Coinage Metal Complexes of the Carbenic Tautomer of a Conjugated Mesomeric Betaine Akin to Nitron †
Abstract
:1. Introduction
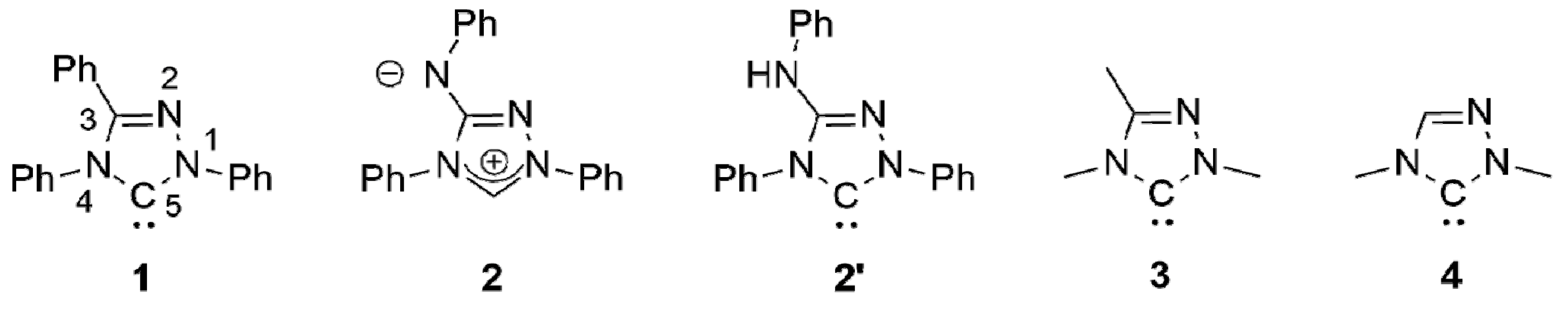
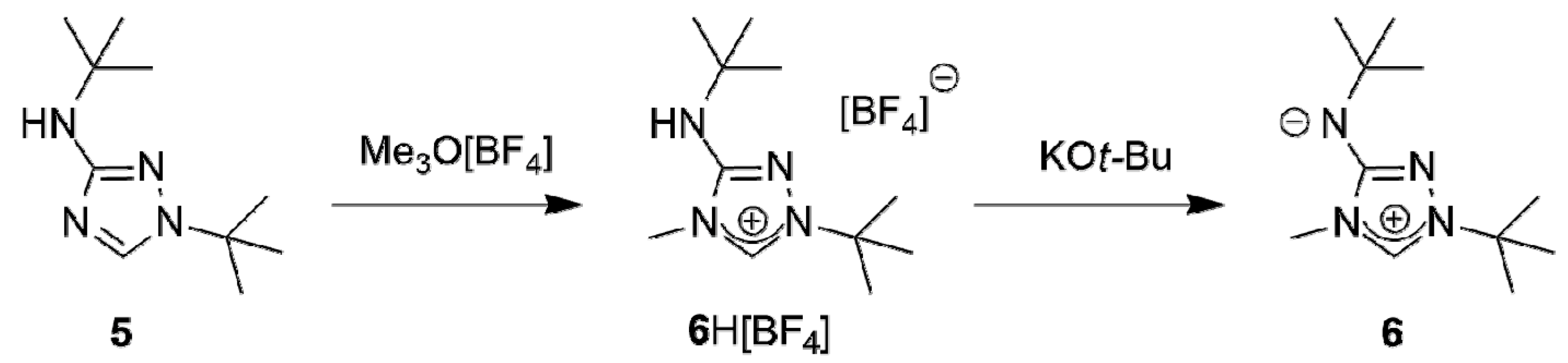
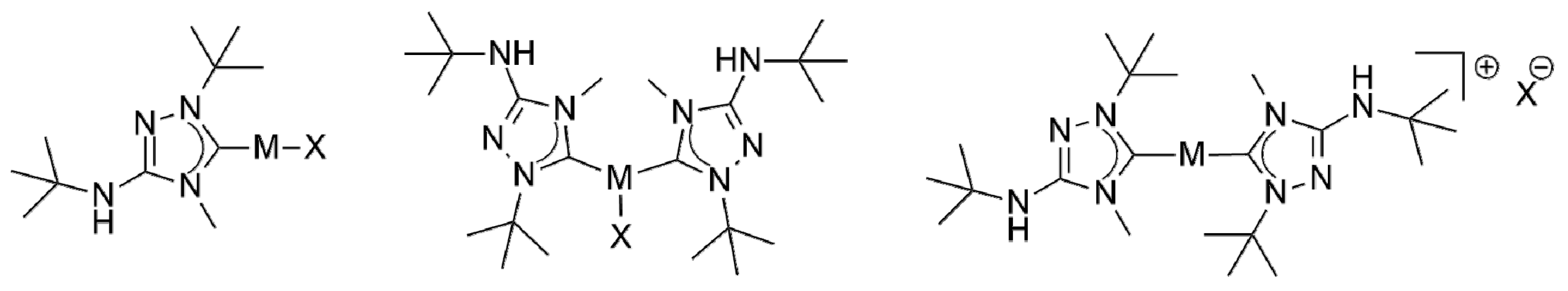
2. Results and Discussion
2.1. Compound Synthesis and Spectroscopic Characterization
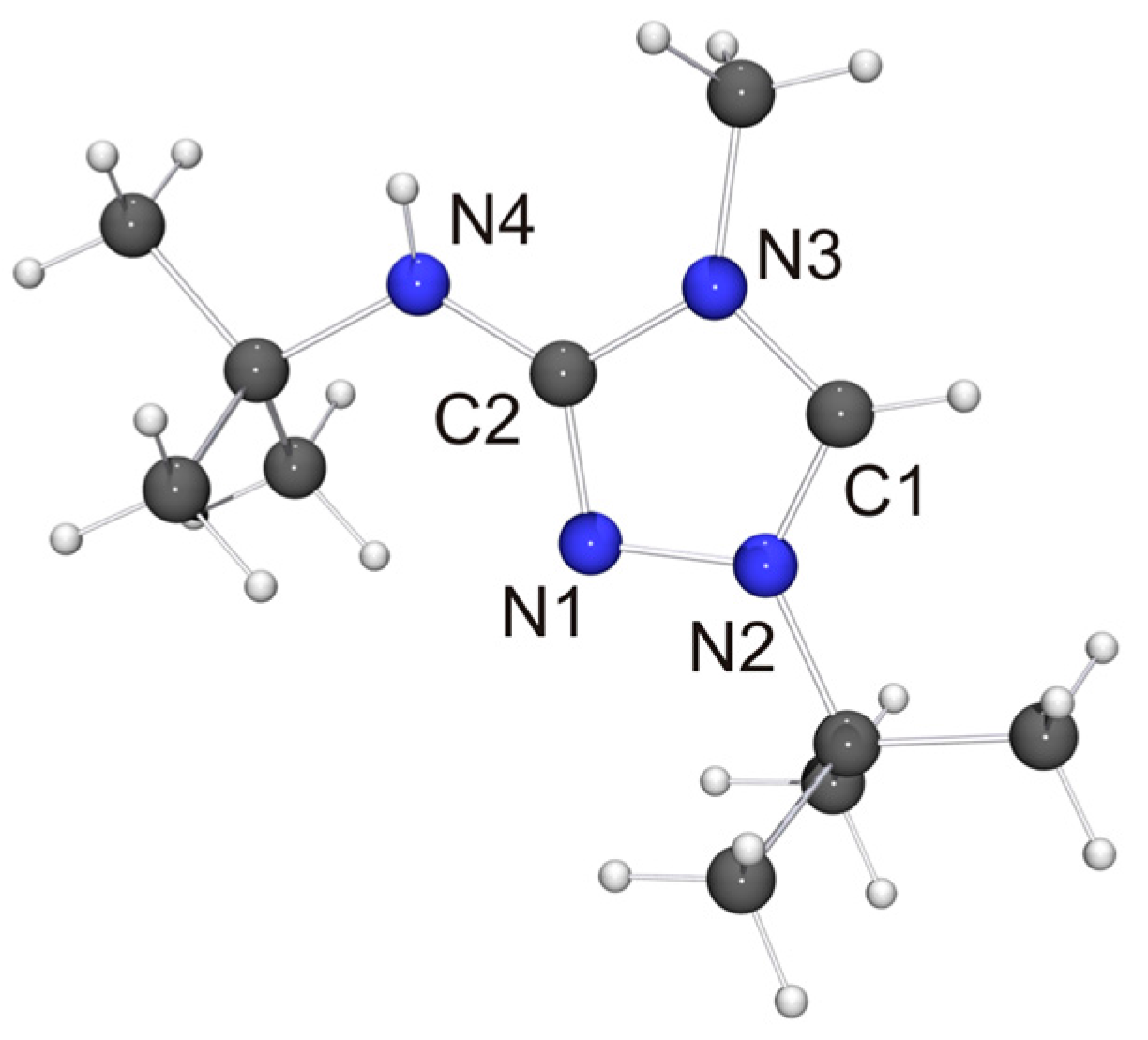
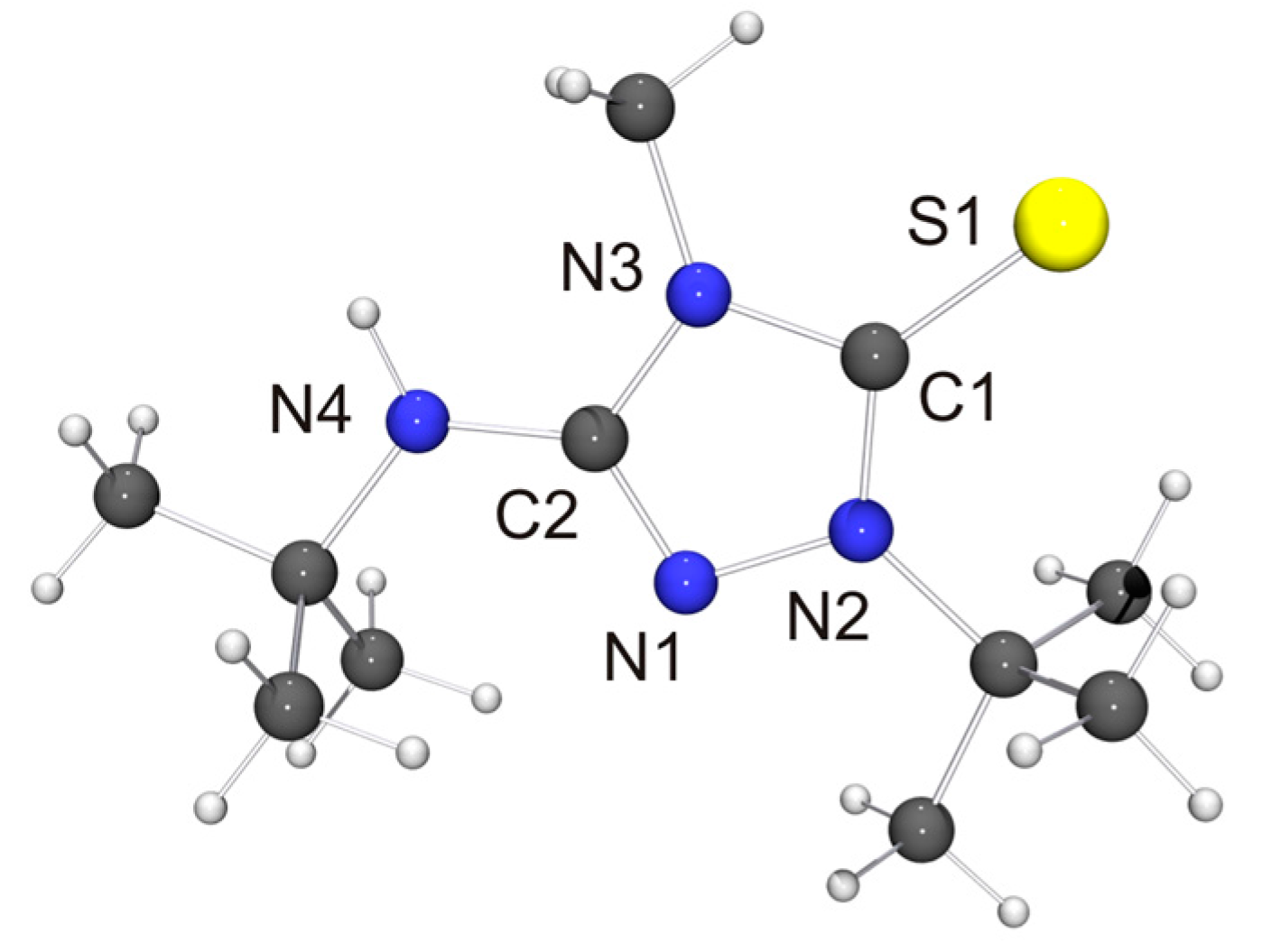
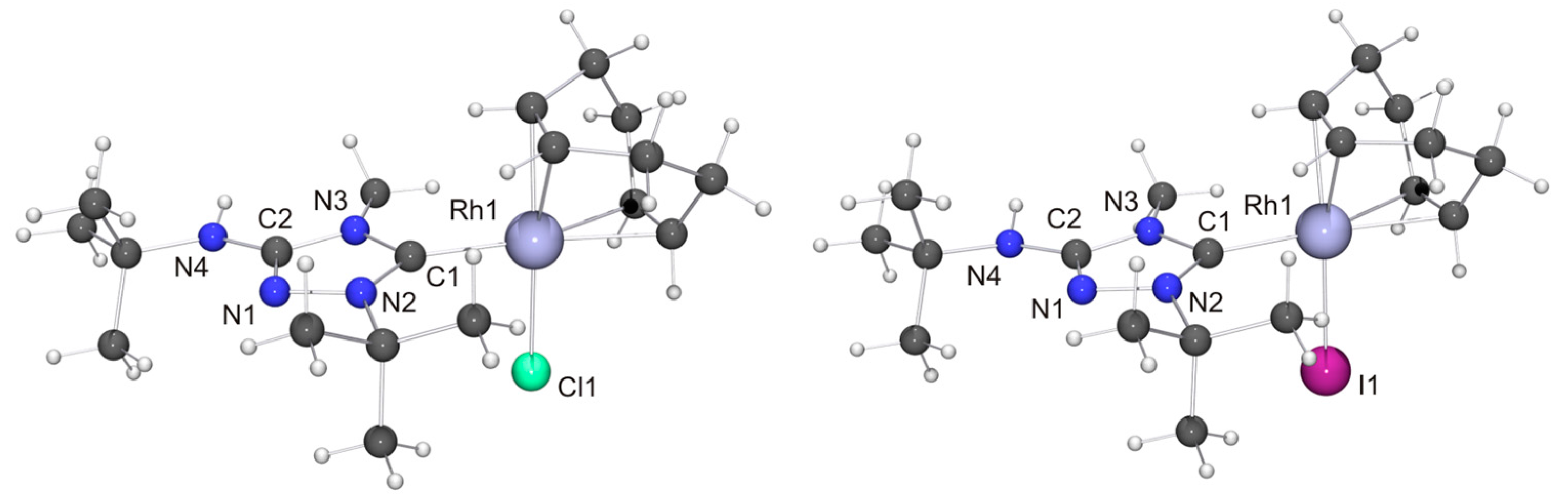
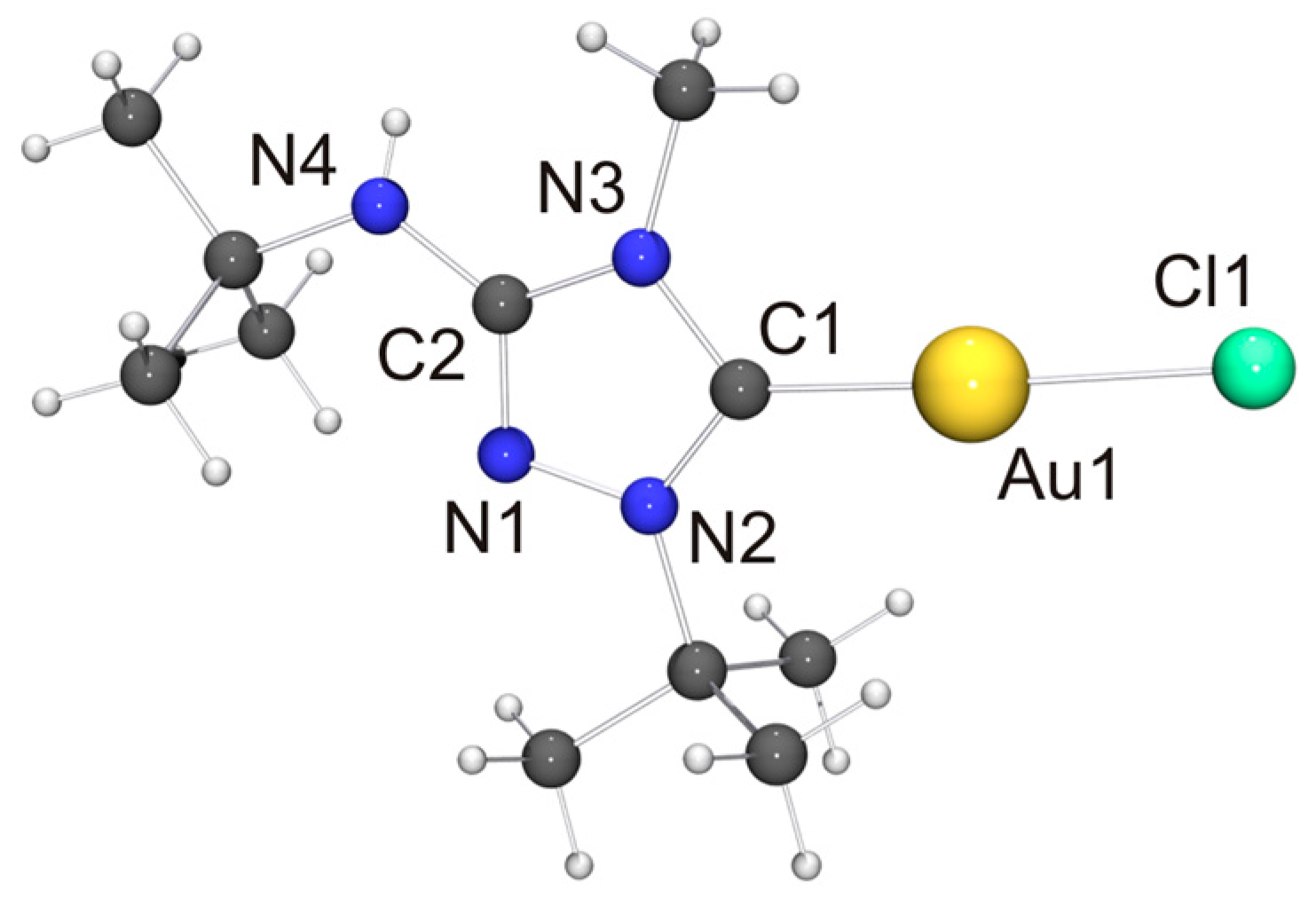
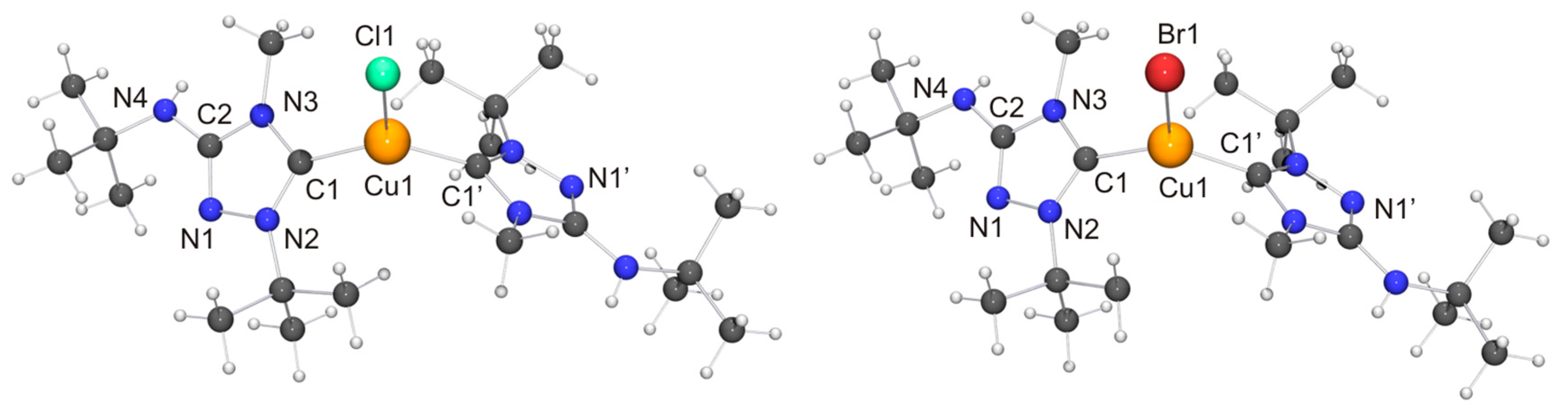
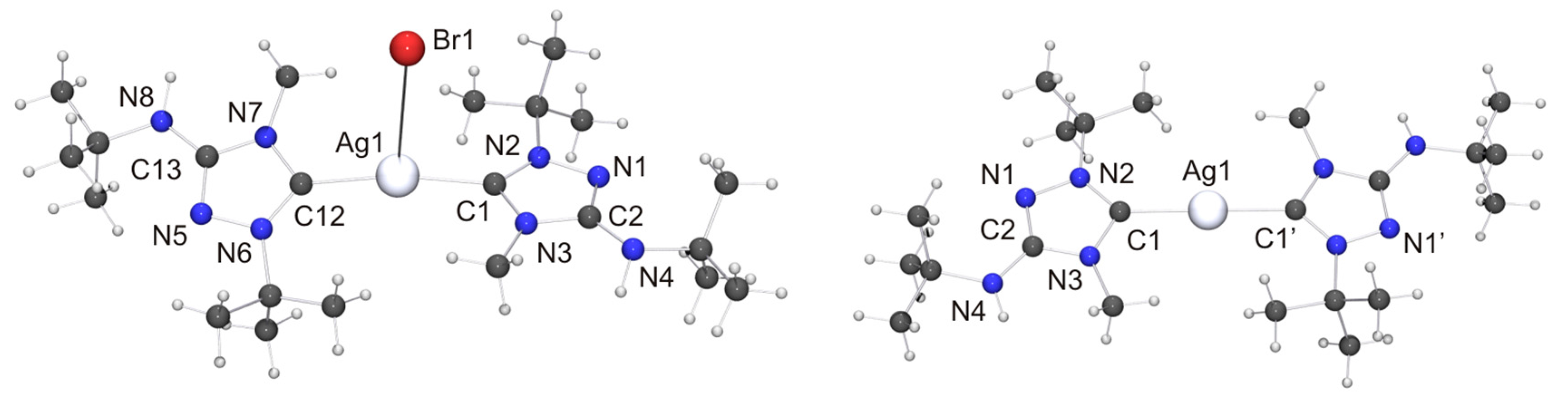
2.2. Structural Characterization
3. Experimental Section
3.1. General
3.2. X-ray Crystallography
3.3. Computational Details
3.4. Synthetic Procedures
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Lin, J.C.Y.; Huang, R.T.W.; Lee, C.S.; Bhattacharyya, A.; Hwang, W.S.; Li, I.J.B. Coinage metal-N-Heterocyclic carbene complexes. Chem. Rev. 2009, 109, 3561–3598. [Google Scholar] [CrossRef] [PubMed]
- Hindi, K.M.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. The medicinal applications of imidazolium carbene-metal complexes. Chem. Rev. 2009, 109, 3859–3884. [Google Scholar] [CrossRef] [PubMed]
- Mercs, L.; Albrecht, M. Beyond catalysis: N-Heterocyclic carbene complexes as components for medicinal. luminescent, and functional materials applications. Chem. Soc. Rev. 2010, 39, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.P. The development and catalytic uses of N-heterocyclic carbene metal complexes. Acc. Chem. Res. 2011, 44, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Deblock, M.C.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. Biologically active N-heterocyclic carbene-metal complexes. In N-Heterocyclic Carbenes. From Laboratory Curiosities to Efficient Synthetic Tools; Díez-González, S., Ed.; Royal Society of Chemistry: Cambridge, UK, 2011; pp. 119–133. [Google Scholar]
- Gautier, A.; Cisnetti, F. Advances in metal-carbene complexes as potent anti-cancer agents. Metallomics 2012, 4, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, S.; Cazin, C.S.J.; Nolan, S.P. N-heterocyclic carbene gold(I) and copper(I) complexes in C–H bond activation. Acc. Chem. Res. 2012, 45, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gust, R. Metal N-Heterocyclic carbene complexes as potential antitumor metallodrugs. Chem. Soc. Rev. 2013, 42, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Oehninger, L.; Rubbiani, R.; Ott, I. N-Heterocyclic carbene metal complexes in medicinal chemistry. Dalton Trans. 2013, 42, 3269–3284. [Google Scholar] [CrossRef] [PubMed]
- Aher, S.B.; Muskawar, P.N.; Thenmozhi, K.; Bhagat, P.R. Recent develoments of metal N-heterocyclic carbenes as anticancer agents. Eur. J. Med. Chem. 2014, 81, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.A.; Willans, C.E. Silver-N-heterocyclic carbene complexes as promising anticancer compounds. In Organometallic Chemistry; Fairlamb, I., Lynam, J., Eds.; Royal Society of Chemistry: Cambridge, UK, 2014; Volume 39, pp. 26–50. [Google Scholar]
- Wagers, P.O.; Shelton, K.L.; Panzner, M.J.; Tessier, C.A.; Youngs, W.J. Synthesis and medicinal properties of silver-NHC complexes and imidazolium salts. In N-Heterocyclic Carbenes: Effective Tools for Organometallic Synthesis; Nolan, S.P., Ed.; Wiley-VCH: Weinheim, Germany, 2014; pp. 151–172. [Google Scholar]
- Lazreg, F.; Cazin, C.S.J. Medical applications of NHC-Gold and -Copper complexes. In N-Heterocyclic Carbenes: Effective Tools for Organometallic Synthesis; Nolan, S.P., Ed.; Wiley-VCH: Weinheim, Germany, 2014; pp. 173–198. [Google Scholar]
- Hu, C.; Li, X.; Wang, W.; Zhang, R.; Deng, L. Metal-N-heterocyclic carbene complexes as antitumor agents. Curr. Med. Chem. 2014, 21, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Visbal, R.; Gimeno, M.C. N-Heterocyclic carbene metal complexes: Photoluminescence and applications. Chem. Soc. Rev. 2014, 43, 3551–3574. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Patil, S.A.; Patil, R.; Keri, R.S.; Budagumpi, S.; Balakrishna, B.R.; Tacke, M. N-Heterocyclic metal carbene complexes as bio-organometallic antimicrobial and anticancer drugs. Future Med. Chem. 2015, 7, 1305–1333. [Google Scholar] [CrossRef] [PubMed]
- Lazreg, F.; Nahra, F.; Cazin, C.S.J. Copper-NHC complexes in catalysis. Coord. Chem. Rev. 2015, 293–294, 48–79. [Google Scholar] [CrossRef]
- Liu, W.; Gust, R. Update on metal N-Heterocyclic carbene complexes as potential anti-tumor metallodrugs. Coord. Chem. Rev. 2016, 329, 191–213. [Google Scholar] [CrossRef]
- Marinelli, M.; Santini, C.; Pellei, M. Recent advances in medicinal applications of coinage-metal (Cu and Ag) N-heterocyclic carbene complexes. Curr. Top. Med. Chem. 2016, 26, 2995–3017. [Google Scholar] [CrossRef]
- Hemmert, C.; Gornitzka, H. Luminescent bioactive NHC-metal complexes to bring light into cells. Dalton Trans. 2016, 45, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Nahra, F.; Gómez-Herrera, A.; Cazin, C.S.J. Copper(I)-NHC complexes as NHC transfer agents. Dalton Trans. 2017, 46, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Beillard, A.; Bantreil, X.; Métro, T.-X.; Martinez, J.; Lamaty, F. Unraveling the synthesis of homoleptic [Ag(N,N-diaryl-NHC)2]Y (Y = BF4, PF6) complexes by ball-milling. Dalton Trans. 2016, 45, 17859–17866. [Google Scholar] [CrossRef] [PubMed]
- Beillard, A.; Bantreil, X.; Métro, T.-X.; Martinez, J.; Lamaty, F. Mechanochemistry for facilitated access to N,N-diaryl NHC metal complexes. New J. Chem. 2017, 41, 1057–1063. [Google Scholar] [CrossRef]
- Dash, C.; Shaikh, M.M.; Butcher, R.J.; Gosh, P. Highly convenient regioselective intermolecular hydroamination of alkynes yielding ketimines catalyzed by Gold(I) complexes of 1,2,4-triazole based N-heterocyclic carbenes. Inorg. Chem. 2010, 49, 4972–4983. [Google Scholar] [CrossRef] [PubMed]
- Dash, C.; Shaikh, M.M.; Gosh, P. Silver complexes of 1,2,4-triazole derived N-heterocyclic carbenes: Synthesis, structure and reactivity studies. J. Chem. Sci. 2011, 123, 97–106. [Google Scholar] [CrossRef]
- Turek, J.; Panov, I.; Švec, P.; Růžičková, Z.; Růžička, A. Non-covalent interactions in coinage metal complexes of 1,2,4-triazole-based N-heterocyclic carbenes. Dalton Trans. 2014, 43, 15465–15474. [Google Scholar] [CrossRef] [PubMed]
- Turek, J.; Růžičková, Z.; Růžička, A. Structural diversity of two 1,2,4-triazole based N-heterocyclic carbene complexes of silver(I). Inorg. Chem. Commun. 2014, 48, 103–106. [Google Scholar] [CrossRef]
- Guo, S.; Bernhammer, J.C.; Huynh, H.V. 1,2,4-Triazole-derived carbene complexes of gold: Characterization, solid-state aggregation and ligand disproportionation. Dalton Trans. 2015, 44, 15157–15165. [Google Scholar] [CrossRef] [PubMed]
- Pellei, M.; Gandin, V.; Marinelli, M.; Orsetti, A.; Del Belo, F.; Santini, C.; Marzano, C. Novel triazolium based 11th group NHCs: Synthesis, characterization and cellular response mechanisms. Dalton Trans. 2015, 44, 21041–21052. [Google Scholar] [CrossRef] [PubMed]
- Turek, J.; Růžičková, Z.; Tloušt′ová, E.; Mertlíková-Kaiserová, H.; Günterová, J.; Rulíšek, L.; Růžička, A. 1,2,4-Triazole-based N-heterocyclic carbene complexes of gold(I): Synthesis, characterization and biological activity. Appl. Organomet. Chem. 2016, 30, 318–322. [Google Scholar] [CrossRef]
- Färber, C.; Leibold, M.; Bruhn, C.; Maurer, M.; Siemeling, U. Nitron: A stable N-heterocyclic carbene that has been commercially available for more than a century. Chem. Commun. 2012, 48, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Hitzel, S.; Färber, C.; Bruhn, C.; Siemeling, U. Reactions of [RuCl2(PPh3)3] with Nitron and with the “Enders Carbene”: Access to Ruthenium(III) complexes. Organometallics 2014, 33, 425–428. [Google Scholar] [CrossRef]
- Thie, C.; Hitzel, S.; Wallbaum, L.; Bruhn, C.; Siemeling, U. Coinage metal complexes of the carbenic tautomer of Nitron. J. Organomet. Chem. 2016, 821, 112–121. [Google Scholar] [CrossRef]
- Ramsden, C.A. Heterocyclic mesomeric betaines: The recognition of five classes and nine sub-classes based on connectivity matrix analysis. Tetrahedron 2013, 69, 4146–4159. [Google Scholar] [CrossRef]
- César, V.; Tourneux, J.C.; Vujkovic, N.; Brousses, R.; Lugan, N.; Lavigne, G. Interplay between an elusive 4-(isopropylamino)imidazole-2-ylidene and its isolable mesoionic tautomer, and associated reactivities. Chem. Commun. 2012, 48, 2349–2351. [Google Scholar] [CrossRef] [PubMed]
- Benhamou, L.; Bastin, S.; Lugan, N.; Lavigne, G.; César, V. Metal-assisted conversion of an N-ylide mesomeric betaine into its carbenic tautomer: Generation of N-(fluoren-9-yl)imidazole-2-ylidene complexes. Dalton Trans. 2014, 43, 4474–4482. [Google Scholar] [CrossRef] [PubMed]
- César, V.; Mallardo, V.; Nano, A.; Dahm, G.; Lugan, N.; Lavigne, G.; Bellemin-Laponnaz, S. IMes-acac: Hybrid combination of diaminocarbene and acetylacetonato sub-units into a new anionic ambidentate NHC ligand. Chem. Commun. 2015, 51, 5271–5274. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, A.A.; Monakhov, K.Y.; Braunstein, P. Anionic N-heterocyclic carbene ligands from mesoionic imidazolium precursors: Remote backbone arylimino substitution directs carbene coordination. Chem. Eur. J. 2013, 19, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, A.A.; Braunstein, P. ‘Janus-type′ organopotassium chemistry observed in deprotonation of mesoionic imidazolium aminides and amino N-heterocyclic carbenes: Coordination and organometallic polymers. Chem. Commun. 2014, 50, 3055–3057. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, A.A.; Braunstein, P.; Rezabal, E.; Frison, G. Unprecedented lateral lithiations of tertiary carbons on NHC platforms. Chem. Commun. 2015, 51, 3049–3052. [Google Scholar] [CrossRef] [PubMed]
- Jonek, M.; Diekmann, J.; Ganter, C. First N-heterocyclic carbenes relying on the triazolone structural motif: Syntheses, modification and reactivity. Chem. Eur. J. 2015, 21, 15759–15768. [Google Scholar] [CrossRef] [PubMed]
- Pidlypnyi, N.; Namyslo, J.C.; Drafz, M.H.H.; Nieger, M.; Schmidt, A. Betaine-carbene interconversions.from N-ylides to zwitterionic n-heterocyclic carbene-borane adducts. J. Org. Chem. 2013, 78, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Pidlypnyi, N.; Uhrner, F.; Nieger, M.; Drafz, M.H.H.; Hübner, E.G.; Namyslo, J.C.; Schmidt, A. N-heterocyclic carbene-betaine interconversions: Tautomeric equilibria of imidazolium-indolates and indole-substituted imidazol-2-ylidenes. Eur. J. Org. Chem. 2013, 7739–7748. [Google Scholar] [CrossRef]
- Zhang, J.; Pidlypnyi, N.; Nieger, M.; Namyslo, J.C.; Schmidt, A. Zwitterionic borane adducts of N-heterocyclic carbenes from mesomeric betaines of uracil. Org. Biomol. Chem. 2014, 12, 2737–2744. [Google Scholar] [CrossRef] [PubMed]
- Pidlypnyi, N.; Wolf, S.; Liu, M.; Rissanen, K.; Nieger, M.; Schmidt, A. N-heterocyclic carbenes from ylides of indolyl-imidazolium, azaindolyl-imidazolium, and indolyl-triazolium salts, and their borane adducts. Tetrahedron 2014, 70, 8672–8680. [Google Scholar] [CrossRef]
- Liu, M.; Nieger, M.; Schmidt, A. Mesomeric betaine–N-heterocyclic carbene interconversions of 1,2,4-triazolium-phenolates. Sulfur, selenium, and borane adduct formation. Chem. Commun. 2015, 51, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Franz, M.; Hübner, E.; Schmidt, A. N-Heterocyclic carbenes by tautomerization of mesomeric betaines. Thione, selone, and borane adduct formations of imidazolium-isocytosinates. Tetrahedron 2016, 72, 525–531. [Google Scholar] [CrossRef]
- Liu, M.; Nieger, M.; Hübner, E.G.; Schmidt, A. Formation of N-heterocyclic carbenes by tautomerization of mesomeric betaines: Cyclic boron adducts and palladium complexes from 2-(imidazolium-1-yl)phenolates. Chem. Eur. J. 2016, 22, 5416–5422. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Namyslo, J.C.; Nieger, M.; Polamo, M.; Schmidt, A. From betaines to anionic N-heterocyclic carbenes. Borane, gold, rhodium, and nickel complexes starting from an imidazoliumphenolate and its carbene tautomer. Beilstein. J. Org. Chem. 2016, 12, 2673–2681. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Wiechmann, S.; Freese, T. Recent advances in neutral and anionic N-heterocyclic carbene-betaine interconversions. Synthesis, characterization, and applications. ARKIVOC 2013, i, 424–469. [Google Scholar] [CrossRef]
- Tolman, C.A. Steric effects of phosphorus ligands in organometallic chemistry and homogeneous catalysis. Chem. Rev. 1977, 77, 313–348. [Google Scholar] [CrossRef]
- Falivene, L.; Cavallo, L. Guidelines to select the n-heterocyclic carbene for the polymerization of monomers with a polar group. Macromolecules 2017, 50, 1394–1401. [Google Scholar] [CrossRef]
- Liske, A.; Verlinden, K.; Buhl, H.; Schaper, K.; Ganter, C. Determining the π-acceptor properties of n-heterocyclic carbenes by measuring the 77Se chemical shifts of their selenium adducts. Organometallics 2013, 32, 5269–5272. [Google Scholar] [CrossRef]
- Koto, Y.; Shibahara, F.; Murai, T. Imidazo[1,5-a]pyridine-3-ylidenes as π-accepting carbene ligands: Substituent effects on properties of N-heterocyclic carbenes. Org. Biomol. Chem. 2017, 15, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Dröge, T.; Glorius, F. The measure of all rings—N-heterocyclic carbenes. Angew. Chem. Int. Ed. 2010, 49, 6940–6952. [Google Scholar] [CrossRef] [PubMed]
- Calculated from IR spectroscopic data for [RhI(CO)2(4)] in dichloromethane solution, see: Herrmann, W.A.; Schütz, J.; Frey, G.D.; Herdtweck, E. N-heterocyclic carbenes: Synthesis, structures, and electronic ligand properties. Organometallics 2006, 25, 2437–2448. [Google Scholar]
- Calculated from IR spectroscopic data for [IrCl(CO)2(1)] in dichloromethane solution, see: Kelly, R.A., III; Clavier, H.; Guidice, S.; Scott, N.M.; Stevens, E.D.; Bordner, J.; Samardjiev, I.; Hoff, C.D.; Cavallo, L.; Nolan, S.P. Determination of N-heterocyclic carbene (NHC) steric and electronic parameters using the [(NHC)IrCl(CO)2] system. Organometallics 2008, 27, 202–208. [Google Scholar]
- Voitekhovich, S.V.; Lyakhov, A.S.; Ivashkevich, L.S.; Matulis, V.E.; Grigoriev, Y.V.; Gaponik, P.N.; Ivashkevich, O.A. Regioselective alkylation of amino- and mercapto-1,2,4-triazoles with t-BuOH–HClO4. Tetrahedron 2012, 68, 4962–4966. [Google Scholar] [CrossRef]
- Verlinden, K.; Buhl, H.; Frank, W.; Ganter, C. Determining the ligand properties of N-heterocyclic carbenes from 77Se NMR parameters. Eur. J. Inorg. Chem. 2015, 2416–2425. [Google Scholar] [CrossRef]
- Buck, D.M.; Kunz, D. Triazine anellated NHC featuring unprecedented coordination ability. Organometallics 2015, 34, 5335–5340. [Google Scholar] [CrossRef]
- Muller, N.; Pritchard, D.E. C13 splittings in proton magnetic resonance spectra. I. hydrocarbons. J. Chem. Phys. 1959, 31, 768–771. [Google Scholar] [CrossRef]
- Tapu, D.; Dixon, D.A.; Roe, C. 13C-NMR spectroscopy of “Arduengo type” carbenes and their derivatives. Chem. Rev. 2009, 109, 3385–3407. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.A.; Schneider, S.K.; Öfele, K.; Sakamoto, M.; Herdtweck, E. First silver complexes of tetrahydropyrimid-2-ylidenes. J. Organomet. Chem. 2004, 689, 2441–2449. [Google Scholar] [CrossRef]
- Arduengo, A.J., III; Dias, H.V.R.; Calabrese, J.C.; Davidson, F. Homoleptic Carbene–Silver(I) and Carbene–Copper(I) Complexes. Organometallics 1993, 12, 3405–3409. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, G.; Li, K.; Shelar, D.P.; Lu, W.; Che, C.-M. Phosphorescent polymeric nanomaterials with metallophilic d10···d10 interactions self-assembled from [Au(NHC)2]+ and [M(CN)2]−. Chem. Sci. 2014, 5, 1348–1353. [Google Scholar] [CrossRef]
- Baker, M.V.; Barnard, P.J.; Berners-Price, S.J.; Brayshore, S.K.; Hickey, J.L.; Skelton, B.W.; White, A.H. Cationic, linear Au(I) N-heterocyclic carbene complexes: Synthesis, structure and anti-mitochondrial activity. Dalton Trans. 2006, 3708–3715. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gangwar, M.K.; Shaikh, M.M.; Gosh, P. Synthesis and structural characterization of the gold complexes of 1,2,4-triazole derived N-heterocyclic carbene ligands. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2016, 86, 605–609. [Google Scholar] [CrossRef]
- Streitberger, M.; Schmied, A.; Hey-Hawkins, E. Selective formation of Bis-phospholane macrocycles, polymeric chains, and nanotubes. Inorg. Chem. 2014, 53, 6794–6804. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Uekusa, H.; Ishii, S.; Otsuka, T.; Kaizu, Y.; Ozawa, Y.; Toriumi, K. Polymorphic crystal approach to changing the emission of [AuCl(PPh3)2], analyzed by direct observation of the photoexcited structures by X-ray photocrystallography. Inorg. Chem. 2010, 49, 7257–7265. [Google Scholar] [CrossRef] [PubMed]
- Khin, C.; Hashmi, A.S.K.; Rominger, S. Gold(I) complexes of P,N ligands and their catalytic activity. Eur. J. Inorg. Chem. 2010, 7, 1063–1069. [Google Scholar] [CrossRef]
- Wile, B.M.; McDonald, R.; Ferguson, M.J.; Stradiotto, M. Au(I) complexes supported by donor-functionalized indene ligands: Synthesis, characterization, and catalytic behavior in aldehyde hydrosilylation. Organometallics 2007, 26, 1069–1076. [Google Scholar] [CrossRef]
- Cerrini, S.; Colapietro, M.; Spagna, R.; Zambonelli, L. The crystal and molecular structure of 1,4-diphenyl-3-phenylamino-1,2,4-triazolium tetrakis(isothiocyanato)cobaltate(II), [C20H17N4]2[Co(NSC)4]: A salt of the conjugate acid of nitron. J. Chem. Soc. A 1971, 1375–1380. [Google Scholar] [CrossRef]
- Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fu, X.; Gao, G. Anion–cation cooperative catalysis by ionic liquids. ChemCatChem 2011, 3, 1359–1364. [Google Scholar] [CrossRef]
- Cannon, J.R.; Raston, C.L.; White, A.H. Crystal structure of nitron and its non-stoichiometric hydrochloride. Aust. J. Chem. 1980, 33, 2237–2247. [Google Scholar] [CrossRef]
- Taylor, R.; Kennard, O. Crystallographic evidence for the existance of C–H···O, C–H···N, and C–H···Cl hydrogen bonds. J. Am. Chem. Soc. 1982, 104, 5063–5070. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Andersen, C.L.; Jensen, C.S.; Mackeprang, K.; Du, L.; Jørgensen, S.; Kjaergaard, H.G. Similar strength of the NH···O and NH···S hydrogen bonds in binary complexes. J. Phys. Chem. A 2014, 118, 11074–11082. [Google Scholar] [CrossRef] [PubMed]
- Phukan, N.; Baruah, J.B. Conformational adjustments over synthons of urea and thiourea based assemblies. CrystEngComm 2016, 18, 7753–7763. [Google Scholar] [CrossRef]
- Laus, G.; Kahlenberg, V.; Wurst, K.; Schottenberger, H. Synthesis and crystal structures of new 1,4-disubstituted 1,2,4-triazoline-5-thiones. Z. Naturforsch. B Chem. Sci. 2014, 69, 950–964. [Google Scholar] [CrossRef]
- Szabo, J.; Karger, K.; Bucher, N.; Maas, G. Derivatives of the triaminoguanidinium ion, 3. Multiple N-functionalization of the triaminoguanidinium ion with isocyanates and isothiocyanates. Beilstein. J. Org. Chem. 2014, 10, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Aullón, G.; Bellamy, D.; Brammer, L.; Bruton, E.A.; Orpen, A.G. Metal-bound chlorine often accepts hydrogen bonds. Chem. Commun. 1998, 653–654. [Google Scholar] [CrossRef]
- Appleton, T.G.; Clark, H.C.; Manzer, L.E. The trans-influence: Its measurement and significance. Coord. Chem. Rev. 1973, 10, 335–422. [Google Scholar] [CrossRef]
- Netland, K.A.; Krivikapic, A.; Tilset, M. Pt(II) complexes with diimine and chelating 5-ring iminocarbene ligands: Synthesis, characterization, and structural and spectroscopic trends. J. Coord. Chem. 2010, 63, 2909–2927. [Google Scholar] [CrossRef]
- Slattery, J.; Thatcher, R.J.; Shi, Q.; Douthwaite, R.E. Comparison of donor properties of N-heterocyclic carbenes and N-donors containing the 1H-pyridin-(2E)-ylidene motif. Pure Appl. Chem. 2010, 82, 1663–1671. [Google Scholar] [CrossRef]
- Datt, M.S.; Nair, J.J.; Otto, S. Synthesis and characterisation of two novel Rh(I) carbene complexes: Crystal structure of [Rh(acac)(CO)(L1)]. J. Organomet. Chem. 2005, 690, 3422–3426. [Google Scholar] [CrossRef]
- Baba, E.; Cundari, T.R.; Firkin, I. N-heterocyclic carbenes of the late transition metals: A computational and structural database study. Inorg. Chim. Acta 2005, 358, 2867–2875. [Google Scholar] [CrossRef]
- Turek, J.; Panov, I.; Horáček, M.; Černošek, Z.; Padĕlková, Z.; Růžička, A. Amino-group functionalized N-heterocyclic 1,2,4-triazole derived carbenes: Structural diversity of rhodium(I) complexes. Organometallics 2013, 32, 7234–7240. [Google Scholar] [CrossRef]
- Paul, S.; Schweizer, W.B.; Rugg, G.; Senn, H.M.; Gilmour, R. The fluorine-NHC gauche effect: A structural and computational study. Tetrahedron 2013, 69, 5647–5659. [Google Scholar] [CrossRef]
- Nichol, G.S.; Rajaseelan, J.; Anna, L.J.; Rajaseelan, E. N-heterocyclic carbene complexes of rhodium and iridium: Steric effects on molecular conformation. Eur. J. Inorg. Chem. 2009, 4320–4328. [Google Scholar] [CrossRef]
- Bonnot, A.; Knorr, M.; Guyon, F.; Kubicki, M.M.; Rousselin, Y.; Strohmann, C.; Fortin, D.; Harvey, P.D. 1,4-Bis(arylthio)but-2-enes as assembling ligands for (Cu2X2)n (X = I, Br; n = 1, 2) coordination polymers: Aryl substitution, olefin configuration, and halide effects on the dimensionality, cluster size, and luminescence properties. Cryst. Growth Des. 2016, 16, 774–788. [Google Scholar] [CrossRef]
- Woidy, P.; Karttunen, A.J.; Widenmeyer, M.; Niewa, R.; Kraus, F. On copper(I) fluorides, the cuprophilic interaction, the preparation of copper nitride at room temperature, and the formation mechanism at elevated temperatures. Chem. Eur. J. 2015, 21, 3290–3303. [Google Scholar] [CrossRef] [PubMed]
- Sevryugina, Y.; Petrukhina, M.A. Breaking infinite CuI carboxylate helix held by cuprophilicity into discrete Cun fragments (n = 6, 4, 2). Eur. J. Inorg. Chem. 2008, 219–229. [Google Scholar] [CrossRef]
- Sugiura, T.; Yoshikawa, H.; Awaga, K. 1D helical polymeric chains with a pseudo-53 screw axis formed by cuprophilicity. synthesis and crystal structure of copper(I) pivalate. Inorg. Chem. 2006, 45, 7584–7586. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.L.; Messerschmidt, M.; Coppens, P. An unstable ligand-supported CuI dimer stabilized in a supramolecular framework. Angew. Chem. Int. Ed. 2005, 44, 4614–4617. [Google Scholar] [CrossRef] [PubMed]
- Markgraf, G.; Bats, J.W.; Bolte, M.; Lerner, H.-W.; Wagner, M. One- and three-dimensional infinite arrays of Cu(I) ions exhibited by [Cu(NH3)2]Br and [Cu(NH3)Cl] in the solid state. Chem. Commun. 2003, 956–957. [Google Scholar] [CrossRef]
- Che, C.-M.; Mao, Z.; Miskowski, V.M.; Tse, M.-C.; Chan, C.-K.; Cheung, K.-K.; Phillips, D.L.; Leung, K.-H. Evidence for Cu–Cu bonding interactions in luminescent dinuclear copper(I) complexes with bridging diphosphane ligands. Angew. Chem. Int. Ed. 2000, 39, 4084–4088. [Google Scholar] [CrossRef]
- Dinda, S.; Samuelson, A.G. The nature of bond critical points in dinuclear copper(I) complexes. Chem. Eur. J. 2012, 18, 3032–3042. [Google Scholar] [CrossRef] [PubMed]
- Pinter, B.; Broeckaert, L.; Turek, J.; Růžička, A.; De Proft, F. Dimers of N-heterocyclic carbene copper, silver, and gold halides: Probing metallophilic interactions through electron density based concepts. Chem. Eur. J. 2014, 20, 734–744. [Google Scholar] [CrossRef] [PubMed]
- We note that the structure of [AuCl(3)] is not contained in the Cambridge Structural Database and that no experimental details are given in ref. [99] concerning this compound. We also note that the structure of the 1,4-dimethyl-1,2,4-triazol-5-ylidene homologue [AuCl(4)] has been published, but suffers from a low resolution of the data (R1 = 0.1692); see: Wang, H.M.J.; Sekhar Vasam, C.; Tsai, T.Y.R.; Chen, S.-H.; Chang, A.H.H.; Lin, I.J.B. Gold(I) N-heterocyclic carbene and carbazolate complexes. Organometallics 2005, 24, 486–493. [Google Scholar]
- Schmidbaur, H.; Schier, A. Aurophilic interactions as a subject of current research: An up-date. Chem. Soc. Rev. 2012, 41, 370–412. [Google Scholar] [CrossRef] [PubMed]
- Pyykkö, P. Theoretical chemistry of gold. III. Chem. Soc. Rev. 2008, 37, 1967–1997. [Google Scholar] [CrossRef] [PubMed]
- Schmidbaur, H.; Schier, A. A briefing on aurophilicity. Chem. Soc. Rev. 2008, 37, 1931–1951. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.J.; Sakai, K.; Leznoff, D.B. The use of aurophilic and other metal–metal interactions as crystal engineering design elements to increase structural dimensionality. Chem. Soc. Rev. 2008, 37, 1884–1895. [Google Scholar] [CrossRef] [PubMed]
- Leitner, S.; List, M.; Monkowius, U. Synthesis, characterization and luminescence of silver(I) and gold(I) complexes bearing a diethyl acetal functionalized n-heterocyclic carbene. Z. Naturforsch. B Chem. Sci. 2011, 66, 1255–1260. [Google Scholar] [CrossRef]
- Cui, F.; Yang, P.; Huang, X.; Yang, X.-J.; Wu, B. Homometallic silver(I) complexes of a heterotopic NHC-bridged bis-bipyridine ligand. Organometallics 2012, 31, 3512–3518. [Google Scholar] [CrossRef]
- Tai, C.C.; Yu, M.-S.; Chen, Y.-L.; Chuang, W.-H.; Lin, T.-H.; Yap, G.P.A.; Ong, T.-G. Synthesis of a guanidine NHC complex and its application in borylation reactions. Chem. Commun. 2014, 50, 4344–4346. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-H.; Lee, C.-S.; Pal, S.; Chen, Y.-N.; Hwang, W.-S.; Lin, I.J.B.; Wang, J.-C. Novel Ag(I), Pd(II), Ni(II) complexes of N,N′-bis-(2,2-diethoxyethyl)imidazole-2-ylidene: Synthesis, structures and their catalytic activity towards Heck reaction. J. Organomet. Chem. 2008, 693, 3729–3740. [Google Scholar] [CrossRef]
- Newman, C.P.; Clarkson, G.J.; Rourke, J.P. Silver(I) N-heterocyclic carbene halide complexes: A new bonding motif. J. Organomet. Chem. 2007, 692, 4962–4968. [Google Scholar] [CrossRef]
- Ming Lee, K.; Wang, H.M.J.; Lin, I.J.B. Structural diversity of N-heterocyclic carbene complexes of silver(I). J. Chem. Soc. Dalton Trans. 2002, 2852–2856. [Google Scholar]
- Uson, R.; Laguna, A.; Laguna, M.; Briggs, D.A.; Murray, H.H.; Fackler, J.P., Jr. (Tetrahydrothiophene)gold(I) or gold(III) complexes. Inorg. Synth. 1989, 26, 85–91. [Google Scholar]
- Duddeck, H. Selenium-77 nuclear magnetic resonance spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 1995, 27, 1–323. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. IL MILIONE: A suite of computer programs for crystal structure solution of proteins. J. Appl. Crystallogr. 2007, 40, 609–613. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behaviour. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thie, C.; Bruhn, C.; Leibold, M.; Siemeling, U. Coinage Metal Complexes of the Carbenic Tautomer of a Conjugated Mesomeric Betaine Akin to Nitron. Molecules 2017, 22, 1133. https://doi.org/10.3390/molecules22071133
Thie C, Bruhn C, Leibold M, Siemeling U. Coinage Metal Complexes of the Carbenic Tautomer of a Conjugated Mesomeric Betaine Akin to Nitron. Molecules. 2017; 22(7):1133. https://doi.org/10.3390/molecules22071133
Chicago/Turabian StyleThie, Charlotte, Clemens Bruhn, Michael Leibold, and Ulrich Siemeling. 2017. "Coinage Metal Complexes of the Carbenic Tautomer of a Conjugated Mesomeric Betaine Akin to Nitron" Molecules 22, no. 7: 1133. https://doi.org/10.3390/molecules22071133




