Advantages of an Electrochemical Method Compared to the Spectrophotometric Kinetic Study of Peroxidase Inhibition by Boroxine Derivative
Abstract
:1. Introduction
2. Results
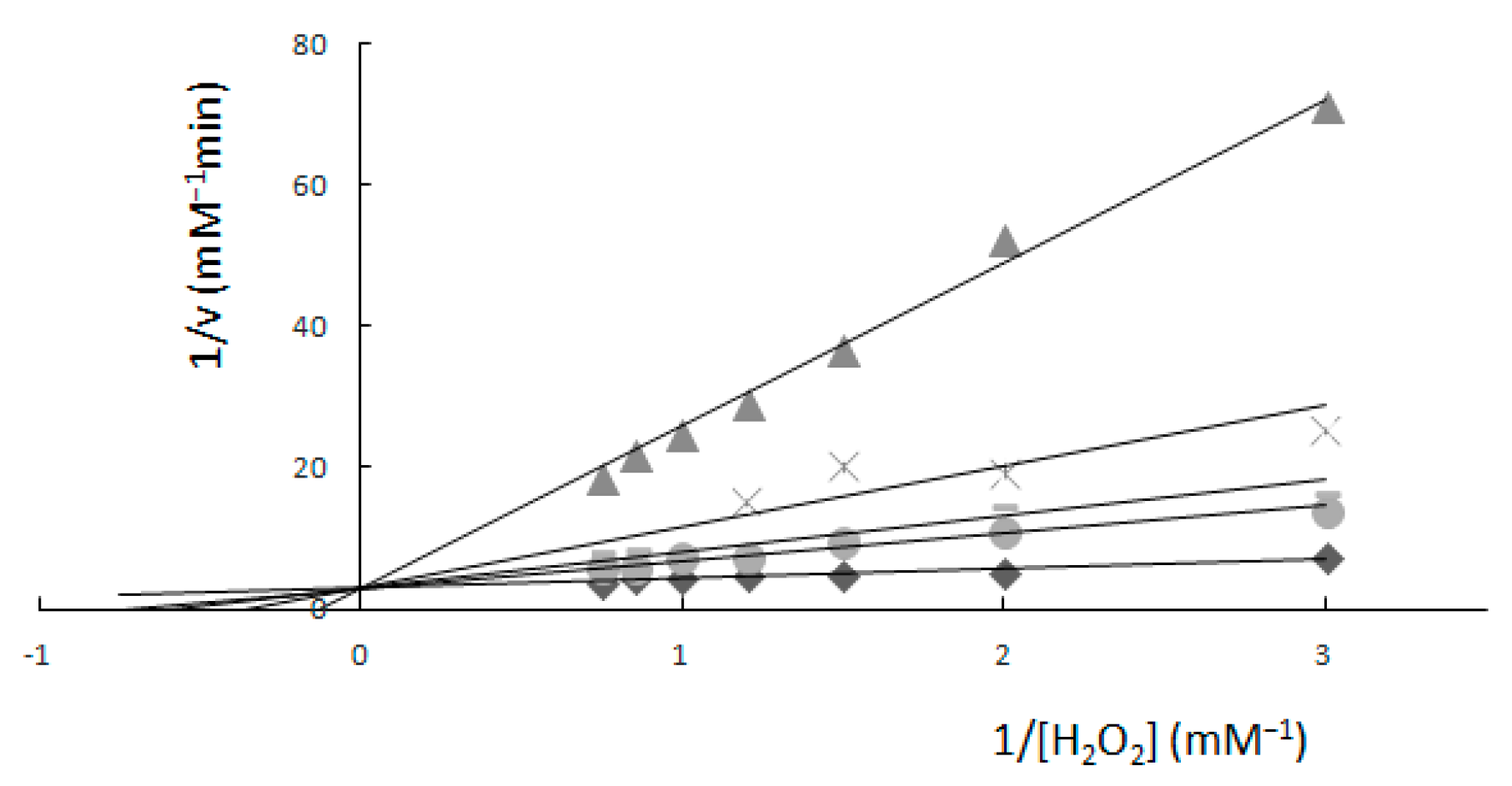
2.1. Determination of Km and Vmax—Spectrophotometric Method
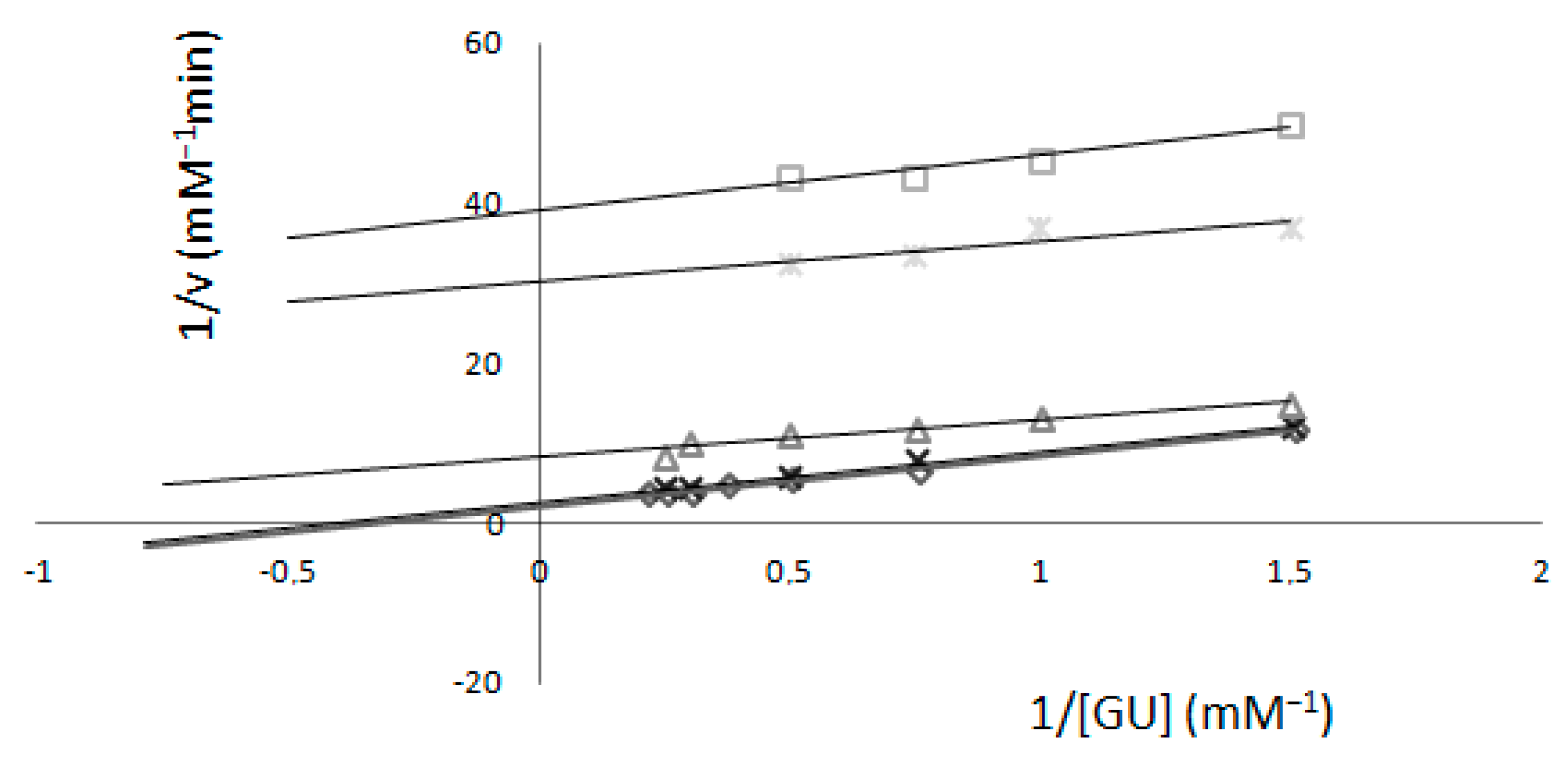
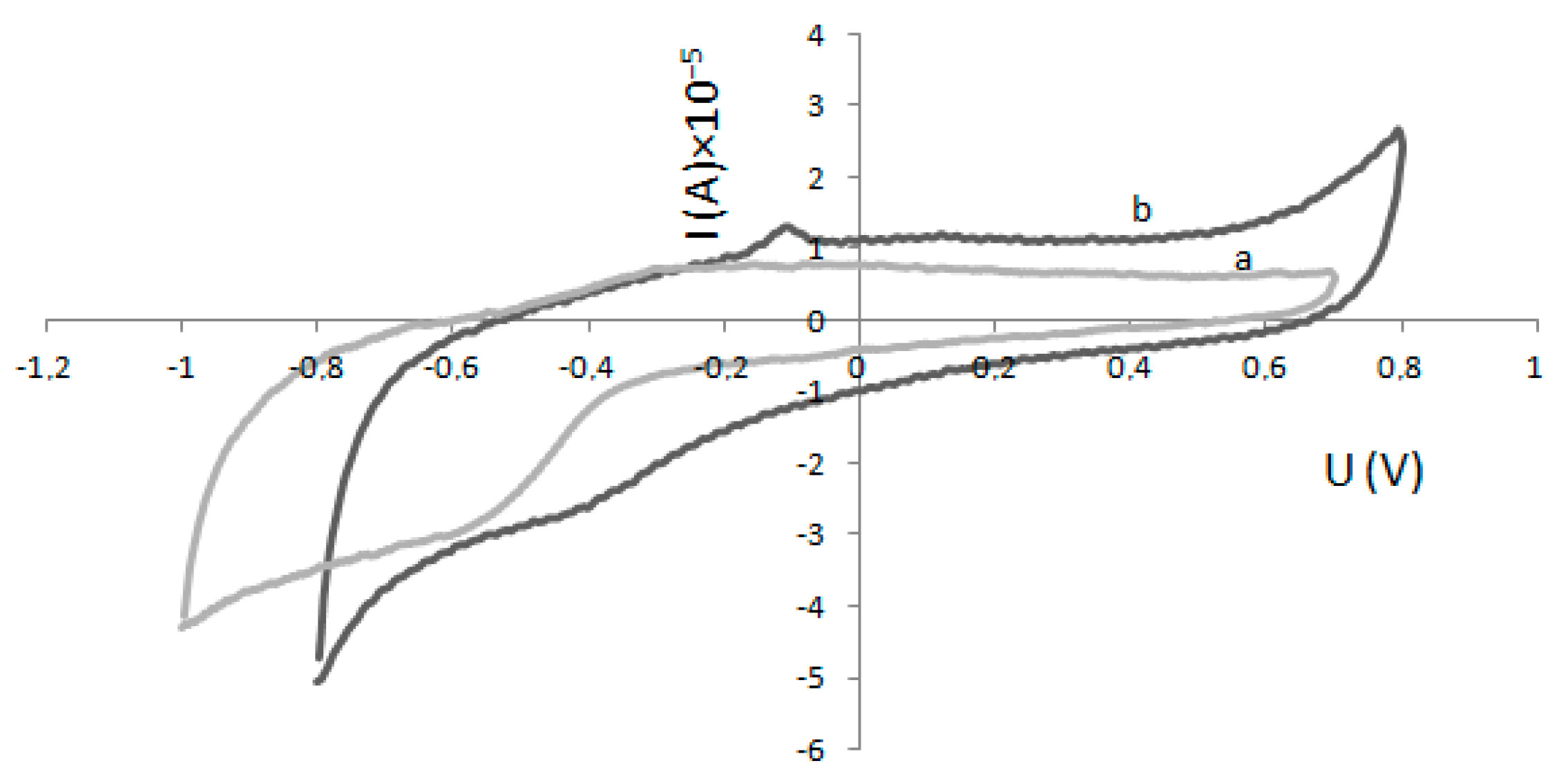
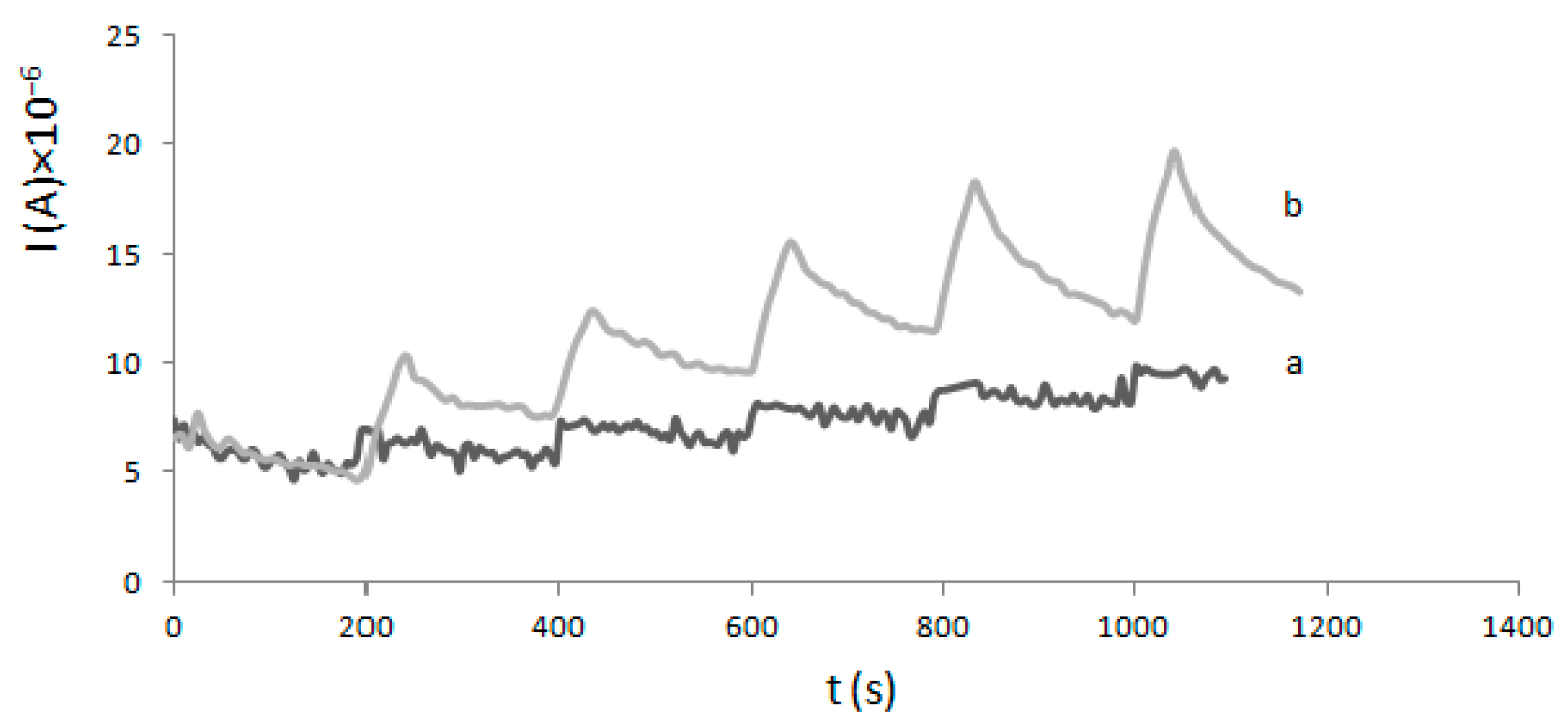
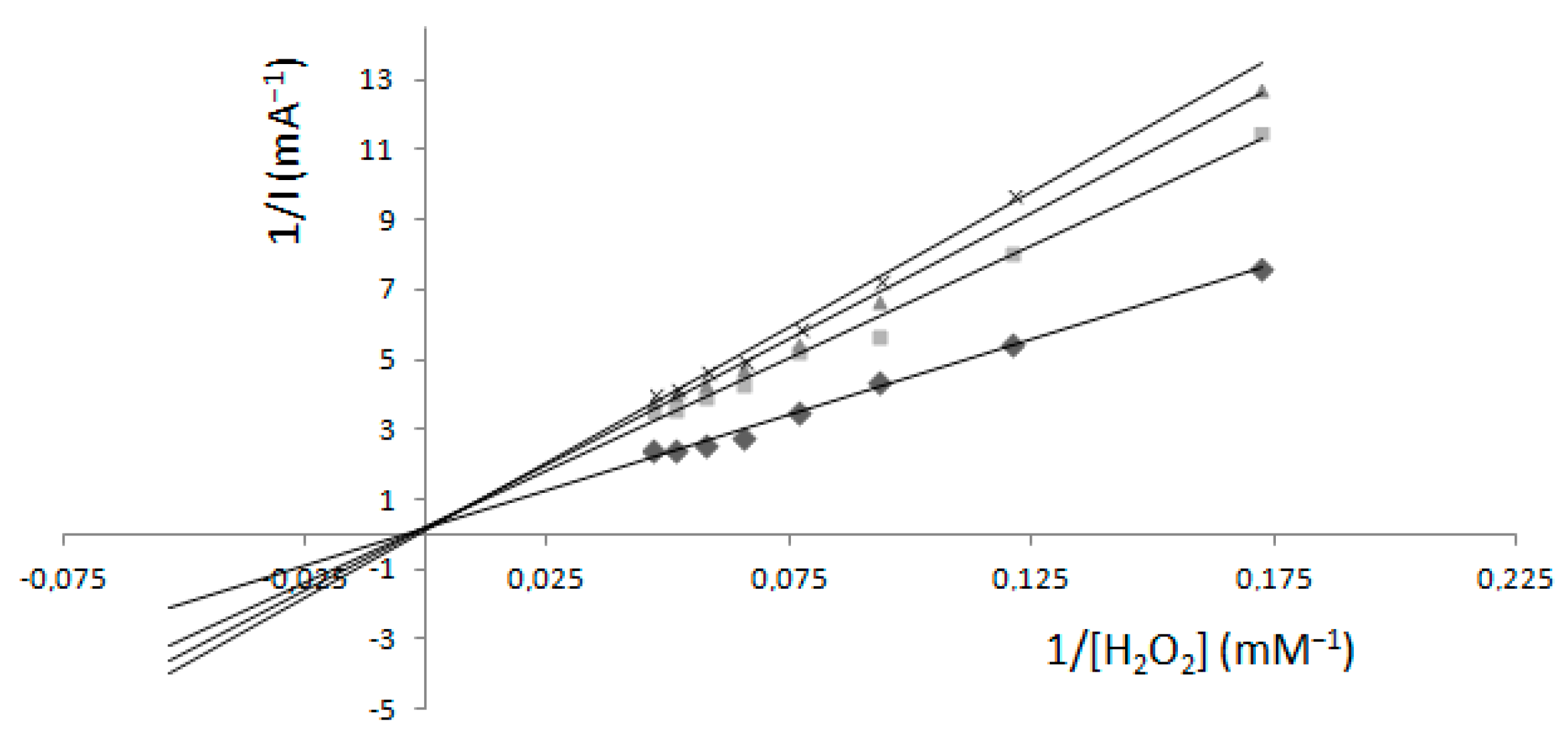
2.2. Determination of Km and Imax—Electrochemical Method
3. Discussion
Comparison of Two Methods
4. Materials and Methods
4.1. Chemicals
4.2. Spectrophotometric Assay of HRP Activity
4.3. Electrode Modification and Electrochemical Experiments
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Islamovic, S.; Galic, B.; Milos, M. A study of the inhibition of catalase by dipotassium trioxohydroxytetrafluorotriborate K2[B3O3F4OH]. J. Enzym. Inhib. Med. Chem. 2014, 29, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Milos, M.; Galic, B.; Scozzafava, A.; Supuran, C.T. Dipotassium-trioxohydroxytetrafluorotriborate, K2[B3O3F4OH], is a potent inhibitor of human carbonic anhydrases. J. Enzym. Inhib. Med. Chem. 2015, 30, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, J.; Herenda, S.; Galijasevic, S.; Galic, B.; Milos, M. Inhibition of horseradish peroxidase activity by boroxine derivative, dipotassium-trioxohydroxytetrafluorotriborate K2[B3O3F4OH]. J. Chem. 2017. [Google Scholar] [CrossRef]
- Lei, C.H.; Deng, J.Q. Hydrogen peroxide sensor based on coimmobilized methylene green and horseradish peroxidase in the same montmorillonite-modified bovine serum albumin-glutaraldehyde matrix on a glassy carbon electrode surface. Anal. Chem. 1996, 68, 3344–3349. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Yan, F.; Kong, J.L.; Deng, J.Q. A reagentless amperometric biosensor based on the coimmobilization of horseradish peroxidase and methylene green in a modified zeolite matrix. Anal. Chim. Acta 1999, 386, 31–39. [Google Scholar] [CrossRef]
- Ruzgas, T.; Csoregi, E.; Emneus, J.; Gorton, L.; MarkoVarga, G. Peroxidase-modified electrodes: Fundamentals and application. Anal. Chim. Acta 1996, 330, 123–138. [Google Scholar] [CrossRef]
- Akkara, J.A.; Senecal, K.J.; Kaplan, D.L. Synthesis and characterization of polymers produced by horseradish peroxidase in dioxane. J. Polym. Sci. Part A: Poly. Chem. 1991, 29, 1561–1574. [Google Scholar] [CrossRef]
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Aghelan, Z.; Shariat, S.Z.S. Partial purification and biochemical characterization of peroxidase from rosemary (Rosmarinus officinalis L.) leaves. Adv. Biomed. Res. 2015, 4, 159. [Google Scholar] [PubMed]
- Hirai, H.; Shibata, H.; Kawai, S.; Nishida, T. Role of 1-hydroxybenzotriazole in oxidation by laccase from trametes versicolor. Kinetic analysis of the laccase-1-hydroxybenzotriazole couple. FEMS Microbiol. Lett. 2006, 265, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.; Banu, U.; Kedilaya, T.; Patheja, M. Characterization of peroxidase from brassica oleracea gongylodes gives a lead for use of bromocresol purple as a novel substrate for peroxidase assay. J. Biochem. Technol. 2012, 4, 502–507. [Google Scholar]
- Tayefi-Nasrabadi, H.; Dehghan, G.; Daeihassani, B.; Movafegi, A.; Samadi, A. Some biochemical properties of guaiacol peroxidases as modified by salt stress in leaves of salt-tolerant and salt-sensitive safflower (Carthamus tinctorius L. cv.) cultivars. Afr. J. Biotechnol. 2011, 10, 751–763. [Google Scholar]
- Zatón, A.M.L.; Ochoa de Aspuru, E. Horseradish peroxidase inhibition by thiouracils. FEBS Lett. 1995, 374, 192–194. [Google Scholar] [CrossRef]
- Andreu, R.; Ferapontova, E.E.; Gorton, L.; Calvente, J.J. Direct electron transfer kinetics in horseradish peroxidase electrocatalysis. J Phys. Chem. B 2007, 111, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, F.A.; Hill, H.A.O.; Walton, N.J. Direct electrochemistry of redox proteins. Acc. Chem. Res. 1988, 21, 407–413. [Google Scholar] [CrossRef]
- Gu, T.T.; Wang, J.L.; Xia, H.Q.; Wang, S.; Yu, X.T. Direct electrochemistry and electrocatalysis of horseradish peroxidase immobilized in a DNA/chitosan-Fe3O4 magnetic nanoparticle bio-complex film. Materials 2014, 7, 1069–1083. [Google Scholar] [CrossRef]
- Li, M.G.; Xu, S.D.; Tang, M.; Liu, L.; Gao, F.; Wang, Y.L. Direct electrochemistry of horseradish peroxidase on graphene-modified electrode for electrocatalytic reduction towards H2O2. Electrochim. Acta 2011, 56, 1144–1149. [Google Scholar] [CrossRef]
- Salimi, A.; Sharifi, E.; Noorbakhsh, A.; Soltanian, S. Direct electrochemistry and electrocatalytic activity of catalase immobilized onto electrodeposited nano-scale islands of nickel oxide. Biophys. Chem. 2007, 125, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Hu, N.F.; Zeng, Y.H.; Zhou, G. Electrochemistry and electrocatalysis with heme proteins in chitosan biopolymer films. Anal. Biochem. 2002, 308, 141–151. [Google Scholar] [CrossRef]
- Upadhyay, A.K.; Ting, T.W.; Chen, S.M. Amperometric biosensor for hydrogen peroxide based on coimmobilized horseradish peroxidase and methylene green in ormosils matrix with multiwalled carbon nanotubes. Talanta 2009, 79, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Q.; Li, J.H. Direct electrochemistry and electrocatalysis of hemoglobin immobilized in bimodal mesoporous silica and chitosan inorganic-organic hybrid film. Electrochem. Commun. 2007, 9, 1530–1535. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Qiao, Y.; Hao, F.; Zhang, L.; Wu, S.Y.; Li, Y.; Li, J.H.; Song, X.M. Fabrication of a biocompatible and conductive platform based on a single-stranded DNA/graphene nanocomposite for direct electrochemistry and electrocatalysis. Chem. Eur. J. 2010, 16, 8133–8139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Sheng, Q.L.; Zheng, J.B. Direct electrochemistry and electrocatalysis of horseradish peroxidase on a gold electrode modified with a polystyrene and multiwalled carbon nanotube composite film. Microchim. Acta 2012, 176, 177–184. [Google Scholar] [CrossRef]
- Dunford, H. Horseradish peroxidase: Structure and kinetic properties. Peroxidases Chem. Biol. 1991, 2, 1–24. [Google Scholar]
- Li, J.; Tan, S.N.; Ge, H. Silica sol-gel immobilized amperometric biosensor for hydrogen peroxide. Anal. Chim. Acta 1996, 335, 137–145. [Google Scholar] [CrossRef]
- Goldstein, L. Microenvironmental effects on enzyme catalysis. Kinetic study of polyanionic and polycationic derivatives of chymotrypsin. Biochemistry 1972, 11, 4072–4084. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T. Understanding Enzymes; Ellis Horwood: New York, NY, USA, 1991. [Google Scholar]
- Ryss, I.; Slutskaya, M. Report on the platinum sector. Akad Nauk SSSR 1951, 26, 216–218. [Google Scholar]
- Chance, B.; Maehly, A. [136] Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostojić, J.; Herenda, S.; Bešić, Z.; Miloš, M.; Galić, B. Advantages of an Electrochemical Method Compared to the Spectrophotometric Kinetic Study of Peroxidase Inhibition by Boroxine Derivative. Molecules 2017, 22, 1120. https://doi.org/10.3390/molecules22071120
Ostojić J, Herenda S, Bešić Z, Miloš M, Galić B. Advantages of an Electrochemical Method Compared to the Spectrophotometric Kinetic Study of Peroxidase Inhibition by Boroxine Derivative. Molecules. 2017; 22(7):1120. https://doi.org/10.3390/molecules22071120
Chicago/Turabian StyleOstojić, Jelena, Safija Herenda, Zerina Bešić, Mladen Miloš, and Borivoj Galić. 2017. "Advantages of an Electrochemical Method Compared to the Spectrophotometric Kinetic Study of Peroxidase Inhibition by Boroxine Derivative" Molecules 22, no. 7: 1120. https://doi.org/10.3390/molecules22071120




