Anti-Inflammatory and Neuroprotective Constituents from the Peels of Citrus grandis
Abstract
:1. Introduction
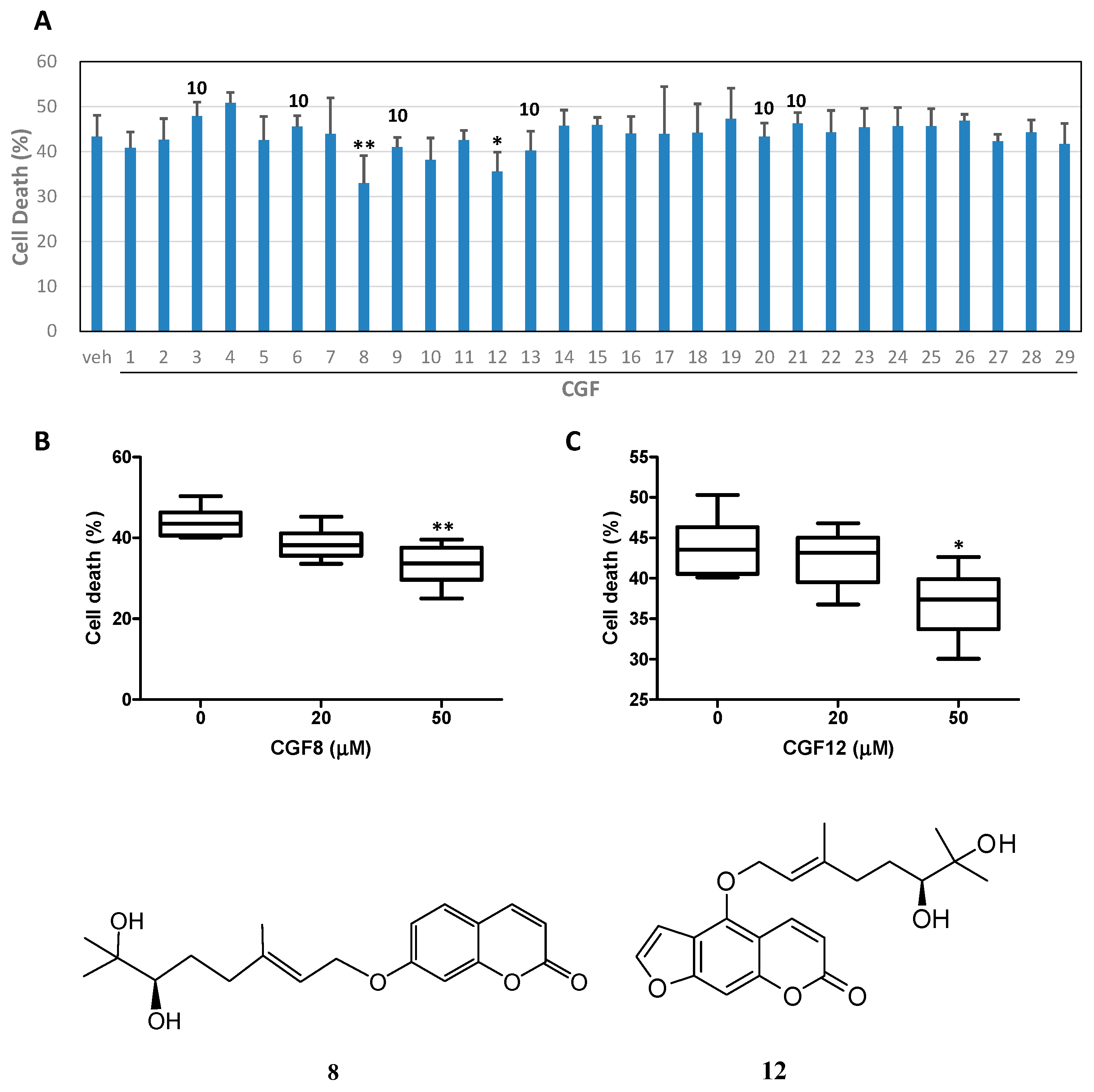
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Materials
3.3. Extraction and Isolation
3.4. Bioactivity Examination
3.4.1. Preparation of Human Neutrophils
3.4.2. Inhibition of Superoxide Anion Generation
3.4.3. Inhibition of Elastase Release
3.4.4. Statistical Analysis
3.4.5. Neuroprotective Activity
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rendeiro, C.; Dong, H.; Saunders, C.; Harkness, L.; Blaze, M.; Hou, Y.; Belanger, R.L.; Corona, G.; Lovegrove, J.A.; Spencer, J.P. Flavanone-rich Citrus beverages counteract the transient decline in postprandial endothelial function in humans: A randomised, controlled, double-masked, cross-over intervention study. Br. J. Nutr. 2016, 116, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Hattori, S.; Shimokoriyama, M.; Kanao, M. Studies on flavanone glycosides. IV. The glycosides of ripe fruit peel and flower petals of Citrus aurantium L. J. Am. Chem. Soc. 1952, 74, 3614–3615. [Google Scholar] [CrossRef]
- Wu, T.S.; Kuoh, C.S.; Furukawa, H. Acridone alkaloids and a coumarin from Citrus grandis. Phytochemistry 1983, 22, 1493–1497. [Google Scholar]
- McPhail, A.T.; Ju-ichi, M.; Fujitani, Y.; Inoue, M.; Wu, T.S.; Furukawa, H. Isolation and structures of citropone-A and -B from Citrus plants, first examples of naturally-occurring homoacridone alkaloids containing a tropone ring system. Tetrahedron Lett. 1985, 26, 3271–3272. [Google Scholar] [CrossRef]
- Wu, T.S.; Huang, S.C.; Jong, T.T.; Lai, J.S.; Furukawa, H. Honyumine, a new linear pyranoacridone alkaloids from Citrus grandis Osbeck. Heterocycles 1986, 24, 41–43. [Google Scholar] [CrossRef]
- Wu, T.S. Baiyumine-A and -B, two acridone alkaloids from Citrus grandis. Phytochemistry 1987, 26, 871–872. [Google Scholar] [CrossRef]
- Wu, T.S.; Huang, S.C.; Jong, T.T.; Lai, J.S.; Kuoh, C.S. Coumarins, acridone alkaloids and a flavone from Citrus grandis. Phytochemistry 1988, 27, 585–587. [Google Scholar] [CrossRef]
- Wu, T.S. Alkaloids and coumarins of Citrus grandis. Phytochemistry 1988, 27, 3717–3718. [Google Scholar] [CrossRef]
- Huang, S.C.; Chen, M.T.; Wu, T.S. Alkaloids and coumarins from stem bark of Citrus grandis. Phytochemistry 1989, 28, 3574–3576. [Google Scholar] [CrossRef]
- Wu, T.S.; Huang, S.C.; Lai, J.S. Stem bark coumarins of Citrus grandis. Phytochemistry 1994, 36, 217–219. [Google Scholar]
- Takemura, Y.; Ju-ichi, M.; Ito, C.; Furukawa, H.; Tokuda, H. Studies on the inhibitory effects of some acridone alkaloids on Epstein-Barr virus activation. Planta Med. 1995, 61, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Peng, S. Introduction to the origin and evolution of Pomelo and its distribution in China. Chin. J. Ecol. 2000, 19, 58–61. [Google Scholar]
- Mokbel, M.S.; Hashinaga, F. Evaluation of the antioxidant activity of extracts from buntan (Citrus grandis Osbeck) fruit tissues. Food Chem. 2006, 94, 529–534. [Google Scholar] [CrossRef]
- Tsai, H.L.; Chang, S.K.; Chang, S.J. Antioxidant content and free radical scavenging ability of fresh red pummelo [Citrus grandis (L.) Osbeck] juice and freeze-dried products. J. Agric. Food Chem. 2007, 55, 2867–2872. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.N.; Shin, J.G.; Jang, H.D. Antioxidant and antidiabetic activity of Dangyuja (Citrus grandis Osbeck) extract treated with Aspergillus saitoi. Food Chem. 2009, 117, 35–41. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the Citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef] [PubMed]
- La, V.D.; Zhao, L.; Epifano, F.; Genovese, S.; Grenier, D. Anti-inflammatory and wound healing potential of Citrus auraptene. J. Med. Food 2013, 16, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Bruschetta, G.; Di Paola, R.; Ahmad, A.; Campolo, M.; Cuzzocrea, S.; Esposito, E.; Navarra, M. The anti-inflammatory and antioxidant effects of bergamot juice extract (BJe) in an experimental model of inflammatory bowel disease. Clin. Nutr. 2014, 33, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Mitoshi, M.; Kuriyama, I.; Nakayama, H.; Miyazato, H.; Sugimoto, K.; Kobayashi, Y.; Jippo, T.; Kuramochi, K.; Yoshida, H.; Mizushina, Y. Suppression of allergic and inflammatory responses by essential oils derived from herbal plants and Citrus fruits. Int. J. Mol. Med. 2014, 33, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.J.; Hwang, D.; Lee, E.S.; Hyun, J.W.; Yi, P.H.; Kim, G.S.; Lee, S.E.; Pang, C.; Park, Y.J.; Chung, K.H.; et al. Anti-inflammatory activity of a new cyclic peptide, citrusin XI, isolated from the fruits of Citrus unshiu. J. Ethnopharmacol. 2015, 163, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Shende, S.; Ingle, A.P.; Gade, A.; Rai, M. Green synthesis of copper nanoparticles by Citrus medica Linn. (Idilimbu) juice and its antimicrobial activity. World J. Microbiol. Biotechnol. 2015, 31, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Chubukov, V.; Mingardon, F.; Schackwitz, W.; Baidoo, E.E.; Alonso-Gutierrez, J.; Hu, Q.; Lee, T.S.; Keasling, J.D.; Mukhopadhyay, A. Acute limonene toxicity in Escherichia coli is caused by limonene hydroperoxide and alleviated by a point mutation in alkyl hydroperoxidase AhpC. Appl. Environ. Microbiol. 2015, 81, 4690–4696. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.C.; Li, H.C.; Wu, P.H.; Huang, P.H.; Wang, Y.T. Assessment of oligogalacturonide from Citrus pectin as a potential antibacterial agent against foodborne pathogens. J. Food Sci. 2014, 79, 1541–1544. [Google Scholar] [CrossRef] [PubMed]
- Vollmerhausen, T.L.; Ramos, N.L.; Dzung, D.T.; Brauner, A. Decoctions from Citrus reticulata Blanco seeds protect the uroepithelium against Escherichia coli invasion. J. Ethnopharmacol. 2013, 150, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015, 124, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K.N.; Jayaprakasha, G.K.; Patil, B.S. Cytotoxicity of obacunone and obacunone glucoside in human prostate cancer cells involves Akt-mediated programmed cell death. Toxicology 2015, 329, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Leclere, L.; Fransolet, M.; Cote, F.; Cambier, P.; Arnould, T.; Van Cutsem, P.; Michiels, C. Heat-modified Citrus pectin induces apoptosis-like cell death and autophagy in HepG2 and A549 cancer cells. PLoS ONE 2015, 10, e0115831. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.Y.; Chen, Y.; Zou, L.; Zhang, Y.; Hu, F.; Feng, J.; Shen, J.; Wei, B. Naringin inhibits growth and induces apoptosis by a mechanism dependent on reduced activation of NF-κB/COX-2 caspase-1 pathway in HeLa cervical cancer cells. Int. J. Oncol. 2014, 45, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Arasteh, E.; Imenshahidi, M.; Iranshahi, M. Antihypertensive effect of auraptene, a monoterpene coumarin from the genus Citrus, upon chronic administration. Iran. J. Basic Med. Sci. 2015, 18, 153–158. [Google Scholar] [PubMed]
- Orhan, I.E.; Nabavi, S.F.; Daglia, M.; Tenore, G.C.; Mansouri, K.; Nabavi, S.M. Naringenin and atherosclerosis: A review of literature. Curr. Pharm. Biotechnol. 2015, 16, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Niu, L.; Cui, L.; Hou, X.; Li, J.; Zhang, X.; Zhang, M. Hesperetin inhibits rat coronary constriction by inhibiting Ca2+ influx and enhancing voltage-gated K+ channel currents of the myocytes. Eur. J. Pharmacol. 2014, 735, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Chanet, A.; Claude, S.; Maier, J.A.; Kamran, K.M.; Rakotomanomana, N.; Shinkaruk, S.; Bérard, A.M.; Bennetau, P.C.; Mazur, A.; Morand, C. Flavanone metabolites decrease monocyte adhesion to TNF-α-activated endothelial cells by modulating expression of atherosclerosis-related genes. Br. J. Nutr. 2013, 110, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, J.; Fang, L.; Zheng, Z.; Zhi, D.; Wang, S.; Li, S.; Ho, C.T.; Zhao, H. Anticancer activities of citrus peel polymethoxyflavones related to angiogenesis and others. Biol. Med. Res. Int. 2014, 30, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.L.; Shih, P.H.; Yen, G.C. Neuroprotective effects of citrus flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.; Bacay, A.F.; Moyer, J.; Webb, A.; Carrico-Moniz, D. Synthesis and biological evaluation of isoprenylated coumarins as potential anti-pancreatic cancer agents. Bioorg. Med. Chem. Lett. 2014, 24, 4654–4658. [Google Scholar] [CrossRef] [PubMed]
- Beare, K.D.; McErlean, C.S.P. Accessing columbianetin-containing natural products via a domino on-water, in-water process. Tetrahedron Lett. 2013, 54, 1056–1058. [Google Scholar] [CrossRef]
- Feger, W.; Brandauer, H.; Gabris, P.; Ziegler, H. Nonvolatiles of commercial lime and grapefruit oils separated by high-speed countercurrent chromatography. J. Agric. Food Chem. 2006, 54, 2242–2252. [Google Scholar] [CrossRef] [PubMed]
- Francis Rouessac, M.A. Syntheses en serie racemique et en serie optiquement active d’une famille de derives oxygenes naturels de l'ombelliferone. Structure spatiale du (−) epoxy-3′6′ auraptene. Tetrahedron 1988, 44, 101–110. [Google Scholar]
- Cai, J.N.; Basnet, P.; Wang, Z.T.; Komatsu, K.; Xu, L.S.; Tani, T. Coumarins from the fruits of Cnidium monnieri. J. Nat. Prod. 2000, 63, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Panthong, K.; Srisud, Y.; Rukachaisirikul, V.; Hutadilok-Towatana, N.; Voravuthikunchai, S.P.; Tewtrakul, S. Benzene, coumarin and quinolinone derivatives from roots of Citrus hystrix. Phytochemistry 2013, 88, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Znati, M.; Ben Jannet, H.; Cazaux, S.; Souchard, J.P.; Harzallah Skhiri, F.; Bouajila, J. Antioxidant, 5-lipoxygenase inhibitory and cytotoxic activities of compounds isolated from the Ferula lutea flowers. Molecules 2014, 19, 16959–16975. [Google Scholar] [CrossRef] [PubMed]
- Marumoto, S.; Miyazawa, M. Structure-activity relationships for naturally occurring coumarins as beta-secretase inhibitor. Bioorg. Med. Chem. 2012, 20, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Ferrino, P.C.; Favela-Hernandez, J.M.; Garza-Gonzalez, E.; Waksman, N.; Rios, M.Y.; del Rayo Camacho-Corona, M. Antimycobacterial activity of constituents from Foeniculum vulgare var. dulce grown in Mexico. Molecules 2012, 17, 8471–8482. [Google Scholar] [PubMed]
- Ohta, T.; Maruyama, T.; Nagahashi, M.; Miyamoti, Y.; Hosoi, S.; Kiuchi, F.; Yamazoe, Y.; Tsukamoto, S.; Paradisin, C. A new CYP3A4 inhibitor from grapefruit juice. Tetrahedron 2002, 58, 6631–6635. [Google Scholar] [CrossRef]
- Abulrob, A.; Suller, M.T.E.; Gumbleton, M.; Simons, C.; Russell, A.D. Identification and biological evaluation of grapefruit oil components as potential novel efflux pump modulators in methicillin-resistant Staphylococcus aureus bacterial strains. Phytochemistry 2004, 65, 3021–3027. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.K.; Wu, T.S. Constituents of flowers of Murraya paniculata. J. Chin. Chem. Soc. 1994, 41, 213–216. [Google Scholar] [CrossRef]
- Youkwan, J.; Sutthivaiyakit, S.; Sutthivaiyakit, P. Citrusosides A–D and furanocoumarins with cholinesterase inhibitory activity from the fruit peels of Citrus hystrix. J. Nat. Prod. 2010, 73, 1879–1883. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, D.; El-Readi, M.Z.; Tahrani, A.; Herrmann, F.; Kaufmann, D.; Farrag, N.; El-Shazly, A.; Wink, M. Chemical composition and biological activity of Citrus jambhiri Lush. Food Chem. 2011, 127, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.Q.; Guo, L.J.; Chen, H.S.; Wu, L.S.; Kong, F.F. Study on the flavonoids constituents of Trachelospermum jasminoides. J. Chin. Med. Mater. 2010, 33, 58–60. [Google Scholar]
- Matsubara, Y.; Kumamoto, H.; Iizuka, Y.; Murakami, T.; Okamoto, K.; Miyake, H.; Yokoi, K. Structure and hypertensive effect of flavonoid glycosides in Citrus unshiu peelings. Agric. Biol. Chem. 1985, 49, 909–914. [Google Scholar]
- Donna, L.D.; Luca, G.D.; Mazzotti, F.; Napoli, A.; Salerno, R.; Taverna, D.; Sindona, G. Statin-like principles of bergamot fruit (Citrus bergamia): Isolation of 3-hydroxymethylglutaryl flavonoid glycosides. J. Nat. Prod. 2009, 72, 1352–1354. [Google Scholar] [CrossRef] [PubMed]
- Lewin, G.; Maciuk, A.; Thoret, S.; Aubert, G.; Dubois, J.; Cresteil, T. Semisynthesis of natural flavones inhibiting tubulin polymerization, from hesperidin. J. Nat. Prod. 2010, 73, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Numonov, S.R.; Usmanova, S.K.; Aisa, H.A. A triterpenoid and flavonoids from Dracocephalum heterophyllum. Chem. Nat. Compd. 2013, 48, 1109–1110. [Google Scholar] [CrossRef]
- Bowen, I.H.; Perera, K.P.W.C. Alkaloids, coumarins and flavonoids of Micromelum zeylanicum. Phytochemistry 1982, 21, 433–437. [Google Scholar] [CrossRef]
- Akhtar, N.; Saleem, M.; Riaz, N.; Ali, M.S.; Yaqoob, A.; Nasim, F.; Jabbar, A. Isolation and characterization of the chemical constituents from Plumeria rubra. Phytochem. Lett. 2013, 6, 291–298. [Google Scholar] [CrossRef]
- Ouyang, X.L.; Wei, L.X.; Fang, X.M.; Wang, H.S.; Pan, Y.M. Flavonoid constituents of Euonymus fortunei. Chem. Nat. Compd. 2013, 49, 428–431. [Google Scholar] [CrossRef]
- Luyen, B.T.; Tai, B.H.; Thao, N.P.; Cha, J.Y.; Lee, H.Y.; Lee, Y.M.; Kim, Y.H. Anti-inflammatory components of Chrysanthemum indicum flowers. Bioorg. Med. Chem. Lett. 2015, 25, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Leu, Y.L.; Hwang, T.L.; Kuo, P.C.; Liou, K.P.; Huang, B.S.; Chen, G.F. Constituents from Vigna vexillata and their anti-inflammatory activity. Int. J. Mol. Sci. 2012, 13, 9754–9768. [Google Scholar] [CrossRef] [PubMed]
- Louche, L.M.M.; Gaydou, E.M.; Lesage, J.C. Determination of phlorin as peel marker in orange (Citrus sinensis) fruits and juices. J. Agric. Food Chem. 1998, 46, 4193–4197. [Google Scholar] [CrossRef]
- Sribuhom, T.; Sriphana, U.; Thongsri, Y.; Yenjai, C. Chemical constituents from the stems of Alyxia schlechteri. Phytochem. Lett. 2015, 11, 80–84. [Google Scholar] [CrossRef]
- Ley, J.P.; Blings, M.; Paetz, S.; Krammer, G.E.; Bertram, H.J. New bitter-masking compounds: Hydroxylated benzoic acid amides of aromatic amines as structural analogues of homoeriodictyol. J. Agric. Food Chem. 2006, 54, 8574–8579. [Google Scholar] [CrossRef] [PubMed]
- Takaya, Y.; Kondo, Y.; Furukawa, T.; Niwa, M. Antioxidant constituents of radish sprout (Kaiware-daikon), Raphanus sativus L. J. Agric. Food Chem. 2003, 51, 8061–8066. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kittaka, H.; Ikegami, S. Novel synthesis of enantiomerically pure natural inositols and their diastereoisomers. J. Org. Chem. 2001, 66, 2705–2716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shu, X.; Jing, F.; Wang, X.; Lin, C.; Luo, A. Preparative separation of alkaloids from Picrasma quassioides (D. Don) Benn. by conventional and pH-zone-refining countercurrent chromatography. Molecules 2014, 19, 8752–8761. [Google Scholar] [CrossRef] [PubMed]
- Strehmel, N.; Böttcher, C.; Schmidt, S.; Scheel, D. Profiling of secondary metabolites in root exudates of Arabidopsis thaliana. Phytochemistry 2014, 108, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Chung, P.J.; Ho, C.M.; Kuo, C.Y.; Hung, M.F.; Huang, Y.T.; Chang, W.Y.; Chang, Y.W.; Chan, K.H.; Hwang, T.L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.P.; Hsieh, P.W.; Chang, Y.J.; Chung, P.J.; Kuo, L.M.; Hwang, T.L. 2-(2-Fluorobenzamido) benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.N.; Chi, C.W.; Lin, Y.L.; Chen, C.F.; Shiao, Y.J. The neuroprotective effects of phytoestrogens on amyloid β protein-induced toxicity are mediated by abrogating the activation of caspase cascade in rat cortical neurons. J. Biol. Chem. 2001, 276, 5287–5295. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the purified compounds are available from the authors. |
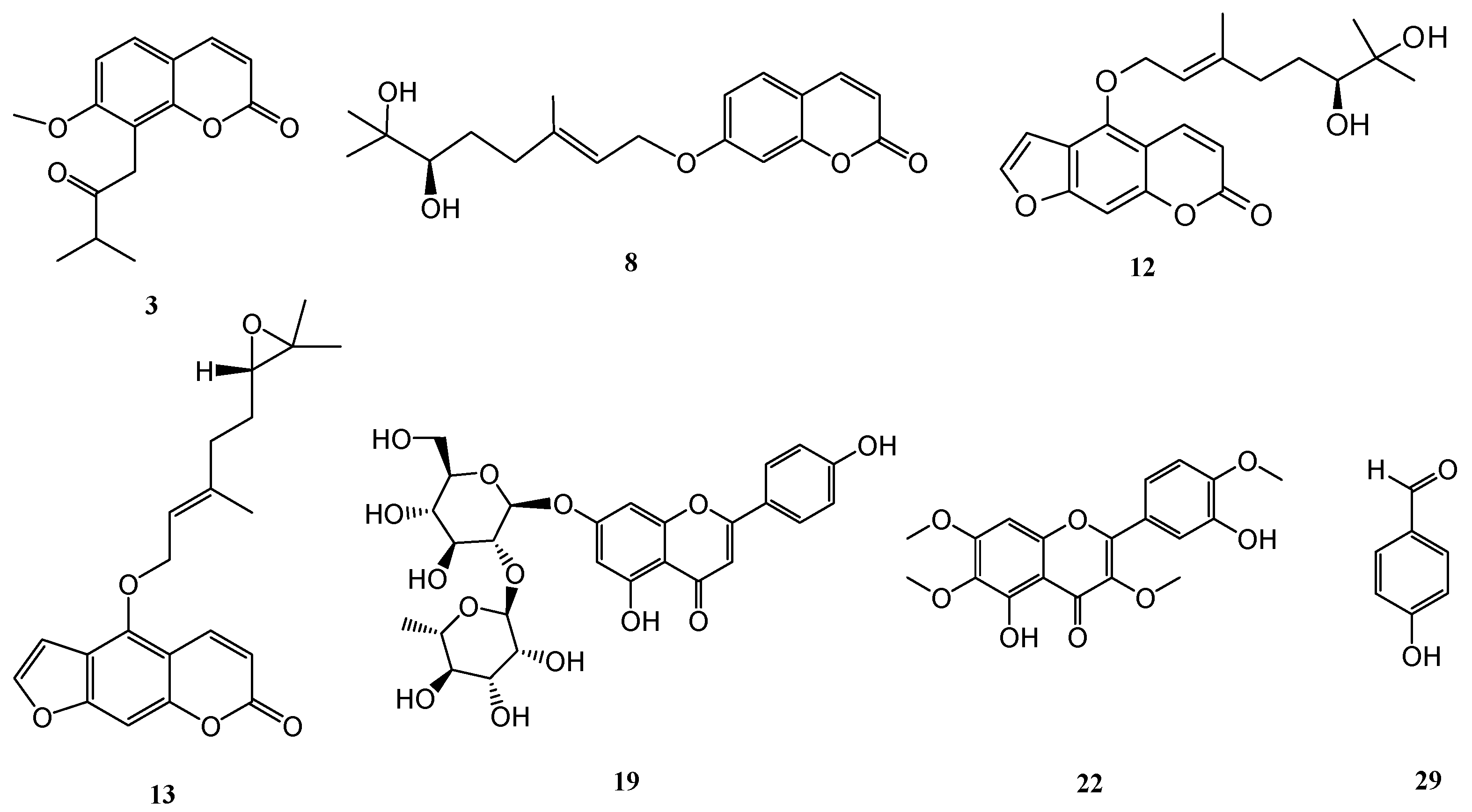
| Compound | Superoxide Anion Generation | Elastase Release |
|---|---|---|
| IC50 (μM) a | IC50 (μM) a | |
| 3 | 3.89 ± 0.45 *** | 4.33 ± 0.83 *** |
| 12 | 6.02 ± 2.46 *** | >10 |
| 13 | 7.57 ± 3.19 *** | 3.58 ± 1.90 *** |
| 19 | 3.79 ± 0.42 *** | >10 |
| 22 | 5.95 ± 1.56 *** | >10 |
| 29 | 0.54 ± 0.24 *** | 0.43 ± 0.09 *** |
| Sorafenib b | 1.49 ± 0.42 | 0.93 ± 0.10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, P.-C.; Liao, Y.-R.; Hung, H.-Y.; Chuang, C.-W.; Hwang, T.-L.; Huang, S.-C.; Shiao, Y.-J.; Kuo, D.-H.; Wu, T.-S. Anti-Inflammatory and Neuroprotective Constituents from the Peels of Citrus grandis. Molecules 2017, 22, 967. https://doi.org/10.3390/molecules22060967
Kuo P-C, Liao Y-R, Hung H-Y, Chuang C-W, Hwang T-L, Huang S-C, Shiao Y-J, Kuo D-H, Wu T-S. Anti-Inflammatory and Neuroprotective Constituents from the Peels of Citrus grandis. Molecules. 2017; 22(6):967. https://doi.org/10.3390/molecules22060967
Chicago/Turabian StyleKuo, Ping-Chung, Yu-Ren Liao, Hsin-Yi Hung, Chia-Wei Chuang, Tsong-Long Hwang, Shiow-Chyn Huang, Young-Ji Shiao, Daih-Huang Kuo, and Tian-Shung Wu. 2017. "Anti-Inflammatory and Neuroprotective Constituents from the Peels of Citrus grandis" Molecules 22, no. 6: 967. https://doi.org/10.3390/molecules22060967






