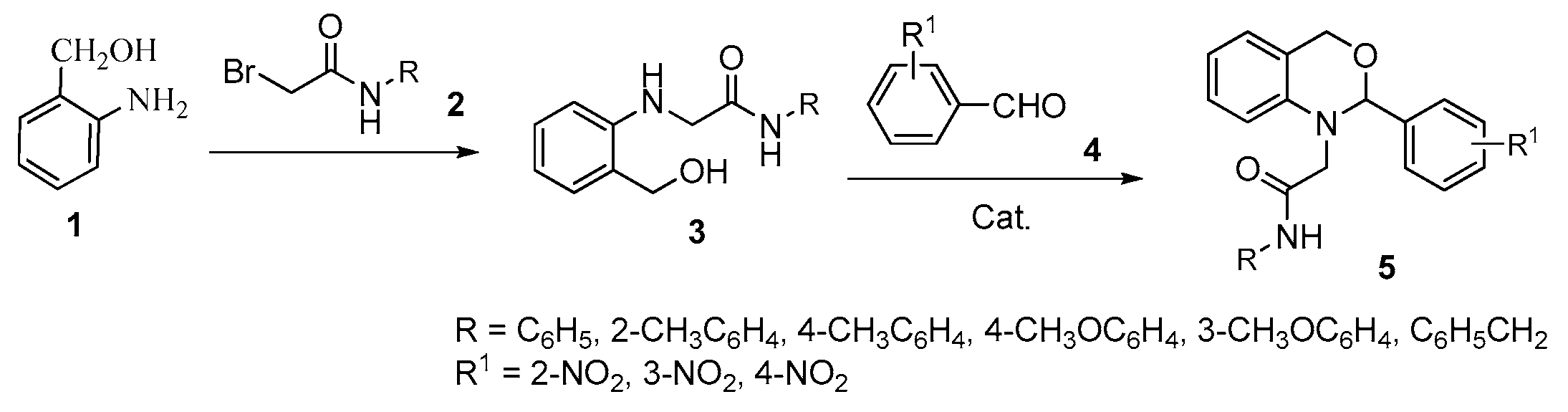
3.2.1. Synthesis of N-Substituted 2-(2-(hydroxymethyl)phenylamino)acetamides 3a–f
General Procedure: Into a 150 mL round bottom flask equipped with a condenser, N-(2-methylphenyl)-2-bromoacetamide (2.270 g, 10 mmol), 2-aminobenzyl alcohol (1.476 g, 12 mmol), potassium carbonate (1.932 g, 14 mmol) and mixed solvent of DMF and THF (45 mL, v:v = 1:2) were added with stirring. The mixture was heated at 65 °C for 12 h (checked by TLC). Then, the solvent was evaporated under reduced pressure. Saturated brine (50 mL) was added to the residue and extracted with ethyl acetate (3 × 50 mL). The organic phase was washed sequentially with water (2 × 50 mL), saturated brine (2 × 50 mL), and dried over Na2SO4, and filtered. The filtrate was evaporated under reduced pressure, the obtained residue purified by silica gel flash chromatography with ethyl acetate–petroleum ether (v/v = 1:2) as eluent, giving the product 3a (73% yield) as a white solid.
N-(2-Methylphenyl)-2-(2-(hydroxymethyl)phenylamino)acetamide (3a): Yield 73%. White solid, m.p.: 115.1–118.3 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.53 (s, 1H), 7.92 (d, J = 5 Hz,1H), 7.23–7.26 (m, 2H), 7.11 (d, J = 7.5 Hz, 2H), 7.09 (t, J = 7.5 Hz, 1H),6.79 (t, J = 7.0 Hz, 1H), 6.67 (d, J = 8.0 Hz, 1H), 5.66 (s, 1H), 4.76 (d, J = 5 Hz, 2H), 3.98 (s, 2H), 2.04 (s, 1H), 1.97 (s, 3H, -CH3); 13C-NMR (125 MHz, CDCl3) δ: 168.69, 146.20, 135.26, 131.52, 130.37, 129.91, 129.24, 126.80, 125.15, 125.00, 122.20, 118.84, 111.48, 64.75, 48.89, 17.11; IR (KBr, cm−1) ν: 3309, 3257, 1670, 1585, 1541, 1264, 1011, 748.
N-(4-Methylphenyl)-2-(2-(hydroxymethyl)phenylamino)acetamide (3b): Eluent: acetate/petroleum ether (v/v = 1:2); Yield 73%. White solid, m.p.: 123.4–124.5 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.51 (s, 1H), 7.33 (d, J = 8.0 Hz, 2H),7.20 (t, J = 8.0 Hz, 1H) 7.06–7.09 (m, 3H), 6.77 (t, J = 6.0 Hz, 1H), 6.58 (s, 1H), 5.52 (s, 1H), 4.72 (d, J = 5 Hz,2H), 3.86 (s, 2H), 2.28 (s,3H, -CH3); 13C-NMR (125 MHz, CDCl3) δ: 169.60, 146.40, 134.60, 134.39, 129.79, 129.48 (2C),129.41, 125.19, 120.31 (2C), 118.59, 111.30, 64.27, 48.87, 20.90; IR (KBr, cm−1) ν: 3398, 3321, 3229, 1677, 1609, 1552, 1525, 1313, 992, 818, 738.
N-(4-Methyloxyphenyl)-2-(2-(hydroxymethyl)phenylamino)acetamide (3c): Eluent: acetate/petroleum ether (v/v = 1:2); Yield 68%. Pale yellow solid, m.p.: 107.0–107.2 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.59 (s, H), 7.34 (d, J = 7.0Hz, 2H), 7.20 (s, 1H), 7.08 (s, 1H), 6.76–6.80 (m, 3H), 6.56 (t, J = 5.0 Hz, 1H), 5.51 (s, 1H), 4.69 (s, 2H), 3.83 (s, 2H), 3.74 (s, 3H, -OCH3); 13C-NMR (125 MHz, CDCl3) δ: 169.38, 156.62, 146.31, 130.17, 129.68, 129.30, 125.06, 121.97, 118.46, 114.00 (2C), 111.16, 64.21, 55.36, 48.67; IR (KBr, cm−1) ν: 3399, 3298, 1669, 1609, 1507, 1453, 1300, 1236, 1000, 825, 743.
N-(3-Methyloxyphenyl)-2-(2-(hydroxymethyl)phenylamino)acetamido (3d): Eluent: acetate/petroleum ether (v/v = 1:2); Yield 65%. Pale yellow solid, m.p.: 120.9–124.4 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.48 (s, 1H), 7.08–7.18 (m, 3H), 7.02 (d, J = 7.0 Hz, 1H), 6.87 (d, J = 8.0 Hz, 1H), 6.71 (t, J = 7.0 Hz, 1H), 6.56–6.58 (m, 1H), 6.52 (d, J = 8.0 Hz, 1H), 4.68 (s, 2H), 3.83 (s, 2H), 3.69 (s, 3H, -OCH3); 13C-NMR (125 MHz, CDCl3) δ: 169.36, 160.11, 146.44, 138.42, 129.96, 129.68, 129.36, 125.12, 118.83, 112.22, 111.51, 110.32, 105.85, 64.62, 55.34, 49.17; IR (KBr, cm−1) ν: 3391, 3336, 1672, 1601, 1560, 1516, 1455, 1430, 1050, 1006, 780, 749.
N-Phenyl-2-(2-(hydroxymethyl)phenylamino)acetamido (3e): Eluent: acetate/petroleum ether (v/v = 1:2); Yield 70%. White solid, m.p.: 117.2–119.3 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.59 (s, 1H), 7.45 (d, J = 8.0 Hz, 2H), 7.26 (t, J = 7.5 Hz, 2H), 7.19 (td, J = 8.0 Hz, 1.0 Hz, 1H), 7.08 (t, J = 6.5 Hz, 2H), 6.77 (t, J = 7.5 Hz, 1H), 6.57(d, J = 8.0 Hz, 1H), 5.52 (br, 1H), 4.72 (s, 2H), 3.86 (s, 2H), 2.77 (br, 1H); 13C-NMR (125 MHz, CDCl3) δ: 169.61, 146.39, 137.18, 129.87, 129.41, 129.00 (2C), 125.18, 124.70, 120.20 (2C), 118.72, 111.37, 64.41, 48.98; IR (KBr, cm−1) ν: 3342, 3265, 1678, 1608, 1563, 1513, 1498, 1444, 1313, 1254, 1003, 751.
N-Benzyl-2-(2-(hydroxymethyl)phenylamino)acetamido (3f): Eluent: acetate/petroleum ether (v/v = 1:2); Yield 66%. Pale yellow solid, m.p.: 107.7–108.8 °C; 1H-NMR (500 MHz, CDCl3) δ: 7.13–7.25 (m, 8H), 7.02 (d, J = 6.0 Hz, 1H), 6.73 (s, 1H, NH), 6.51 (s, 1H, NH), 4.60 (s, 2H), 4.37 (s, 2H), 3.77 (s, 2H); 13C-NMR (125 MHz, CDCl3) δ: 171.14, 146.48, 138.01, 129.63, 129.28, 128.62 (2C), 127.45 (2C), 127.41, 125.07, 118.27, 111.11, 64.35, 48.19, 43.01; IR (KBr, cm−1) ν: 3375, 3254, 1645, 1585, 1530, 1505, 1450, 1427, 1365, 1311, 1252, 1016, 751.
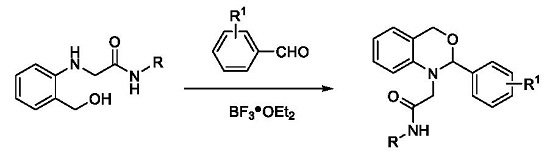
3.2.2. Synthesis of 2,4-Dihydro-1H-3,1-benzoxazines 5a–r
General Procedure: Under nitrogen, into a 100 mL three-necked round bottom flask equipped with a condenser, N-(2-methylphenyl)-2-(2-(hydroxymethyl)phenylamino)acetamide 3a (0.405 g, 1.5 mmol), 3-nitrobenzaldehyde (0.339 g, 2.25 mmol), THF (30 mL), BF3·OEt2 (0.031 g, 0.3 mmol) and molecular sieve 4Å (0.250 g) were added with stirring. The solution was heated at 65 °C for 10 h (checked by TLC). Then, the solvent was evaporated under reduced pressure. Ethyl acetate (70 mL) was added to the residue, and the obtained solution washed sequentially with water (2 × 40 mL) and saturated brine (2 × 40 mL). The organic phase was dried over Na2SO4 and filtered. The filtrate was evaporated under reduced pressure and the obtained residue purified by silica gel flash chromatography with ethyl acetate–petroleum ether (v/v = 1:5) as eluent, giving the product 5a (55% yield) as a yellow solid.
1-((2-Methylphenyl)carbamoylmethyl)-2-(3-nitrophenyl)-2,4-dihydro-1H-3,1-benzoxazine (5a): Yield 55%. Yellow solid; m.p.: 151.2–152.2 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.50 (s, 1H), 8.34 (s, 1H), 8.23 (d, J = 9.0 Hz, 1H), 7.84 (d, J = 8.0 Hz, 1H), 7.78 (d, J = 7.5 Hz, 1H), 7.58 (t, J = 8.0 Hz, 1H), 7.24 (d, J = 7.0 Hz, 1H), 7.19 (t, J = 8.0 Hz, 1H), 7.13 (t, J = 7.0 Hz, 1H), 7.05 (t, J = 7.5 Hz, 1H), 6.93–6.97 (m, 3H), 5.88 (s, 1H, NCHO), 4.97 (d, J = 15 Hz, 1H), 4.74 (d, J = 15 Hz, 1H), 4.13 (d, J = 18 Hz, 1H), 3.88 (d, J = 18 Hz, 1H), 2.06 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 167.30 (C=O), 148.67, 141.92, 139.47, 135.04, 133.57, 130.53, 130.08, 128.70, 128.54, 126.86, 125.32, 125.09, 124.28, 122.82, 122.47, 122.23, 121.18, 115.20, 88.59, 64.74, 54.86, 17.37; IR (KBr, cm−1) ν: 3268, 1661 (C=O), 1586, 1536, 1497, 1458, 1397, 1346, 1259, 1208, 1070, 757, 733; Anal. Calcd. for C23H21N3O4: C, 68.47; H, 5.25; N, 10.42; Found: C, 68.16; H, 5.22; N, 10.37.
1-((2-Methylphenyl)carbamoylmethyl)-2-(2-nitrophenyl)-2,4-dihydro-1H-3,1-benzoxazine (5b): Eluent: acetate/petroleum ether (v/v = 1:5); Yield 45%. Yellow solid, m.p.: 162.6–162.9 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.51(s, 1H), 7.88 (d, J = 7.5 Hz, 1H), 7.78 (d, J = 8.0 Hz, 1H), 7.55–7.59 (m, 3H), 7.16–7.24 (m, 2H), 7.09 (d, J = 7.0 Hz, 1H), 7.03 (t, J = 7.0 Hz,1H), 6.89–6.91 (m, 3H), 6.44 (s, 1H, NCHO), 4.91 (d, J = 15 Hz, 1H), 4.61 (d, J = 15 Hz, 1H), 4.13 (d, J = 18 Hz, 1H) 3.96 (d, J = 18 Hz, 1H), 1.99 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 167.39 (C=O), 149.06, 141.80, 135.07, 133.07, 131.15, 130.50, 130.40, 129.34, 128.75, 128.58, 126.76, 125.24, 125.07, 125.05, 122.34, 121.99, 120.76, 114.34, 85.18, 65.30, 54.26, 17.29; IR (KBr, cm−1) ν: 3396, 2846, 1677 (C=O), 1606, 1533, 1515, 1499, 1466, 1459, 1354, 1326, 1186, 959, 759; Anal. Calcd. for C23H21N3O4: C, 68.47; H, 5.25; N, 10.42; Found: C, 68.75; H, 5.23; N, 10.38.
1-((2-Methylphenyl)carbamoylmethyl)-2-(4-nitrophenyl)-2,4-dihydro-1H-3,1-benzoxazine (5c): Eluent: acetate/petroleum ether (v/v = 1:5); Yield 49%. Yellow solid, m.p.: 153.4–153.8 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.47 (s, 1H), 8.24 (d, J = 8.5 Hz, 2H), 7.84 (d, J = 8.0 Hz, 1H), 7.62 (d, J = 8.5 Hz, 2H), 7.18–7.25 (m, 2H), 7.13 (d, J = 7.5 Hz, 1H), 7.06 (t, J = 7.5 Hz, 1H), 6.93–6.96 (m, 3H), 5.89 (s, 1H, NCHO), 4.94 (d, J = 15 Hz, 1H), 4.69 (d, J = 15 Hz, 1H), 4.14 (d, J = 18 Hz, 1H), 3.88 (d, J = 18 Hz, 1H), 2.03 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 167.28 (C=O), 148.42, 144.07, 141.68, 135.03, 130.54, 128.68 (3C), 128.50, 126.90, 125.36, 125.12, 124.15 (2C), 122.36, 122.29, 121.06, 114.90, 88.52, 64.46, 54.78, 17.39; IR (KBr, cm−1) ν: 3308, 1663 (C=O), 1608, 1585, 1531, 1502, 1459, 1354, 1291, 1257, 1080, 859, 741; Anal. Calcd. for C23H21N3O4: C, 68.47; H, 5.25; N, 10.42; Found: C, 68.15; H, 5.27; N, 10.46.
1-((4-Methylphenyl)carbamoylmethyl)-2-(3-nitrophenyl)-2,4-dihydro-1H-3,1-benzoxazine (5d): Eluent: acetate/petroleum ether (v/v = 1:5); Yield 56%. Yellow solid, m.p.: 168.5–169.1 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.49 (s, 1H), 8.31 (s, 1H), 8.13 (d, J = 8.0 Hz, 1H), 7.72 (d, J = 8.0 Hz, 1H), 7.49 (t, J = 8.0 Hz, 1H), 7.25 (d, J = 8.0 Hz, 2H), 7.16–7.19 (m, 1H), 7.03 (d, J = 8.0 Hz, 2H), 6.88 (d, J = 6.5 Hz, 2H), 6.85 (d, J = 6.5 Hz, 1H), 5.76 (s, 1H, NCHO), 4.96 (d, J = 15Hz, 1H), 4.72 (d, J = 15 Hz, 1H), 3.92 (d, J = 18 Hz, 1H), 3.78 (d, J = 18 Hz, 1H), 2.22 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 167.36 (C=O), 148.64, 142.61, 139.42, 134.48, 134.44, 133.57, 130.05, 129.57 (2C), 128.65, 124.99, 124.31, 122.80 (2C), 121.41, 119.84 (2C), 115.95, 88.81, 65.27, 55.33, 20.89; IR (KBr, cm−1) ν: 3318, 1681 (C=O), 1602, 1528, 1505, 1349, 1309, 1257, 1241, 1056, 964, 814, 739; Anal. Calcd. for C23H21N3O4: C, 68.47; H, 5.25; N, 10.42; Found: C, 68.13; H, 5.27; N, 10.48.
1-((4-Methylphenyl)carbamoylmethyl)-2-(2-nitrophenyl)-2,4-dihydro-1H-3,1-benzoxazine (5e): Eluent: acetate/petroleum ether (v/v = 1:5); Yield 40%. Yellow solid, m.p.: 136.0–138.5 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.65 (s, 1H), 7.81 (d, J = 8.0 Hz, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.54 (t, J = 7.5 Hz, 1H), 7.45 (t, J = 7.5 Hz, 1H), 7.27 (d, J = 8.5 Hz, 2H), 7.15–7.19 (m, 1H), 7.01 (d, J = 7.0 Hz, 2H), 6.88 (d, J = 3.5 Hz, 2H), 6.83 (d, J = 8.5 Hz, 1H), 6.28 (s, 1H, NCHO), 4.94 (d, J = 15 Hz, 1H), 4.67 (d, J = 15 Hz, 1H), 3.92 (d, J = 18 Hz, 1H), 3.86 (d, J = 18 Hz, 1H), 2.22 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 167.67 (C=O), 148.88, 143.17, 134.66, 134.24, 133.18, 131.02, 130.40, 129.55, 129.49 (2C), 128.47, 124.99, 124.94, 122.92, 121.42, 119.75 (2C), 116.10, 85.39, 66.14, 55.03, 20.90; IR (KBr, cm−1) ν: 3337, 2919, 1671 (C=O), 1587, 1524, 1488, 1455, 1352, 1206, 1029, 923, 738; Anal. Calcd. for C23H21N3O4: C, 68.47; H, 5.25; N, 10.42; Found: C, 68.90; H, 5.22; N, 10.36.
1-((4-Methylphenyl)carbamoylmethyl)-2-(4-nitrophenyl)-2,4-dihydro-1H-3,1-benzoxazine (5f): Eluent: acetate/petroleum ether (v/v = 1:5); Yield 48%. Yellow solid, m.p.: 157.2–158.5 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.53 (s, 1H), 8.22 (d, J = 8.5 Hz, 2H), 7.64 (d, J = 8.5 Hz, 2H), 7.31 (d, J = 8.5 Hz, 2H), 7.21–7.25 (m, 1H), 7.10 (d, J = 8.0 Hz, 2H), 6.93–6.95 (m, 3H), 5.85 (s, 1H, NCHO), 4.99 (d, J = 15 Hz, 1H), 4.75 (d, J = 15 Hz, 1H), 4.03 (d, J = 18Hz, 1H), 3.85 (d, J = 18 Hz, 1H), 2.30 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 167.38 (C=O), 148.38, 144.01, 142.34, 134.52, 134.49, 129.59 (2C), 128.66 (2C), 128.63, 125.04, 124.13 (2C), 122.78, 121.34, 119.83 (2C), 115.77, 88.68, 64.88, 55.34, 20.89; IR (KBr, cm−1) ν: 3369, 2973, 1672 (C=O), 1605, 1519, 1348, 1329, 1191, 1067, 966, 813, 745; Anal. Calcd. for C23H21N3O4: C, 68.47; H, 5.25; N, 10.42; Found: C, 68.08; H, 5.22; N, 10.47.
1-((4-Methyloxyphenyl)carbamoylmethyl)-2-(3-nitrophenyl)-2,4-dihydro-1H-3,1-benzoxazine (5g): Eluent: acetate/petroleum ether (v/v = 1:5); Yield 85%. Yellow solid, m.p.: 167.0–167.2 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.49 (s, 1H), 8.31 (s, 1H), 8.13 (d, J = 8.0 Hz, 1H), 7.72 (d, J = 7.5 Hz, 1H), 7.49 (t, J = 8.0 Hz, 1H), 7.25 (d, J = 7.5 Hz, 2H), 7.18 (t, J = 6.5 Hz, 1H), 7.02 (d, J = 8.0 Hz, 2H), 6.84–6.88 (m, 3H), 5.76 (s, 1H, NCHO), 4.96 (d, J = 15Hz, 1H), 4.71 (d, J = 15 Hz, 1H), 3.91 (d, J = 18 Hz, 1H), 3.77 (d, J = 18 Hz, 1H), 2.22 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 167.36 (C=O), 148.63, 142.62, 139.42, 134.47, 134.45, 133.59, 130.06, 129.57 (2C), 128.65, 124.99, 124.31, 122.80 (2C), 121.40, 119.84 (2C), 115.95, 88.81, 65.29, 55.32, 20.91; IR (KBr, cm−1) ν: 3319, 1682 (C=O), 1603, 1527, 1504, 1444, 1404, 1350, 1309, 1257, 1242, 1179, 1057, 965, 931, 814, 740; Anal. Calcd. for C23H21N3O5: C, 65.86; H, 5.05; N, 10.02; Found: C, 65.56; H, 5.03; N, 10.06.
1-((4-Methyloxyphenyl)carbamoylmethyl)-2-(2-nitrophenyl)-2,4-dihydro-1H-3,1-benzoxazine (5h): Eluent: acetate/petroleum ether (v/v = 1:5); Yield 66%. Yellow solid, m.p.: 156.1–158.2 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.67 (s, 1H), 7.89 (d, J = 8.0 Hz, 1H), 7.70 (d, J = 7.5 Hz, 1H), 7.62 (t, J = 7.5 Hz, 1H), 7.53 (t, J = 7.5 Hz, 1H), 7.36 (d, J = 9.0 Hz, 2H), 7.22–7.23 (m, 1H), 6.96 (d, J = 4.5 Hz, 2H), 6.92 (d, J = 8.5 Hz, 1H), 6.83 (d, J = 9.0 Hz, 2H), 6.36 (s, 1H, NCHO), 5.01 (d, J = 15 Hz, 1H), 4.74 (d, J = 15 Hz, 1H), 3.99 (d, J = 15 Hz, 1H), 3.93 (d, J = 15 Hz, 1H), 3.77 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 167.53 (C=O), 156.67, 148.88, 143.15, 133.17, 131.03, 130.40, 130.37, 129.56, 128.46, 124.99, 124.95, 122.89, 121.47 (2C), 121.39, 116.04, 114.14 (2C), 85.38, 66.11, 55.49, 54.93; IR (KBr, cm−1) ν: 3339, 2942, 1674 (C=O), 1605, 1531, 1506, 1465, 1405, 1346, 1316, 1246, 1175, 1038, 962, 839, 744; Anal. Calcd. for C23H21N3O5: C, 65.86; H, 5.05; N, 10.02; Found: C, 65.46; H, 5.08; N, 9.97.
1-((4-Methyloxyphenyl)carbamoylmethyl)-2-(4-nitrophenyl)-2,4-dihydro-1H-3,1-benzoxazine (5i): Eluent: acetate/petroleum ether (v/v = 1:5); Yield 80%. Pale yellow solid, m.p.: 156.6–158.5 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.42 (s, 1H), 8.16 (d, J = 8.5 Hz, 2H), 7.57 (d, J = 8.5 Hz, 2H), 7.26 (d, J = 9.0 Hz, 2H), 7.15–7.19 (m, 1H), 6.85–6.88 (m, 3H), 6.76 (d, J = 9.0 Hz, 2H), 5.78 (s, 1H, NCHO), 4.92 (d, J = 15 Hz, 1H), 4.66 (d, J = 15 Hz, 1H), 3.96 (d, J = 18 Hz, 1H), 3.78 (d, J = 18 Hz, 1H), 3.71 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 167.22 (C=O), 156.75, 148.39, 144.00, 142.30, 130.61, 130.13, 128.65 (2C), 125.04, 124.14 (2C), 122.72, 121.58 (2C), 121.31, 115.69, 114.25 (2C), 88.67, 64.84, 55.51, 55.24; IR (KBr, cm−1) ν: 3379, 2934, 1684 (C=O), 1601, 1523, 1494, 1347, 1307, 1264, 1240, 1039, 865, 763; Anal. Calcd. for C23H21N3O5: C, 65.86; H, 5.05; N, 10.02; Found: C, 66.24; H, 5.02; N, 10.07.
1-((3-Methyloxyphenyl)carbamoylmethyl)-2-(3-nitrophenyl)-2,4-dihydro-1H-3,1-benzoxazine (5j): Eluent: acetate/petroleum ether (v/v = 1:5); Yield 74%. Pale yellow solid, m.p.: 56.6–58.4 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.65 (s, 1H), 8.39 (s, 1H), 8.21 (d, J = 8.0 Hz, 1H), 7.80 (d, J = 7.5 Hz, 1H), 7.58 (t, J = 7.5 Hz, 1H), 7.18–7.26 (m, 3H), 6.92–6.97 (m, 4H), 6.66 (d, J = 8.0 Hz, 1H), 5.84 (s, 1H, NCHO), 5.04 (d, J = 15 Hz, 1H), 4.80 (d, J = 15 Hz, 1H) ,4.00 (d, J = 18 Hz, 1H), 3.85 (d, J = 18 Hz, 1H), 3.79 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 167.60 (C=O), 160.17, 148.62, 142.57, 139.36, 138.21, 133.62, 130.13, 129.83, 128.70, 125.05, 124.37, 122.87, 122.79, 121.55, 116.09, 111.87, 110.61, 105.41, 88.81, 65.29, 55.50, 55.39; IR (KBr, cm−1) ν: 3327, 3078, 2937, 2837, 1674 (C=O), 1606, 1530, 1494, 1458, 1349, 1290, 1220, 1155, 1085, 1046, 960, 754; Anal. Calcd. for C23H21N3O5: C, 65.86; H, 5.05; N, 10.02; Found: C, 65.52; H, 5.07; N, 9.98.
1-((3-Methyloxyphenyl)carbamoylmethyl)-2-(2-nitrophenyl)-2,4-dihydro-1H-3,1-benzoxazine (5k): Eluent: acetate/petroleum ether (v/v = 1:5); Yield 41%. Brown yellow solid, m.p.: 50.9–52.4 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.73 (s, 1H), 7.80 (d, J = 8.0 Hz, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.54 (t, J = 7.5 Hz, 1H), 7.44 (t, J = 8.0 Hz, 1H), 7.18 (s, 1H), 7.12–7.15 (m, 2H), 7.09 (d, J = 8.0 Hz, 1H), 6.86–6.88 (m, 2H), 6.83 (d, J = 8.0 Hz, 1H), 6.57 (d, J = 8.0 Hz, 1H), 6.27 (s, 1H, NCHO), 4.94 ( d, J = 15 Hz, 1H), 4.67 (d, J = 15 Hz, 1H), 3.87 (d, J = 5 Hz, 2H), 3.70 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 168.00 (C=O), 160.12, 148.84, 143.23, 138.40, 133.26, 130.98, 130.46, 129.73, 129.60, 128.50, 125.03, 124.99, 123.06, 121.59, 116.31, 111.88, 110.44, 105.38, 85.44, 66.21, 55.36, 55.19; IR (KBr, cm−1) ν: 3361, 2974, 2895, 1680 (C=O), 1607, 1532, 1494, 1457, 1377, 1088, 1049, 881, 753; Anal. Calcd. for C23H21N3O5: C, 65.86; H, 5.05; N, 10.02; Found: C, 65.55; H, 5.03; N, 10.06.
1-((3-methyloxyphenyl)carbamoylmethyl)-2-(4-nitrophenyl)-2,4-dihydro-1H-3,1-benzoxazine (5l): Eluent: acetate/petroleum ether (v/v = 1:5); Yield 60%. Yellow solid, m.p.: 128.7–129.9 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.54 (s, 1H), 8.14 (d, J = 8.5 Hz, 2H),7.56(d,J =8.5 Hz, 2H), 7.13–7.18 (m, 2H), 7.10 (d, J = 8.0 Hz, 1H), 6.81–6.87 (m, 4H), 6.58 (td, J = 8.0, 2.0 Hz, 1H), 5.78 (s, 1H, NCHO), 4.92 (d, J = 15 Hz, 1H), 4.67 (d, J = 15 Hz, 1H), 3.96 (d, J = 18 Hz, 1H), 3.78 (d, J = 18 Hz, 1H) ,3.70 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 167.63 (C=O), 160.20, 148.35, 143.99, 142.30, 138.30, 129.80, 128.67 (2C), 128.62, 125.07, 124.13 (2C), 122.85, 121.40, 115.86, 111.85, 110.39, 105.64, 88.65, 64.88, 55.44, 55.35; IR (KBr, cm−1) ν: 3366, 1666 (C=O), 1602, 1593, 1520, 1456, 1434, 1348, 1330, 1270, 1157, 1073, 1052, 972, 855, 776; Anal. Calcd. for C23H21N3O5: C, 65.86; H, 5.05; N, 10.02; Found: C, 66.15; H, 5.03; N, 9.99.
2-(3-Nitrophenyl)-1-(phenylcarbamoylmethyl)-2,4-dihydro-1H-3,1-benzoxazine (5m): Eluent: acetate/petroleum ether (v/v = 1:5); Yield 52%. Yellow solid, m.p.: 130.6–131.5 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.59 (s, 1H), 8.35 (s, 1H), 8.18 (d, J = 8.0 Hz, 1H), 7.76 (d, J = 8.0 Hz, 1H), 7.53 (t, J = 8.0 Hz, 2H), 7.42 (d, J = 8.0 Hz, 2H), 7.27 (t, J = 7.5 Hz, 2H), 7.19–7.23 (m, 1H), 7.08 (t, J = 7.5 Hz, 1H), 6.90–6.94 (m, 2H), 5.81 (s, 1H, NCHO), 5.01 (d, J = 15 Hz, 1H), 4.76 (d, J = 15 Hz, 1H), 3.99 (d, J = 18 Hz, 1H), 3.84 (d, J = 15 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ: 167.61 (C=O), 148.68, 142.59, 139.42, 137.05, 133.61, 130.11, 129.14 (2C), 128.71, 125.06, 124.83, 124.35, 122.89, 122.83, 121.52, 119.83 (2C), 116.05, 88.84, 65.27, 55.44; IR (KBr, cm−1) ν: 3302, 3069, 1669 (C=O), 1602, 1532, 1496, 1444, 1349, 1301, 1256, 1174, 1079, 751; Anal. Calcd. for C22H19N3O4: C, 67.86; H, 4.92; N, 10.79; Found: C, 67.59; H, 4.90; N, 10.75.
2-(2-Nitrophenyl)-1-(phenylcarbamoylmethyl)-2,4-dihydro-1H-3,1-benzoxazine (5n): Eluent: acetate/petroleum ether (v/v = 1:5); Yield 44%. Yellow solid, m.p.: 157.5–158.8 °C; 1H-NMR (500 MHz, CDCl3), δ: 8.73 (s, 1H), 7.80 (d, J = 8.5 Hz, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.53 (t, J = 7.5 Hz, 1H), 7.44 (t, J = 7.5 Hz, 1H), 7.39 (d, J = 8.0 Hz, 2H), 7.18–7.23 (m, 2H), 7.12–7.14 (m, 1H), 7.02 (t, J = 7.5 Hz, 1H), 6.86 (d, J = 4.5 Hz, 2H), 6.84 (d, J = 8.0 Hz, 1H), 6.28 (s, 1H, NCHO), 4.95 (d, J = 15 Hz, 1H), 4.67 (d, J = 15 Hz, 1H), 3.93 (d, J = 18 Hz, 1H), 3.88 (d, J = 18 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ: 167.95 (C=O), 148.91, 143.17, 137.25, 133.23, 131.01, 130.46, 129.61, 129.05 (2C), 128.52, 125.02 (2C), 124.63, 123.00, 121.53, 119.76 (2C), 116.18, 85.46, 66.16, 55.13; IR (KBr, cm−1) ν: 3316, 3207, 1676 (C=O), 1603, 1553, 1528, 1495, 1441, 1346, 1311, 1252, 1197, 1075, 750; Anal. Calcd. for C22H19N3O4: C, 67.86; H, 4.92; N, 10.79; Found: C, 67.54; H, 4.90; N, 10.74.
2-(4-Nitrophenyl)-1-(phenylcarbamoylmethyl)-2,4-dihydro-1H-3,1-benzoxazine (5o): Eluent: acetate/petroleum ether (v/v = 1:5); Yield 46%. Yellow solid, m.p.: 142.6–144.0 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.66 (s, 1H), 8.22 (d, J = 8.5 Hz, 2H), 7.64 (d, J = 8.5 Hz, 2H), 7.44 (d, J = 7.5 Hz, 2H), 7.30 (t, J = 8.0 Hz, 2H), 7.21–7.25 (m, 1H), 7.12 (t, J = 7.5 Hz, 1H), 6.92–6.95 (m, 3H), 5.86 (s, 1H, NCHO), 5.01 (d, J = 15 Hz, 1H), 4.75 (d, J = 15 Hz, 1H), 4.05 (d, J = 18 Hz, 1H), 3.86 (d, J = 18Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ: 167.56 (C=O), 148.46, 143.99, 142.29, 137.10, 129.19 (2C), 128.73, 128.70 (2C), 125.13, 124.88, 124.23 (2C), 122.87, 121.51, 119.79 (2C), 115.91, 88.75, 64.90, 55.52; IR (KBr, cm−1) ν: 3365, 1666 (C=O), 1600, 1519, 1444, 1350, 1331, 1263, 853, 762; Anal. Calcd. for C22H19N3O4: C, 67.86; H, 4.92; N, 10.79; Found: C, 67.55; H, 4.94; N, 10.83.
1-(Benzylcarbamoylmethyl)-2-(3-nitrophenyl)-2,4-dihydro-1H-3,1-benzoxazine (5p): Eluent: acetate/petroleum ether (v/v = 1:5); Yield 56%. Yellow solid, m.p.: 103.3–105.7 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.28 (s, 1H), 8.20 (d, J = 8.0 Hz, 1H), 7.68 (d, J = 7.5 Hz, 1H), 7.48 (t, J = 8.0 Hz, 1H), 7.22–7.26 (m, 4H), 7.15 (s, 1H), 7.07–7.09 (m, 2H), 6.92–6.93 (m, 2H), 6.85 (d, J = 8.0 Hz, 1H), 5.75 (s, 1H, NCHO), 4.91 (d, J = 15 Hz, 1H), 4.69 (d, J = 15 Hz, 1H), 4.49 (dd, J = 14.5, 6.0 Hz, 1H), 4.32 (dd, J = 14.5, 5.0 Hz, 1H), 3.95 (d, J = 18 Hz, 1H), 3.78 (d, J = 18 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ: 169.25 (C=O), 148.56, 142.52, 139.52, 137.69, 133.65, 130.00, 128.75 (2C), 128.53, 127.62, 127.56 (2C), 124.96, 124.26, 122.85, 122.47, 120.96, 115.43, 88.80, 65.24, 54.46, 43.37; IR (KBr,cm-1) ν: 3329, 1650 (C=O), 1530, 1494, 1459, 1426, 1353, 1328, 1248, 1084, 744; Anal. Calcd. for C23H21N3O4: C, 68.47; H, 5.25; N, 10.42; Found: C, 68.09; H, 5.22; N, 10.37.
1-(Benzylcarbamoylmethyl)-2-(2-nitrophenyl)-2,4-dihydro-1H-3,1-benzoxazine (5q): Eluent: acetate/petroleum ether (v/v = 1:5); Yield 58%. Yellow solid, m.p.: 96.2–97.3 °C; 1H-NMR (500 MHz, CDCl3) δ: 7.82 (s, 1H), 7.47–7.53 (m, 3H), 7.21–7.25 (m, 4H), 7.05 (d, J = 4.5 Hz, 2H), 6.88–6.91 (m, 2H), 6.84 (d, J = 8.0 Hz, 1H), 6.32 (s, 1H, NCHO), 4.84 (d, J = 15.0 Hz, 1H), 4.57 (d, J = 15.0 Hz, 1H), 4.52 (dd, J = 15.0, 7.0 Hz, 1H), 4.27 (dd, J = 15.0, 5.0 Hz, 1H), 4.03 (d, J = 18.0 Hz, 1H), 3.89 (d, J = 18.0 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ: 169.42 (C=O), 148.95, 142.46, 137.84, 133.02, 131.15, 130.24, 129.46, 128.64 (2C), 128.39, 127.49 (2C), 127.41, 124.91 (2C), 122.20, 120.67, 114.97, 85.14, 65.48, 54.20, 43.18; IR (KBr, cm−1) ν: 3394, 1675 (C=O), 1607, 1530, 1501, 1466, 1358, 1325, 1263, 1188, 1067, 960, 847, 755; Anal. Calcd. for C23H21N3O4: C, 68.47; H, 5.25; N, 10.42; Found: C, 68.13; H, 5.23; N, 10.38.
1-(Benzylcarbamoylmethyl)-2-(4-nitrophenyl)-2,4-dihydro-1H-3,1-benzoxazine (5r): Eluent: acetate/petroleum ether (v/v = 1:5); Yield 65%. Yellow solid, m.p.: 147.7–148.5 °C; 1H-NMR (500 MHz, CDCl3) δ: 8.03 (d, J = 8.5 Hz, 2H), 7.45 (d, J = 8.5 Hz, 2H), 7.14–7.20 (m, 4H), 7.05 (br, 1H, NH), 6.99–7.01 (m, 2H), 6.82–6.85 (m, 2H), 6.77 (d, J = 8.5 Hz, 1H), 5.67 (s, 1H, NCHO), 4.81 (d, J = 15 Hz, 1H), 4.59 (d, J = 15 Hz, 1H), 4.4 (dd, J = 15.0, 7.0 Hz, 1H), 4.22 (dd, J = 15.0, 5.0 Hz, 1H), 3.86 (d, J = 18.0 Hz, 1H), 3.69 (d, J = 18.0 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ: 169.15 (C=O), 148.31, 144.11, 142.51, 137.75, 128.72 (2C), 128.67 (2C), 128.48, 127.69, 127.62 (2C), 124.95, 124.06 (2C), 122.49, 120.89, 115.33, 88.78, 65.06, 54.52, 43.32; IR (KBr, cm−1) ν: 3405, 1681 (C=O), 1604, 1518, 1495, 1463, 1425, 1347, 1298, 1074, 1027, 857, 761; Anal. Calcd. for C23H21N3O4: C, 68.47; H, 5.25; N, 10.42; Found: C, 68.20; H, 5.23; N, 10.39.