | F: CGACAGGAAGGTGAAGAGCA |
| R: ATTCCACCACCAGCTTGTCA | ||
| β-actin | NM_007393 | F: GGACCTGACAGACTACCTCA |
| R: GTTGCCAATAGTGATGACCT | ||
| CEBP-α | NM_007678 | F: ATAGACATCAGCGCCTACAT |
| R: TCCCGGGTAGTCAAAGTCAC | ||
| FAS | AF127033 | F: CTGGCATTCGTGATGGAGTC |
| R: TGTTTCCCCTGAGCCATGTA | ||
| IL-6 | NM_031168 | F: CCTTCCTACCCCAATTTCCA |
| R: TAACGCACTAGGTTTGCCGA | ||
| iNOS | BC062378.1 | F: GCTACTGGGTCAAAGACAAG |
| R: GCTGAACTTCCAGTCATTGT | ||
| MCP-1 | NM_019812 | F: TGCTGACCCCAAGAAGGAAT |
| R: TGAGGTGGTTGTGGAAAAGG | ||
| PPAR-γ | NM_011146 | F: TTGATTTCTCCAGCATTTCT |
| R: TGTTGTAGAGCTGGGTCTTT | ||
| SCD-1 | AF509567 | F: ATGGATATCGCCCCTACGAC |
| R: GATGTGCCAGCGGTACTCAC | ||
| TNF-α | NM_013693 | F: AGCACAGAAAGCATGATCCG |
| R: CCACAAGCAGGAATGAGAA |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-S.; Shin, Y.; Jung, S.; Kim, S.-Y.; Jo, Y.-H.; Kim, C.-T.; Yun, M.-K.; Lee, S.-J.; Sohn, J.; Yu, H.-J.; et al. The Inhibitory Effect of Tartary Buckwheat Extracts on Adipogenesis and Inflammatory Response. Molecules 2017, 22, 1160. https://doi.org/10.3390/molecules22071160
Lee M-S, Shin Y, Jung S, Kim S-Y, Jo Y-H, Kim C-T, Yun M-K, Lee S-J, Sohn J, Yu H-J, et al. The Inhibitory Effect of Tartary Buckwheat Extracts on Adipogenesis and Inflammatory Response. Molecules. 2017; 22(7):1160. https://doi.org/10.3390/molecules22071160
Chicago/Turabian StyleLee, Mak-Soon, Yoonjin Shin, Sunyoon Jung, Seog-Young Kim, Young-Hee Jo, Chong-Tai Kim, Min-Kyu Yun, Sung-Jin Lee, Johann Sohn, Heui-Jong Yu, and et al. 2017. "The Inhibitory Effect of Tartary Buckwheat Extracts on Adipogenesis and Inflammatory Response" Molecules 22, no. 7: 1160. https://doi.org/10.3390/molecules22071160





