Spectroscopic Investigation of the Interaction of the Anticancer Drug Mitoxantrone with Sodium Taurodeoxycholate (NaTDC) and Sodium Taurocholate (NaTC) Bile Salts
Abstract
:1. Introduction
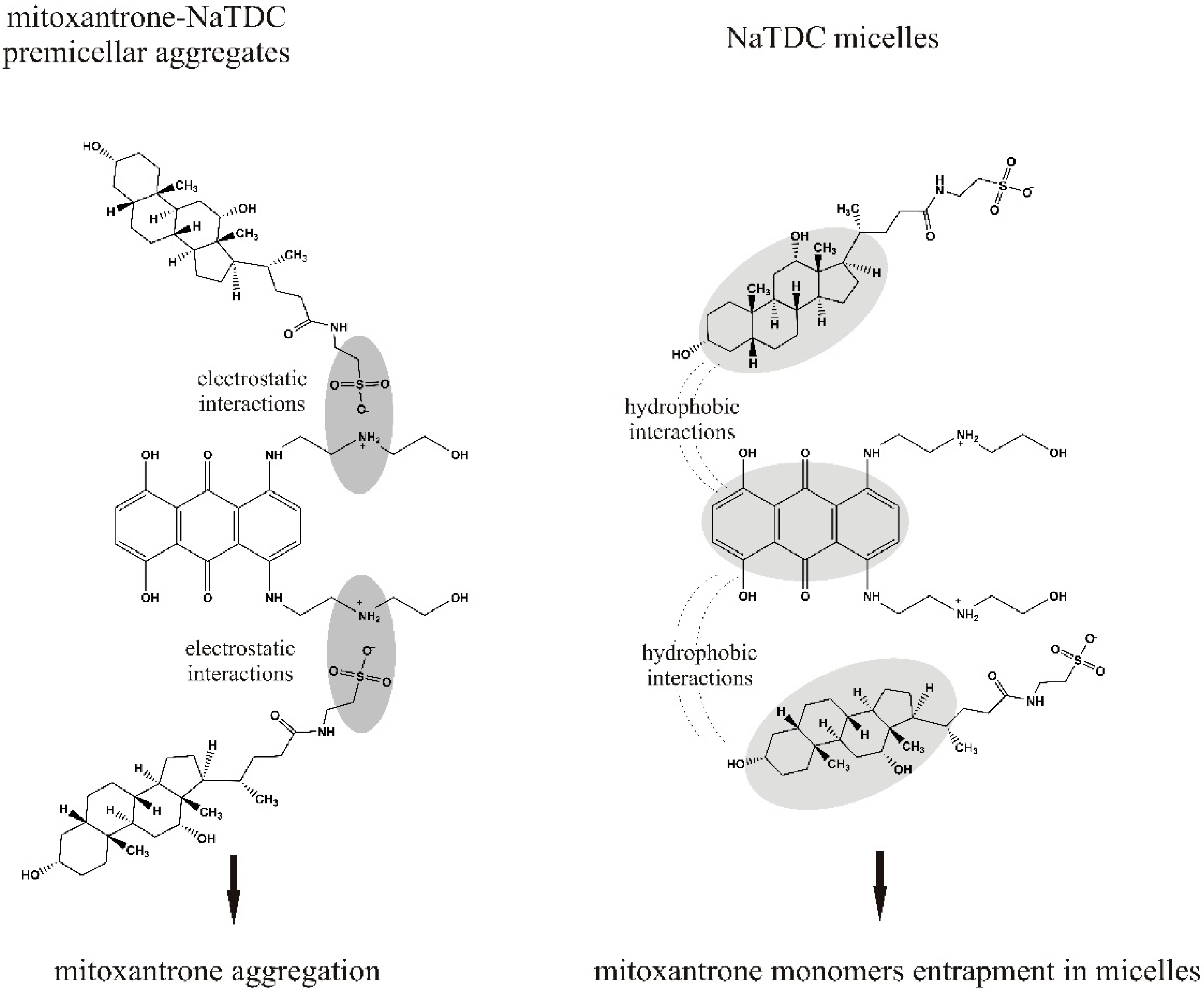
2. Results and Discussion
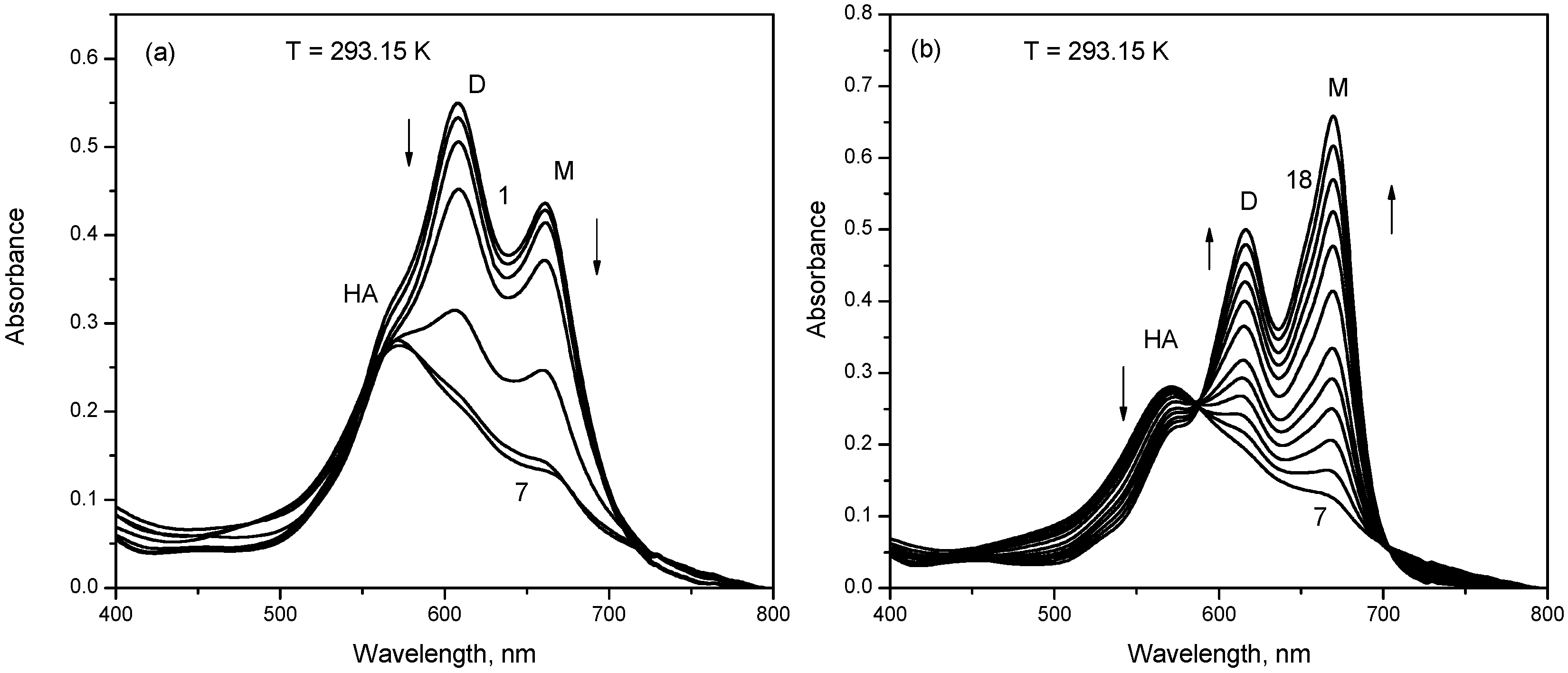
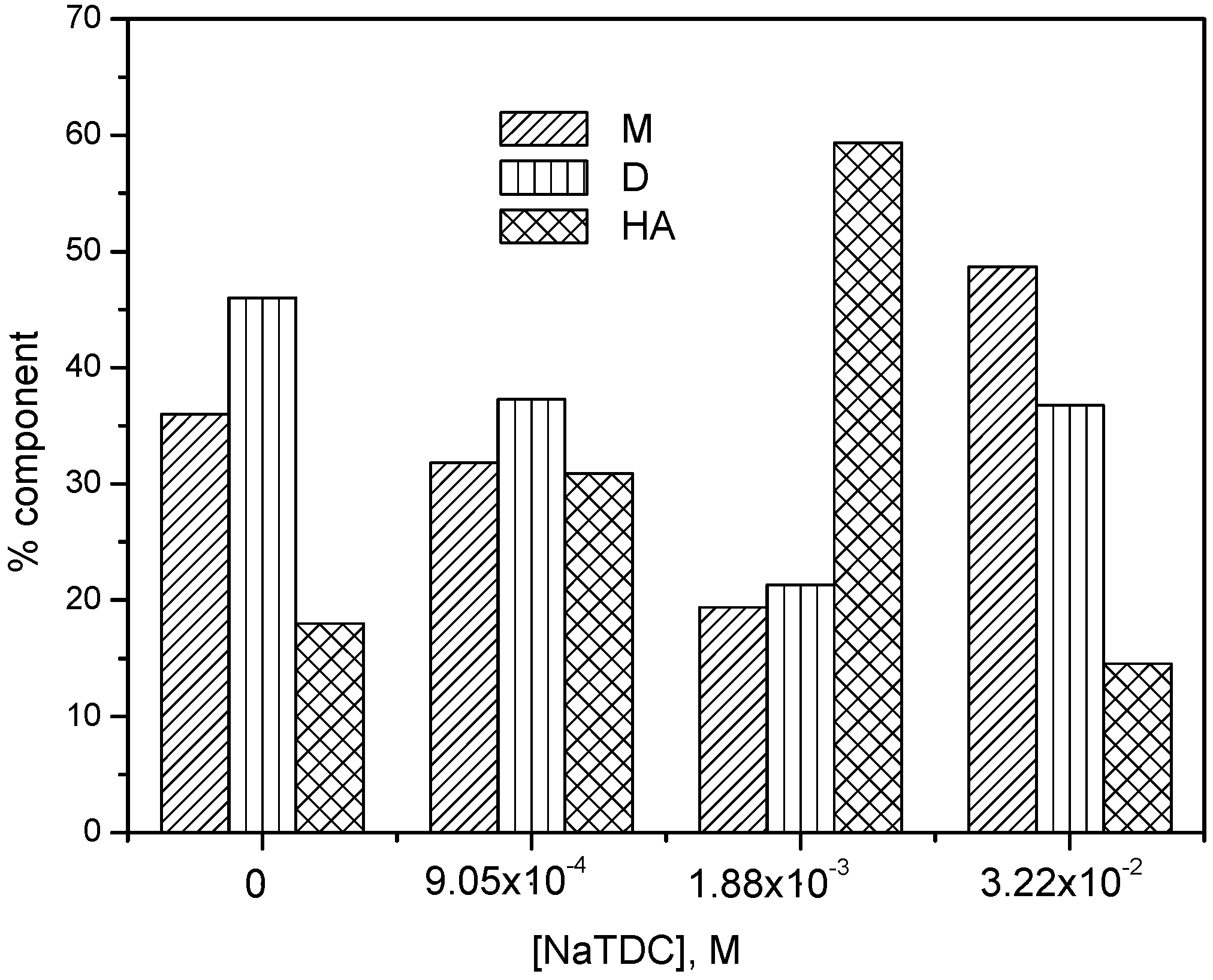
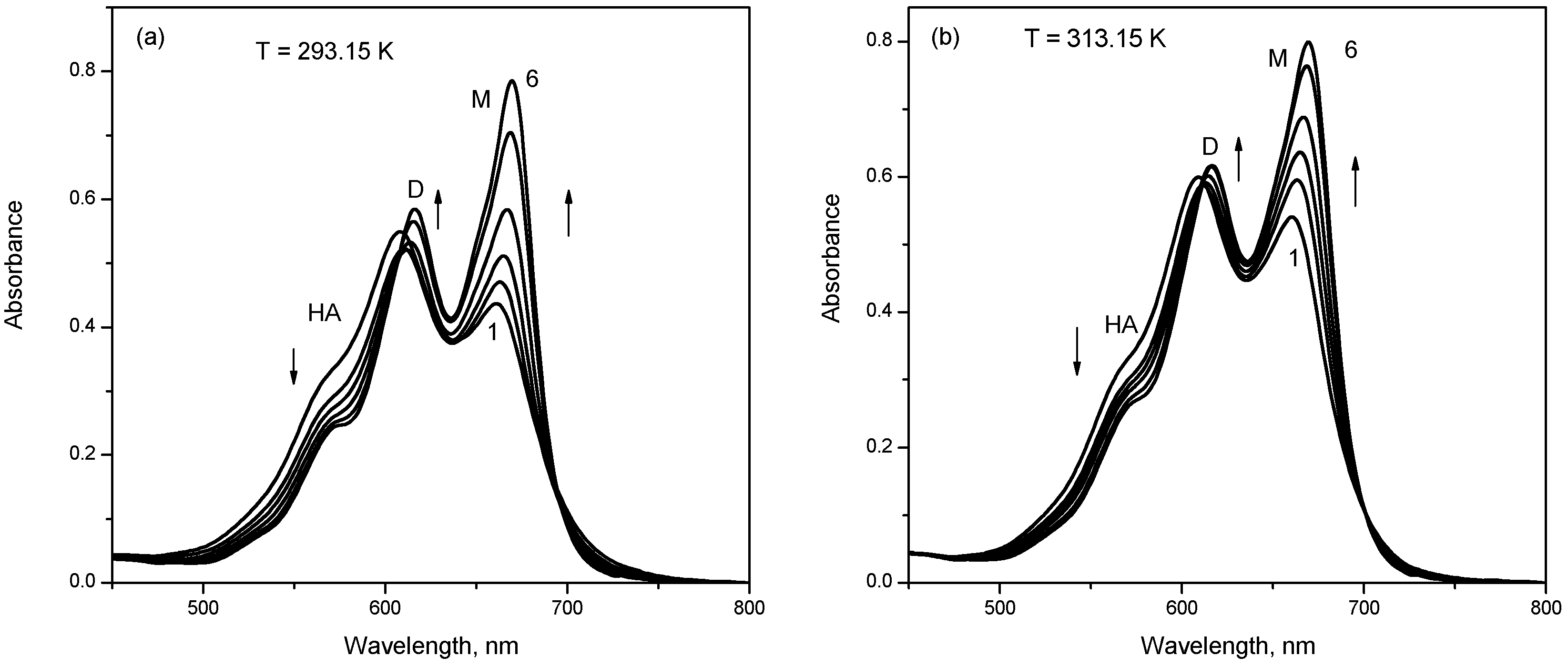
2.1. UV-Vis Absorption Studies
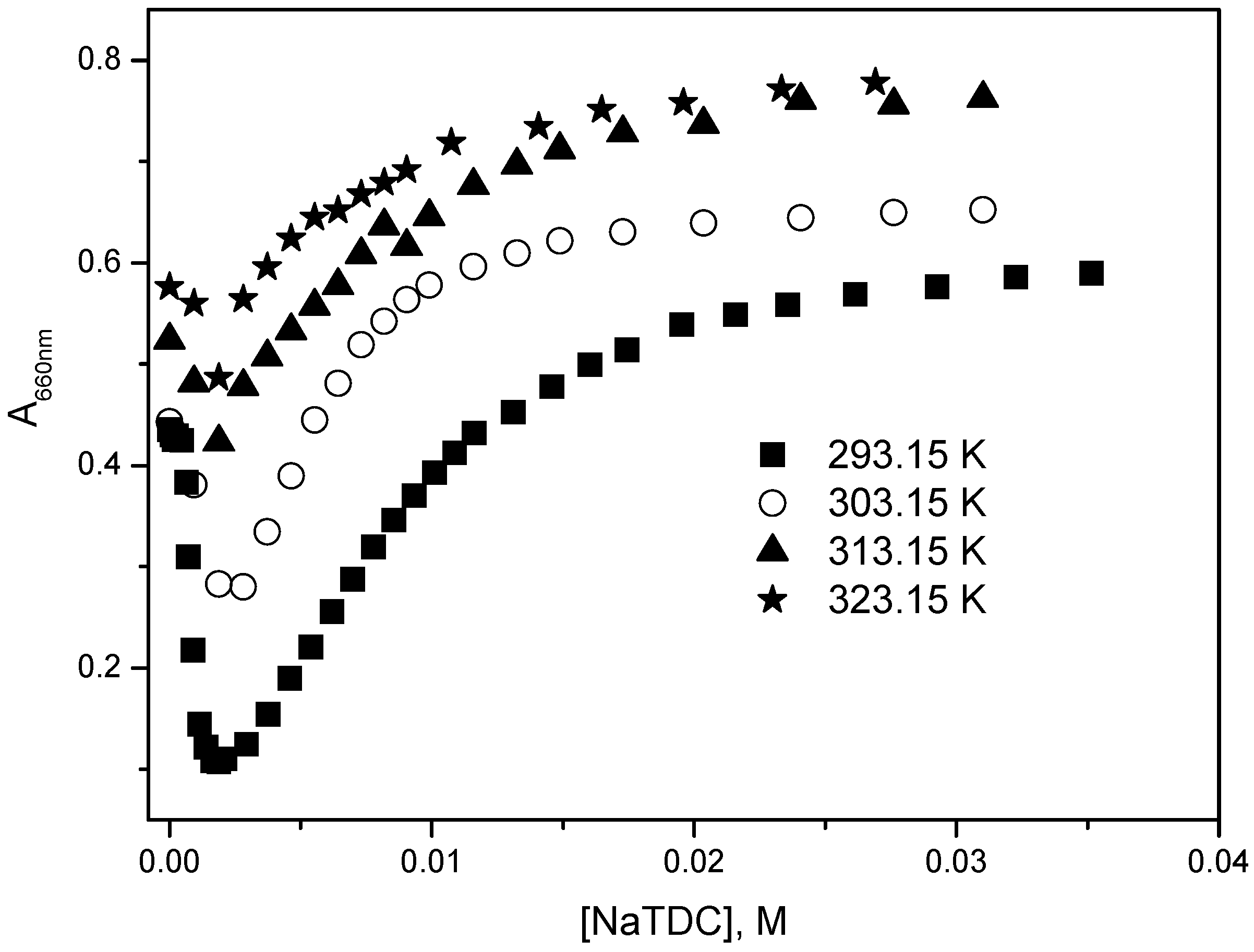
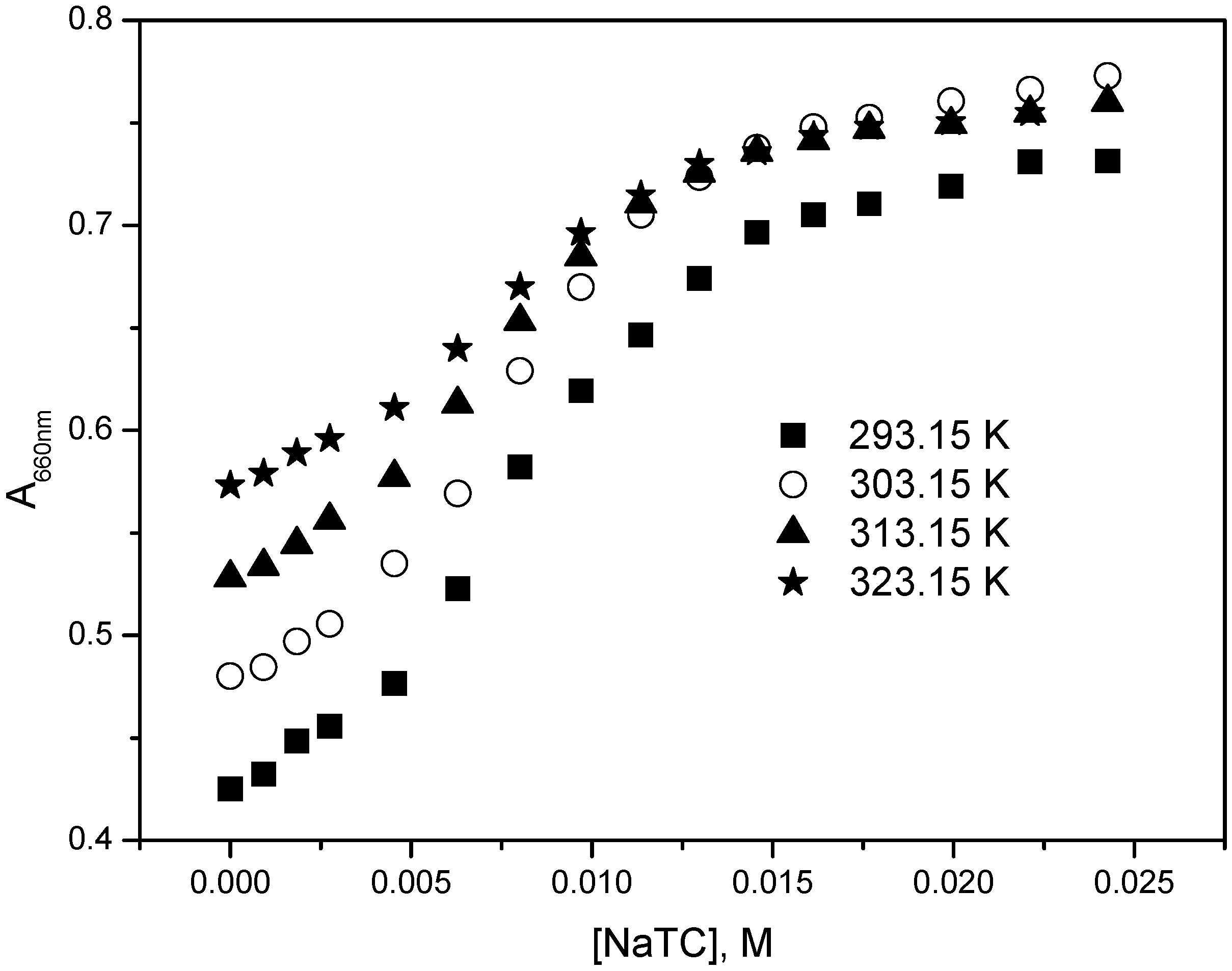
2.2. Determination of Binding Constant, Partition Coefficient and Thermodynamic Parameters
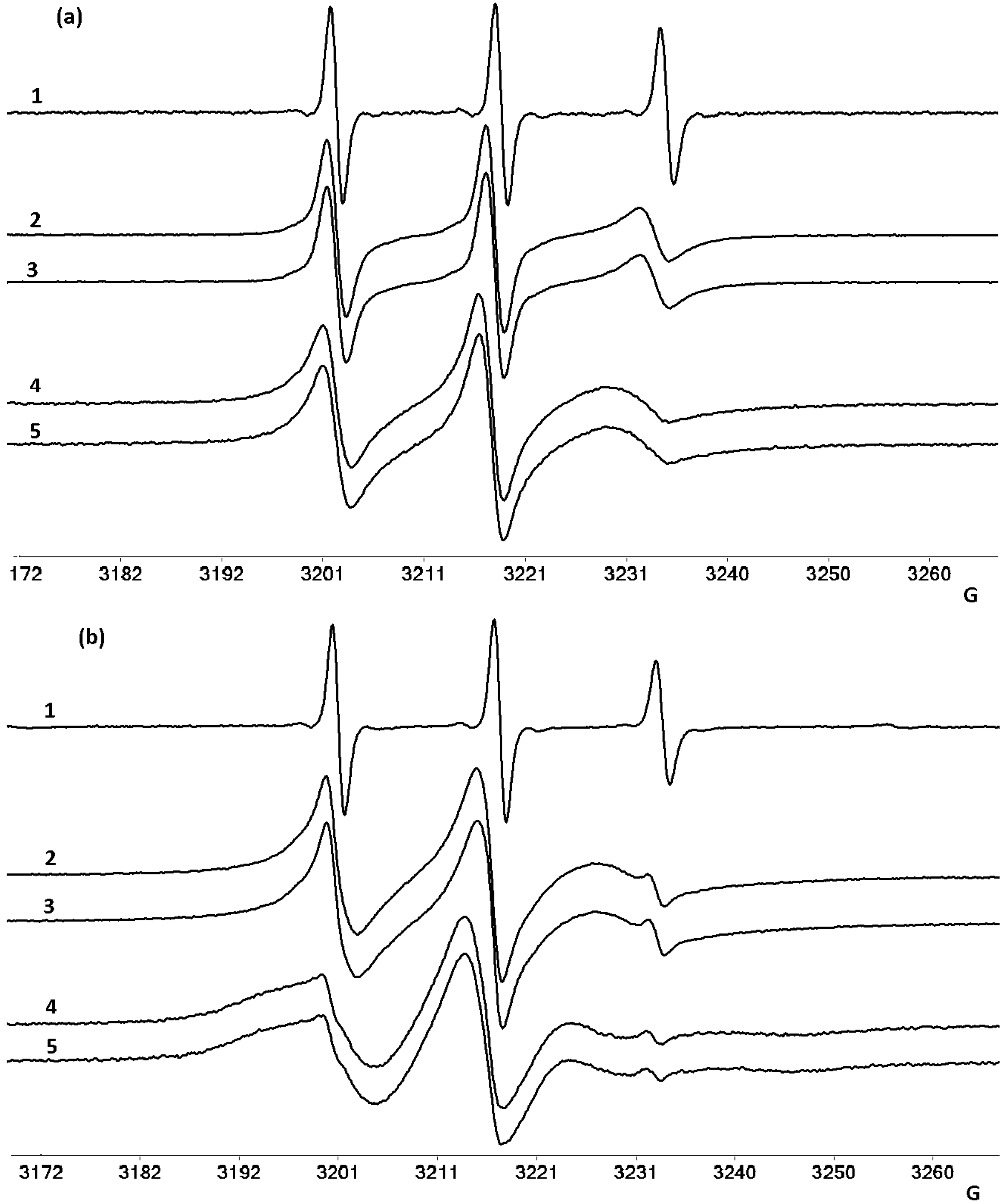
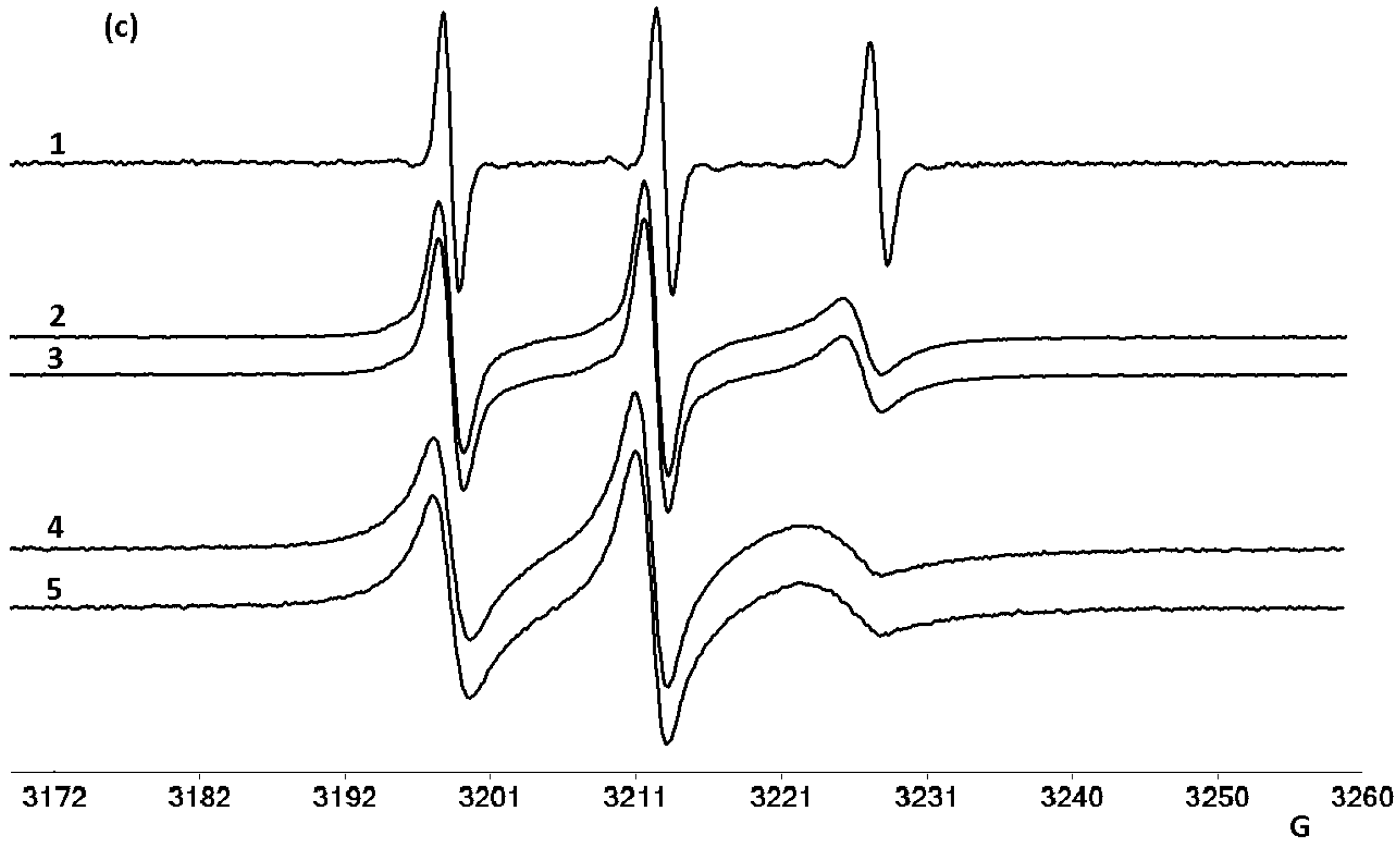
2.3. EPR Studies on Mitoxantrone/Micellar Bile Salts Systems
3. Materials and Methods
3.1. Materials
3.2. UV-Vis Absorption Spectroscopy
3.3. EPR Spectroscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gomez-Mendoza, M.; Nuin, E.; Andreu, I.; Luisa Marin, M.; Miranda, M.A. Photophysical probes to assess the potential of cholic acid aggregates as drug carriers. J. Phys. Chem. B 2012, 116, 10213–10218. [Google Scholar] [CrossRef] [PubMed]
- Dongowski, G.; Fritzsch, B.; Giessler, J.; Hartl, A.; Kuhlmann, O.; Neubert, R.H.H. The influence of bile salts and mixed micelles on the pharmacokinetics of quinine in rabbits. Eur. J. Pharm. Biopharm. 2005, 60, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Natalini, B.; Sardella, R.; Gioiello, A.; Ianni, F.; Di Michele, A.; Marinozzi, M. Determination of bile salt critical micellization concentration on the road to drug discovery. J. Pharm. Biomed. Anal. 2014, 87, 62–81. [Google Scholar] [CrossRef] [PubMed]
- Din, K.-U.; Rub, M.A.; Naqvi, A.Z. Aqueous amphiphilic drug (amitriptyline hydrochloride)-bile salt mixtures at different temperatures. Coll. Surf. B Biointerfaces 2011, 84, 285–291. [Google Scholar] [CrossRef]
- Holm, R.; Mullertz, A.; Mu, H. Bile salts and their importance for drug absorption. Int. J. Pharm. 2013, 453, 44–55. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.J.; Wallace, R.G. Physico-chemical behavior of bile salts. Adv. Coll. Interface Sci. 1985, 22, 1–111. [Google Scholar] [CrossRef]
- Madenci, D.; Egelhaaf, S.U. Self-assembly in aqueous bile salt solutions. Curr. Opin. Coll. Interface Sci. 2010, 15, 109–115. [Google Scholar] [CrossRef]
- Thakur, R.; Das, A.; Adhikari, C.; Chakraborty, A. Partitioning of prototropic species of an anticancer drug ellipticine in bile salt aggregates of different head groups and hydrophobic skeleton: A photophysical study to probe bile salt as multisite drug carrier. Phys. Chem. Chem. Phys. 2012, 14, 15369–15378. [Google Scholar] [CrossRef] [PubMed]
- Amundson, L.L.; Li, R.; Bohne, C. Effect of the guest size and shape on its binding dynamics with sodium cholate aggregates. Langmuir 2008, 24, 8491–8500. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Ghosh, S.; Banik, D.; Banerjee, C.; Kuchlyan, J.; Sarkar, N. An investigation into the effect of the structure of bile salt aggregates on the binding interactions and ESIHT dynamics of curcumin, a photophysical approach to probe bile salt aggregates a potential drug carrier. J. Phys. Chem. B 2013, 117, 13795–13807. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Ghosh, S.; Banerjee, C.; Rao, V.G.; Sarkar, N. Modulation of photophysics and photodynamics of 1’-hydroxy-2’-acetonaphtone (HAN) in bile salt aggregates: A study of polarity and nanoconfinement effects. J. Phys. Chem. B 2012, 116, 8780–8792. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M. Size and structure of bile salt micelles: Influence of structure, concentration, counterion concentration, pH and temperature. In Advances in Chemistry Series; Goddard, E.D., Ed.; Volume 84, Chapter 4; Plenum Press: New York, NY, USA, 1968; pp. 31–42. [Google Scholar]
- Velasquez, W.S.; Lew, D.; Grogan, T.M.; Spiridonidis, C.H.; Balcerzak, S.P.; Dakhil, S.R.; Miller, T.P.; Lanier, K.S.; Chapman, R.A.; Fisher, R.I.; et al. Combination of fludarabine and mitoxantrone in untreated stages III and IV low-grade lymphoma: S9501. J. Clin. Oncol. 2003, 21, 1996–2003. [Google Scholar] [CrossRef] [PubMed]
- Thomas, X.; Archimbaud, E. Mitoxantrone in the treatment of acute myelogenous leukemia. Hematol. Cell Ther. 1997, 39, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Shenkenberg, T.D.; Von Hoff, D.D. Mitoxantrone: A new anticancer drug with significant clinical activity. Ann. Intern. Med. 1986, 105, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Berger, T. Current therapeutic recommendations in multiple sclerosis. J. Neurol. Sci. 2009, 287, S37–S45. [Google Scholar] [CrossRef]
- Bhattacharyya, J.; Basu, A.; Kumar, G.S. Intercalative interaction of the anticancer drug mitoxantrone with double stranded DNA: A calorimetric characterization of the energetics. J. Chem. Thermodyn. 2014, 75, 45–51. [Google Scholar] [CrossRef]
- Agarwal, S.; Jangir, D.K.; Mehrotra, R. Spectroscopic studies of the effects of anticancer drug mitoxantrone interaction with calf-thymus DNA. J. Photochem. Photobiol. B 2013, 120, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Awasthi, P.; Nair, M.; Barthwal, R. Interaction of anticancer drug mitoxantrone with DNA hexamer sequence d-(CTCGAG)2 by absorption, fluorescence and circular dichroism spectroscopy. J. Photochem. Photobiol. B 2013, 123, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Hajihassan, Z.; Rabbani-Chadegani, A. Interaction of mitoxantrone, as an anticancer drug, with chromatin proteins, core histones and H1, in solution. Int. J. Biol. Macromol. 2011, 48, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ma, Y.; Yang, C.; Guo, L.; Yang, X. Interaction of anticancer drug mitoxantrone with DNA analyzed by electrochemical and spectroscopic methods. Biophys. Chem. 2005, 116, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Enache, M.; Volanschi, E. Electrochemical and spectral study of the interaction of antitumoral drug mitoxantrone with DNA. Rev. Roum. Chim. 2005, 50, 131–140. [Google Scholar]
- Pommier, Y.; Leo, E.; Zhang, H.L.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Hande, K.R. Topoisomerase II inhibitors. Update Cancer Ther. 2008, 3, 13–26. [Google Scholar] [CrossRef]
- Seiter, K. Toxicity of the topoisomerase II inhibitors. Expert Opin. Drug Saf. 2005, 4, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.J. The cardiac effects of mitoxantrone: Do the benefits in multiple sclerosis outweigh the risks? Expert Opin. Drug Saf. 2006, 5, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Nieth, C.; Lage, H. Induction of the ABC-Transporters Mdr1/P-gp (Abcb1), Mrp1 (Abcc1), and Bcrp (Abcg2) during establishment of multidrug resistance following exposure to mitoxantrone. J. Chemother. 2005, 17, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Mukhija, A.; Kishore, N. Partitioning of drugs in micelles and effect on micellization: Physicochemical insights with tryptophan and diclofenac sodium. Colloid Surf. A Physicochem. Eng. Asp. 2017, 513, 204–214. [Google Scholar] [CrossRef]
- Banipal, T.S.; Kaur, R.; Banipal, P.K. Interactions of diazepam with sodium dodecylsulfate and hexadecyl trimethyl ammonium bromide. J. Mol. Liq. 2017, 236, 331–337. [Google Scholar] [CrossRef]
- Khan, M.S.; Sheikh, M.; Rub, A.; Azum, N.; Asiri, A.M. Antidepressant drug amitriptyline hydrochloride (AMT) interaction with anionic surfactant sodium dodecyl sulfate in aqueous/brine/urea solutions at different temperatures. J. Mol. Liq. 2016, 222, 1020–1030. [Google Scholar] [CrossRef]
- De Souza Santos, M.; de Morais Del Lama, M.P.F.; Ito, A.S.; Zumstein Georgetto Naal, R.M. Binding of chloroquine to ionic micelles: Effect of pH and micellar surface charge. J. Lumin. 2014, 147, 49–58. [Google Scholar] [CrossRef]
- Din, K.-U.; Khan, A.B.; Naqvi, A.Z. A study of the interaction between a phenothiazine drug promazine hydrochloride with cationic surfactants. J. Mol. Liq. 2013, 187, 374–380. [Google Scholar] [CrossRef]
- Erdinc, N.; Gokturk, S.; Tunkay, M. Interaction of epirubicin HCl with surfactants: Effect of NaCl and glucose. J. Pharm. Sci. 2004, 93, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, H.; Sarkar, M. Interaction of piroxicam with micelles: Effect of hydrophobic chain length on structural switchover. Biophys. Chem. 2005, 117, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Yagui, C.O.; Pessoa, A., Jr.; Tavares, L.C. Micellar solubilization of drugs. J. Pharm. Pharm. Sci. 2005, 8, 147–165. [Google Scholar] [PubMed]
- Bonacucina, G.; Cespi, M.; Misici-Falzi, M.; Palmieri, G.F. Colloidal soft matter as drug delivery system. J. Pharm. Sci. 2009, 98, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Enache, M.; Anghelache, I.; Volanschi, E. Coupled spectral and electrochemical evaluation of the anticancer drug mitoxantrone-sodium dodecyl sulfate interaction. Int. J. Pharm. 2010, 390, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Enache, M.; Volanschi, E. Spectral studies on the molecular interaction of anticancer drug mitoxantrone with CTAB micelles. J. Pharm. Sci. 2011, 100, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Enache, M.; Volanschi, E. Spectroscopic investigations of the molecular interaction of anticancer drug mitoxantrone with non-ionic surfactant micelles. J. Pharm. Pharmacol. 2012, 64, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Enache, M.; Toader, A.M.; Enache, M.I. Mitoxantrone—Surfactant interactions: A physicochemical overview. Molecules 2016, 21, 1356. [Google Scholar] [CrossRef] [PubMed]
- Chechik, V.; Caragheorgheopol, A. Getting an Inside View of Nanomaterials with Spin Labels and Spin Probes in Electron Paramagnetic Resonance; Gilbert, B.C., Davies, M.J., Murphy, D.M., Eds.; RSC Publishing: Cambridge, UK, 2007; Volume 20, pp. 96–130. [Google Scholar]
- Caragheorgheopol, A.; Caldararu, H. EPR Spin-Labelling and Spin-Probe Studies of Self-Assembled Systems in Electron Paramagnetic Resonance; Gilbert, B.C., Davies, M.J., McLauchlan, K.A., Eds.; RSC Publishing: Cambridge, UK, 2000; Volume 17, pp. 205–245. [Google Scholar]
- Enache, M.; Volanschi, E. Spectral characterization of self-association of antitumor drug mitoxantrone. Rev. Roum. Chim. 2010, 55, 255–262. [Google Scholar]
- Wiedmann, T.S.; Kamel, L. Examination of the solubilization of drugs by bile salt micelles. J. Pharm. Sci. 2002, 91, 1743–1764. [Google Scholar] [CrossRef] [PubMed]
- Raghunand, N.; Mahoney, B.P.; Gillies, R.J. Tumor acidity, ion trapping and chemotherapeutics II. pH-dependent partition coefficients predict importance of ion trapping on pharmacokinetics of weakly basic chemotherapeutic agents. Biochem. Pharmacol. 2003, 66, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.S.; Subuddhi, U. Spectroscopic investigation of interaction of Nile Blue A, a potent photosensitizer, with bile salts in aqueous medium. J. Photochem. Photobiol. B 2014, 141, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Tunc, S.; Duman, O.; Kanc, B. Spectrophotometric investigation of the interactions between cationic dye (C.I. Basic Yellow 2) and anionic surfactant (sodium dioctylsulfosuccinate) in the premicellar and micellar region. Dyes Pigm. 2012, 94, 233–238. [Google Scholar] [CrossRef]
- Sarkar, M.; Poddar, S. Studies on the interaction of surfactants with cationic dye by absorption spectroscopy. J. Colloid Interface Sci. 2000, 221, 181–1885. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Zeng, H.; Durocher, G.; Girard, D.; Giasson, R.; Blanchard, L.; Gaboury, L.; Villeneuve, L. Spectroscopic and photophysical properties of some new rhodamine derivatives in cationic, anionic and neutral micelles. J. Photochem. Photobiol. A 1996, 98, 65–72. [Google Scholar] [CrossRef]
- Mahajan, S.; Mahajan, R.K. Interactions of phenothiazines drugs with bile salts: Micellization and binding studies. J. Coll. Interface Sci. 2012, 387, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Holm, R.; Schonbeck, C.; Askjaer, S.; Westh, P. Thermodynamics of the interaction of c-cyclodextrin and tauro- and glyco-conjugated bile salts. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 223–233. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, X. Spectrometric study on the interaction of sodium cholate aggregates with quercitin. Colloid Surf. A Physicochem. Eng. Asp. 2015, 481, 31–37. [Google Scholar] [CrossRef]
- Funasaki, N.; Fukuba, M.; Hattori, T.; Ishikawa, S.; Okuno, T.; Hirota, S. Micelles formation of bile salts and zwitterionic derivative as studied by two-dimensional NMR spectroscopy. Chem. Phys. Lipids 2006, 142, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Funasaki, N.; Fukuba, M.; Kitagawa, T.; Nomura, M.; Ishikawa, S.; Hirota, S.; Neya, S. Two-dimensional NMR study on the structures of micelles of sodium taurocholate. J. Phys. Chem. B 2004, 108, 438–443. [Google Scholar] [CrossRef]
- Stones, T.J.; Buckman, T.; Nordio, P.L.; McConnell, H.M. Spin-labeled biomolecules. Proc. Natl. Acad. Sci. USA 1965, 54, 1010–1017. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Sumiya, S.; Kohno, Y.; Hirai, T. A rhodamine-cyclen conjugate as a highly sensitive and selective fluorescent chemosensor for Hg(II). J. Org. Chem. 2008, 73, 8571–8574. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, L.; Lissi, E.; Quina, F. Interactions of neutral molecules with ionic micelles. Adv. Coll. Int. Sci. 1986, 25, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, H.; Manabe, M.; Miyamoto, Y.; Fujita, Y.; Tokunaga, S. Partititon coefficients of homologous ω-phenylalkanols between water and sodium dodecyl sulfate micelles. J. Phys. Chem. 1989, 93, 5536–5540. [Google Scholar] [CrossRef]
- Budil, D.E.; Lee, S.; Saxena, S.; Freed, J.H. Nonlinear-least-squares analysis of slow-motion EPR spectra in one and two dimensions using a modified Levenberg–Marquardt algorithm. J. Magn. Reson. 1996, 120, 155–189. [Google Scholar] [CrossRef]
Sample Availability: Not available. |





| NaTDC | NaTC | |||||||
|---|---|---|---|---|---|---|---|---|
| T (K) | Kb/104 (M−2) | (kJ mol−1) | (kJ mol−1) | (J mol−1 K−1) | Kb/103 (M−2) | (kJ mol−1) | (kJ mol−1) | (J mol−1 K−1) |
| 293.15 | 0.64 ± 0.05 | −21.35 | 26.85 | 164.42 | 1.41 ± 0.08 | −23.27 | 20.49 | 149.27 |
| 303.15 | 1.21 ± 0.09 | −23.68 | 166.68 | 1.89 ± 0.07 | −24.81 | 149.43 | ||
| 313.15 | 1.52 ± 0.09 | −25.06 | 165.77 | 2.17 ± 0.08 | −25.98 | 148.40 | ||
| 323.15 | 1.83 ± 0.08 | −26.36 | 164.66 | 3.21 ± 0.09 | −27.87 | 149.65 | ||
| NaTDC | NaTC | |||||||
|---|---|---|---|---|---|---|---|---|
| T (K) | Kx/103 | (kJ mol−1) | (kJ mol−1) | (J mol−1 K−1) | Kx/104 | (kJ mol−1) | (kJ mol−1) | (J mol−1 K−1) |
| 293.15 | 1.32 ± 0.04 | −17.50 | 42.39 | 204.30 | 3.03 ± 0.04 | −25.14 | 20.87 | 156.95 |
| 303.15 | 3.16 ± 0.08 | −20.30 | 206.80 | 4.10 ± 0.07 | −26.76 | 157.11 | ||
| 313.15 | 4.29 ± 0.07 | −21.77 | 204.89 | 4.67 ± 0.02 | −27.98 | 156.00 | ||
| 323.15 | 7.11 ± 0.09 | −23.82 | 204.89 | 7.06 ± 0.05 | −29.98 | 157.36 | ||
| System | 5-DSA | 12-DSA | 16-DSA | |
|---|---|---|---|---|
| τ1 (s) | τ1 (s) | τ2 (s) | τ1 (s) | |
| Phosphate buffer, pH 7.4 | 2.55 × 10−10 | 2.50 × 10−10 | 1.23 × 10−10 | |
| NaTDC | 2.9 × 10−9 | 5.63 × 10−11 | 6.39 × 10−9 | 2.02 × 10−9 |
| mitoxantrone + NaTDC | 3.50 × 10−9 | 7.32 × 10−11 | 6.72 × 10−9 | 2.44 × 10−9 |
| NaTC | 2.13 × 10−9 | 5.87 × 10−11 | 3.26 × 10−9 | 1.18 × 10−9 |
| mitoxantrone + NaTC | 2.56 × 10−9 | 3.85 × 10−10 | 3.44 × 10−9 | 1.28 × 10−9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enache, M.; Toader, A.M.; Neacsu, V.; Ionita, G.; Enache, M.I. Spectroscopic Investigation of the Interaction of the Anticancer Drug Mitoxantrone with Sodium Taurodeoxycholate (NaTDC) and Sodium Taurocholate (NaTC) Bile Salts. Molecules 2017, 22, 1079. https://doi.org/10.3390/molecules22071079
Enache M, Toader AM, Neacsu V, Ionita G, Enache MI. Spectroscopic Investigation of the Interaction of the Anticancer Drug Mitoxantrone with Sodium Taurodeoxycholate (NaTDC) and Sodium Taurocholate (NaTC) Bile Salts. Molecules. 2017; 22(7):1079. https://doi.org/10.3390/molecules22071079
Chicago/Turabian StyleEnache, Mirela, Ana Maria Toader, Victoria Neacsu, Gabriela Ionita, and Madalin I. Enache. 2017. "Spectroscopic Investigation of the Interaction of the Anticancer Drug Mitoxantrone with Sodium Taurodeoxycholate (NaTDC) and Sodium Taurocholate (NaTC) Bile Salts" Molecules 22, no. 7: 1079. https://doi.org/10.3390/molecules22071079





