Characterization of Aliphatic Polyesters Synthesized via Enzymatic Ring-Opening Polymerization in Ionic Liquids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Copolymerization of rac-LA and CL
2.2. Chemical Composition of the Copolymers
2.3. Chain Microstructures of Synthesized Copolymers
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Copolymers of rac-Lactide and ε-Caprolactone
3.3. Spectroscopic Analysis of Polymers
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Seyednejad, H.; Ghassemi, A.H.; van Nostrum, C.F.; Vermonden, T.; Hennink, W.E. Functional aliphatic polyesters for biomedical and pharmaceutical applications. J. Controll. Release 2011, 152, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Bergsma, J.E.; de Bruijn, W.; Rozema, F.; Bos, R.; Boering, G. Late degradation tissue response to poly (l-lactide) bone plates and screws. Biomaterials 1995, 16, 25–31. [Google Scholar] [CrossRef]
- Oledzka, E.; Horeglad, P.; Gruszczyńska, Z.; Plichta, A.; Nałęcz-Jawecki, G.; Sobczak, M. Polylactide conjugates of camptothecin with different drug release abilities. Molecules 2014, 19, 19460–19470. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, M.; Kolodziejski, W. Polymerization of cyclic esters initiated by carnitine and tin (II) octoate. Molecules 2009, 14, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Shoda, S.-I.; Uyama, H. Polymer Synthesis/Polymer Engineering; Springer: New York, NY, USA, 1995; pp. 1–30. [Google Scholar]
- Albertsson, A.-C.; Srivastava, R.K. Recent developments in enzyme-catalyzed ring-opening polymerization. Adv. Drug Deliv. Rev. 2008, 60, 1077–1093. [Google Scholar] [CrossRef] [PubMed]
- Varma, I.K.; Albertsson, A.-C.; Rajkhowa, R.; Srivastava, R.K. Enzyme catalyzed synthesis of polyesters. Prog. Polym. Sci. 2005, 30, 949–981. [Google Scholar] [CrossRef]
- Sobczak, M. Enzyme-catalyzed ring-opening polymerization of cyclic esters in the presence of poly(ethylene glycol). J. Appl. Polym. Sci. 2012, 125, 3602–3609. [Google Scholar] [CrossRef]
- Sobczak, M.; Kamysz, W.; Tyszkiewicz, W.; Dębek, C.; Kozłowski, R.; Olędzka, E.; Piotrowska, U.; Nałęcz-Jawecki, G.; Plichta, A.; Grzywacz, D. Biodegradable macromolecular conjugates of citropin: Synthesis, characterization and in vitro efficiency study. React. Funct. Polym. 2014, 83, 54–61. [Google Scholar] [CrossRef]
- Piotrowska, U.; Sobczak, M.; Oledzka, E.; Combes, C. Effect of ionic liquids on the structural, thermal, and in vitro degradation properties of poly(ε-caprolactone) synthesized in the presence of Candida antarctica lipase B. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Domenek, S.; Ducruet, V. Biodegradable and Biobased Polymers for Environmental and Biomedical Applications; Wiley: Hoboken, NJ, USA, 2016; pp. 171–224. [Google Scholar] [CrossRef]
- Matsumura, S.; Mabuchi, K.; Toshima, K. Lipase-catalyzed ring-opening polymerization of lactide. Macromol. Rapid Commun. 1997, 18, 477–482. [Google Scholar] [CrossRef]
- Hans, M.; Keul, H.; Moeller, M. Ring-opening polymerization of DD-Lactide catalyzed by novozyme 435. Macromol. Biosci. 2009, 9, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Düşkünkorur, H.Ö.; Bégué, A.; Pollet, E.; Phalip, V.; Güvenilir, Y.; Avérous, L. Enzymatic ring-opening (co)polymerization of lactide stereoisomers catalyzed by lipases. Toward the in situ synthesis of organic/inorganic nanohybrids. J. Mol. Catal. B Enzym. 2015, 115, 20–28. [Google Scholar] [CrossRef]
- Piotrowska, U.; Sobczak, M. Enzymatic polymerization of cyclic monomers in ionic liquids as a prospective synthesis method for polyesters used in drug delivery systems. Molecules 2014, 20, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Uyama, H.; Takamoto, T.; Kobayashi, S. Enzymatic synthesis of polyesters in ionic liquids. Polym. J. 2002, 34, 94–96. [Google Scholar] [CrossRef]
- Yoshizawa-Fujita, M.; Saito, C.; Takeoka, Y.; Rikukawa, M. Lipase-catalyzed polymerization of l-lactide in ionic liquids. Polym. Adv. Technol. 2008, 19, 1396–1400. [Google Scholar] [CrossRef]
- Chanfreau, S.; Mena, M.; Porras-Domínguez, J.R.; Ramírez-Gilly, M.; Gimeno, M.; Roquero, P.; Tecante, A.; Bárzana, E. Enzymatic synthesis of poly-l-lactide and poly-l-lactide-co-glycolide in an ionic liquid. Bioproc. Biosyst. Eng. 2010, 33, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Mena, M.; López-Luna, A.; Shirai, K.; Tecante, A.; Gimeno, M.; Bárzana, E. Lipase-catalyzed synthesis of hyperbranched poly-l-lactide in an ionic liquid. Bioproc. Biosyst. Eng. 2013, 36, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Kumar, A.; Gross, R.A. Probing water-temperature relationships for lipase-catalyzed lactone ring-opening polymerizations. Macromolecules 2002, 35, 5444–5448. [Google Scholar] [CrossRef]
- Fernández, J.; Etxeberria, A.; Sarasua, J.-R. Synthesis, structure and properties of poly (l-lactide-co-ε-caprolactone) statistical copolymers. J. Mech. Behav. Biomed. 2012, 9, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Dakshinamoorthy, D.; Peruch, F. Block and random copolymerization of ε-caprolactone, l-, and rac-lactide using titanium complex derived from aminodiol ligand. J. Polym. Sci. A2 2012, 50, 2161–2171. [Google Scholar] [CrossRef]
- Wahlberg, J.; Persson, P.V.; Olsson, T.; Hedenström, E.; Iversen, T. Structural characterization of a lipase-catalyzed copolymerization of ε-caprolactone and d, l-lactide. Biomacromolecules 2003, 4, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Nalampang, K.; Molloy, R.; Punyodom, W. Synthesis and characterization of poly (l-lactide-co-ε-caprolactone) copolymers: Influence of sequential monomer addition on chain microstructure. Polym. Adv. Technol. 2007, 18, 240–248. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Kreiser, I. Polylactones. 13. Transesterification of Poly (l-Lactide) with Poly (Glycoude), Poly (β-Propio-Lactone), and Poly (ε-Caprolactone). J. Macromol. Sci. Chem. 1987, 24, 1345–1356. [Google Scholar] [CrossRef]
- Kasperczyk, J.; Bero, M. Coordination polymerization of lactides, 4. The role of transesterification in the copolymerization of L, L-lactide and ε-caprolactone. Macromol. Chem. Physic. 1993, 194, 913–925. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Boettcher, C.; Tönnes, K.-U. Polylactones: 23. Polymerization of racemic and mesod, l-lactide with various organotin catalysts—Stereochemical aspects. Polymer 1992, 33, 2817–2824. [Google Scholar] [CrossRef]
- Bero, M.; Adamus, G.; Kasperczyk, J.; Janeczek, H. Synthesis of block copolymers of ε-caprolactone and lactide in the presence of lithium t-butoxide. Polym. Bull. 1993, 31, 9–14. [Google Scholar] [CrossRef]
- Zhou, X.; Hong, L. Controlled ring-opening polymerization of cyclic esters with phosphoric acid as catalysts. Colloid Polym. Sci. 2013, 291, 2155–2162. [Google Scholar] [CrossRef]
- Bero, M.; Kasperczyk, J.; Adamus, G. Coordination polymerization of lactides, 3. Copolymerization of L, l-lactide and ε-caprolactone in the presence of initiators containing Zn and Al. Macromol. Chem. Phys. 1993, 194, 907–912. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, L.; Qu, C.; Qi, M. Microstructure analysis and thermal properties of l-lactide/ε-caprolactone copolymers obtained with magnesium octoate. Polymer 2009, 50, 1423–1429. [Google Scholar] [CrossRef]
Sample Availability: Samples of the synthesized compounds are available from the authors. |
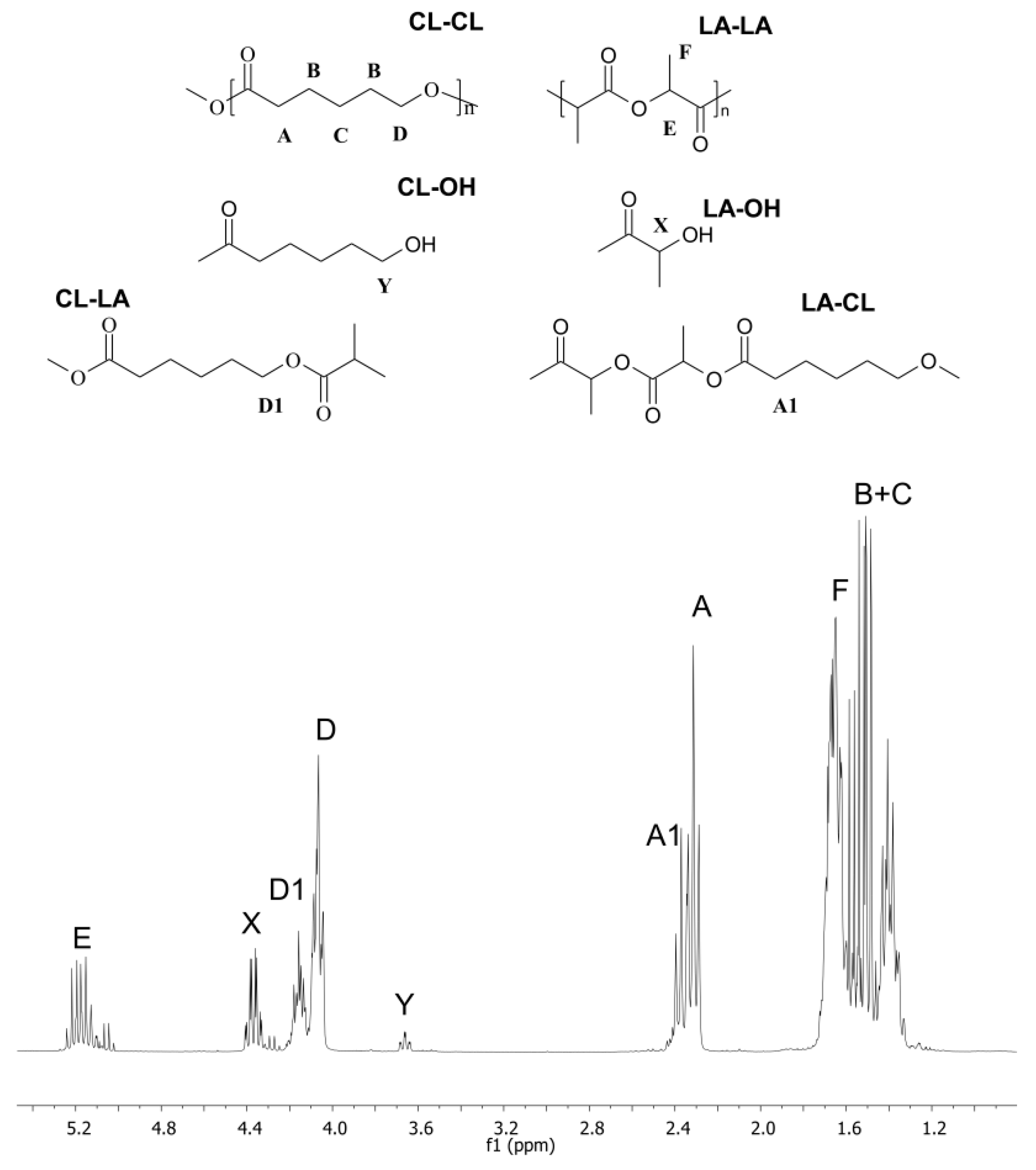
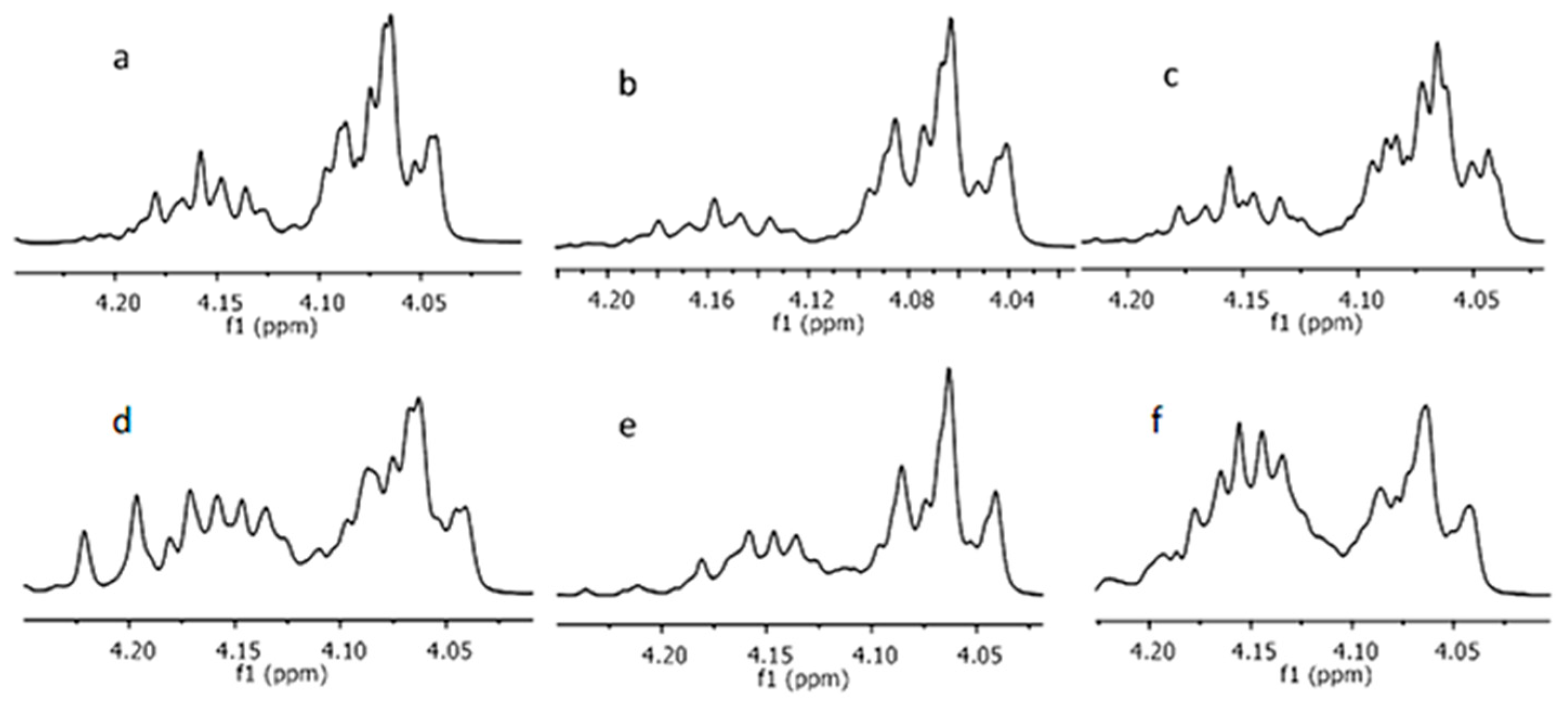


| Entry | Solvent (1 mL) | Temp. (°C) | Time (days) | fLA a | FLA b | rac-LAconv. c (%) | Yield (%) | Mn d (g/mol) | Mw/Mn e | Average Dyad Relative Molar Fractions f | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LA-LA | LA-CL | CL-CL | ||||||||||
| A1 | [bmim][BF4] | 80 | 7 | 0.50 | 0.29 | 0.18 | 35 | 0.029 | 0.516 | 0.456 | ||
| A2 | [bmim][BF4] | 80 | 14 | 0.50 | 0.27 | 0.75 | 43 | 1100 | 1.98 | 0.051 | 0.462 | 0.487 |
| A3 | [bmim][BF4] | 110 | 14 | 0.50 | 0.31 | 0.69 | 45 | 2500 | 2.58 | 0.168 | 0.349 | 0.483 |
| A4 | [bmim][BF4] | 80 | 7 | 0.33 | 0.25 | 0.65 | 42 | 700 | 1.54 | 0.023 | 0.464 | 0.513 |
| A5 | [bmim][BF4] | 80 | 14 | 0.33 | 0.25 | 0.79 | 55 | 3100 | 3.82 | 0.028 | 0.343 | 0.630 |
| A6 | [bmim][BF4] | 110 | 14 | 0.33 | 0.29 | 0.82 | 59 | 2800 | 3.10 | 0.108 | 0.352 | 0.540 |
| A7 | [bmim][BF4] | 80 | 7 | 0.67 | 0.30 | 0.16 | 54 | 0.106 | 0.395 | 0.499 | ||
| A8 | [bmim][BF4] | 80 | 14 | 0.67 | 0.28 | 0.18 | 56 | 1000 | 2.20 | 0.111 | 0.362 | 0.527 |
| A9 | [bmim][BF4] | 110 | 14 | 0.67 | 0.42 | 0.65 | 57 | 2100 | 2.18 | 0.211 | 0.516 | 0.272 |
| B1 | [bmim][NTf2] | 80 | 7 | 0.50 | 0.46 | 0.49 | 60 | 0.2212 | 0.479 | 0.300 | ||
| B2 | [bmim][NTf2] | 80 | 14 | 0.50 | 0.46 | 0.86 | 63 | 1700 | 2.41 | 0.2698 | 0.391 | 0.340 |
| B3 | [bmim][NTf2] | 110 | 14 | 0.50 | 0.44 | 0.90 | 69 | 2700 | 2.82 | 0.2247 | 0.432 | 0.344 |
| B4 | [bmim][NTf2] | 80 | 7 | 0.33 | 0.31 | 0.92 | 55 | 0.0592 | 0.488 | 0.453 | ||
| B5 | [bmim][NTf2] | 80 | 14 | 0.33 | 0.33 | 0.94 | 53 | 2400 | 1.80 | 0.1242 | 0.486 | 0.390 |
| B6 | [bmim][NTf2] | 110 | 14 | 0.33 | 0.30 | 0.92 | 57 | 2900 | 3.28 | 0.1040 | 0.596 | 0.492 |
| B7 | [bmim][NTf2] | 80 | 7 | 0.67 | 0.36 | 0.26 | 63 | 0.1119 | 0.498 | 0.390 | ||
| B8 | [bmim][NTf2] | 80 | 14 | 0.67 | 0.62 | 0.59 | 52 | 3000 | 1.68 | 0.4438 | 0.375 | 0.181 |
| B9 | [bmim][NTf2] | 110 | 14 | 0.67 | 0.60 | 0.92 | 61 | 1700 | 1.94 | 0.4000 | 0.412 | 0.188 |
| Sample | Microstructural Magnitudes of the Copolymers | |||
|---|---|---|---|---|
| R | TII | |||
| A2 | 1.96 | 7.41 | 0.50 | 0.08 |
| A3 | 1.52 | 12.88 | 0.25 | 0 |
| A5 | 2.25 | 23.56 | 0.17 | 0.03 |
| A6 | 1.23 | 16.14 | 0.21 | 0 |
| A8 | 2.87 | 4.44 | 0.80 | 0 |
| A9 | - | - | - | 0 |
| B2 | 2.04 | 5.67 | 0.38 | 0.04 |
| B3 | 3.52 | 5.53 | 0.41 | 0 |
| B5 | 2.20 | 10.66 | 0.28 | 0.01 |
| B6 | 2.08 | 14.38 | 0.23 | 0 |
| B8 | 6.47 | 2.66 | 0.99 | 0 |
| B9 | 5.81 | 2.48 | 0.67 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowska, U.; Sobczak, M.; Oledzka, E. Characterization of Aliphatic Polyesters Synthesized via Enzymatic Ring-Opening Polymerization in Ionic Liquids. Molecules 2017, 22, 923. https://doi.org/10.3390/molecules22060923
Piotrowska U, Sobczak M, Oledzka E. Characterization of Aliphatic Polyesters Synthesized via Enzymatic Ring-Opening Polymerization in Ionic Liquids. Molecules. 2017; 22(6):923. https://doi.org/10.3390/molecules22060923
Chicago/Turabian StylePiotrowska, Urszula, Marcin Sobczak, and Ewa Oledzka. 2017. "Characterization of Aliphatic Polyesters Synthesized via Enzymatic Ring-Opening Polymerization in Ionic Liquids" Molecules 22, no. 6: 923. https://doi.org/10.3390/molecules22060923






