Systematic Review of Chemical Constituents in the Genus Lycium (Solanaceae)
Abstract
:1. Introduction
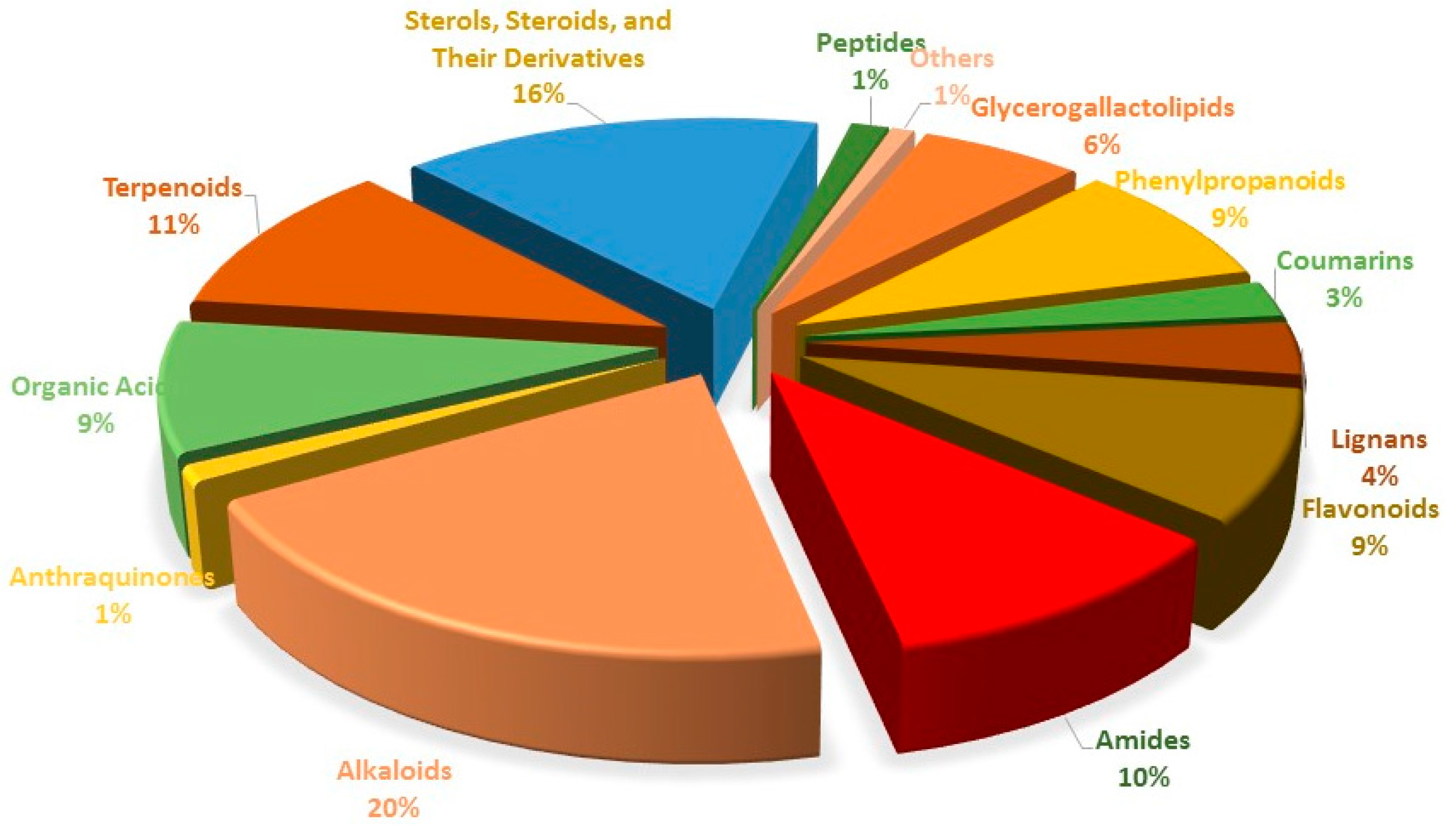
2. Constituents
2.1. Macromolecules in the Lycium Genus
Polysaccharides
2.2. Small Molecule Substances
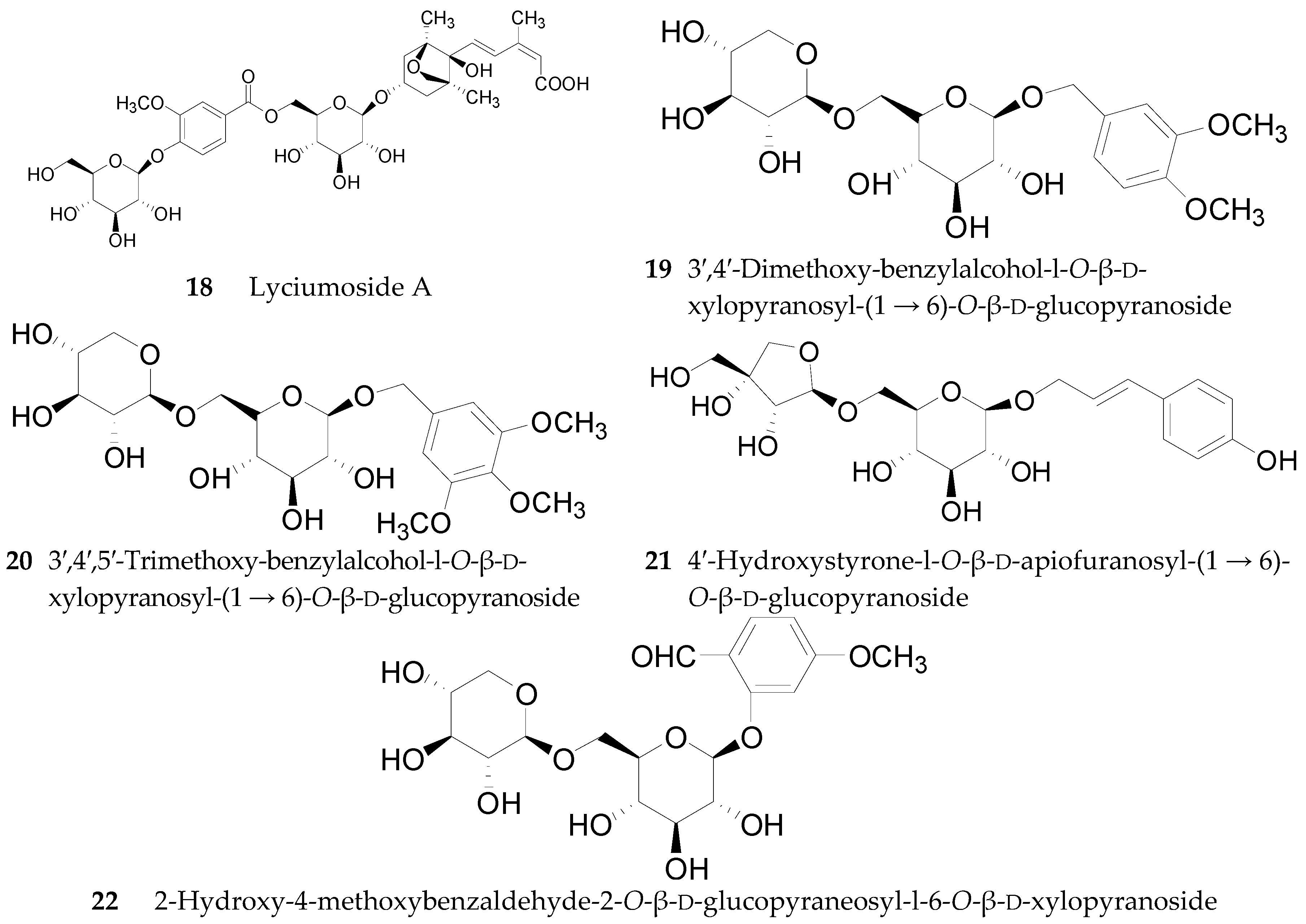
2.2.1. Glycerogalactolipids 1–22
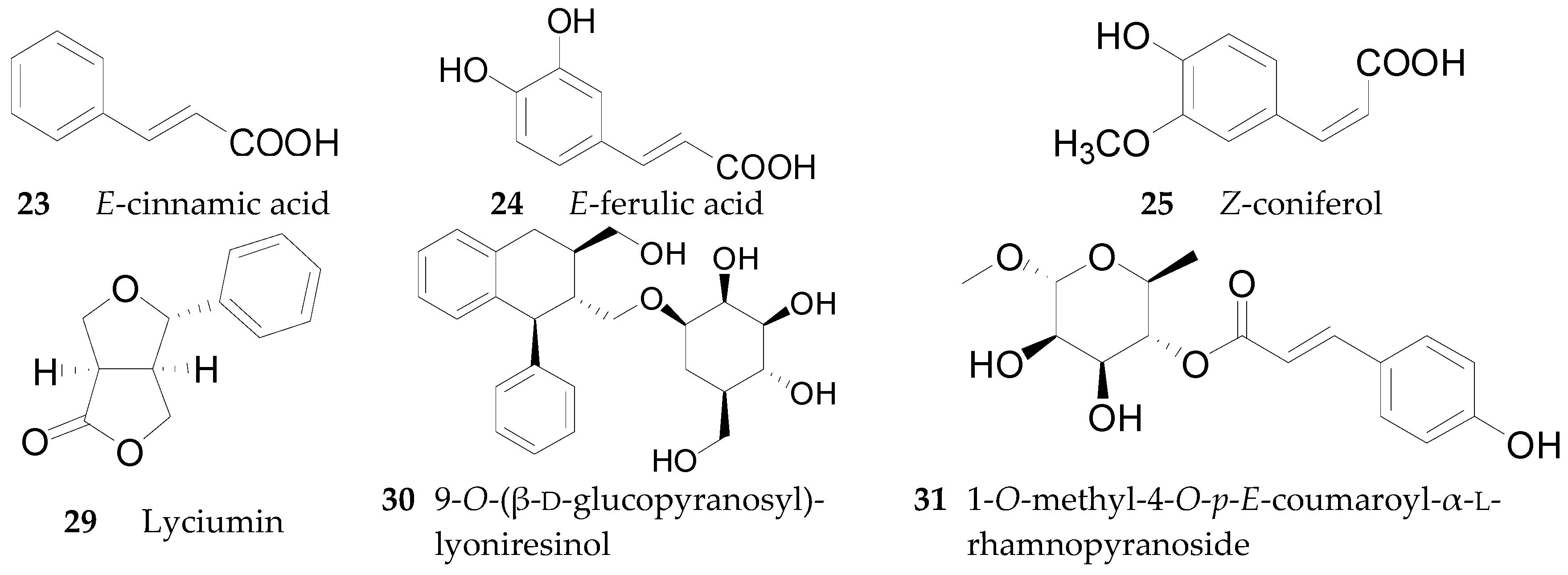
2.2.2. Phenylpropanoids 23–51
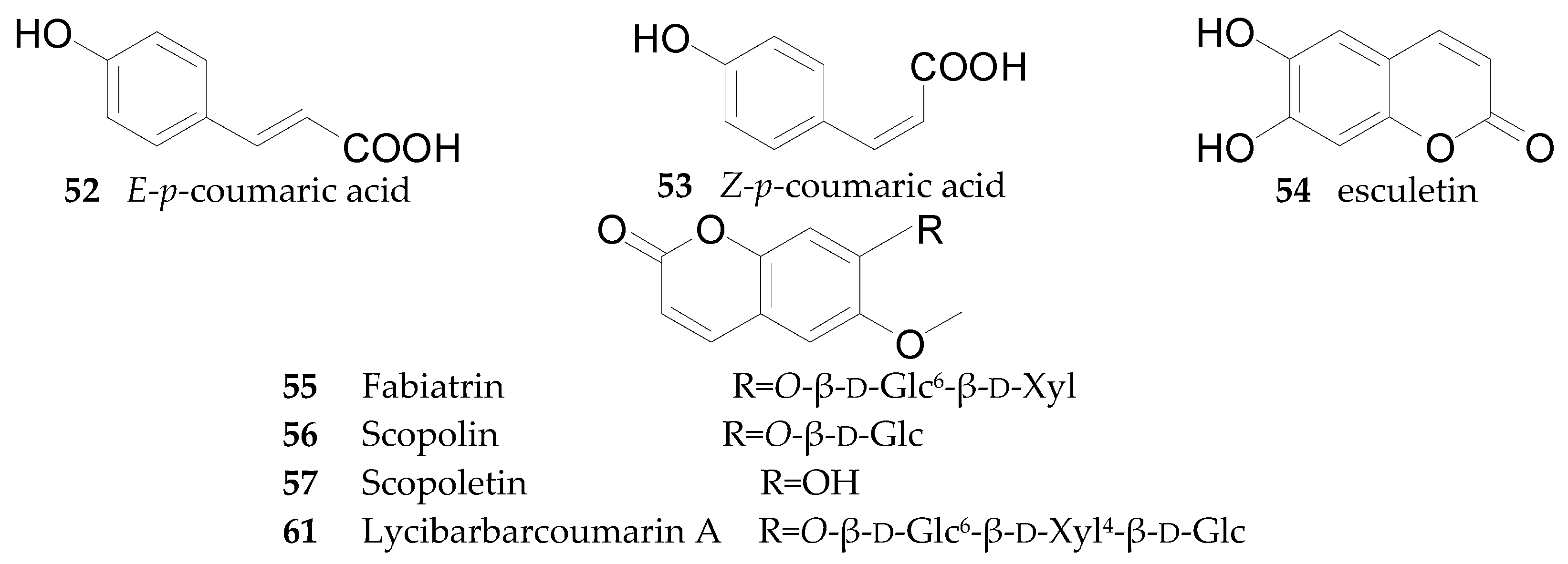
2.2.3. Coumarins 52–61
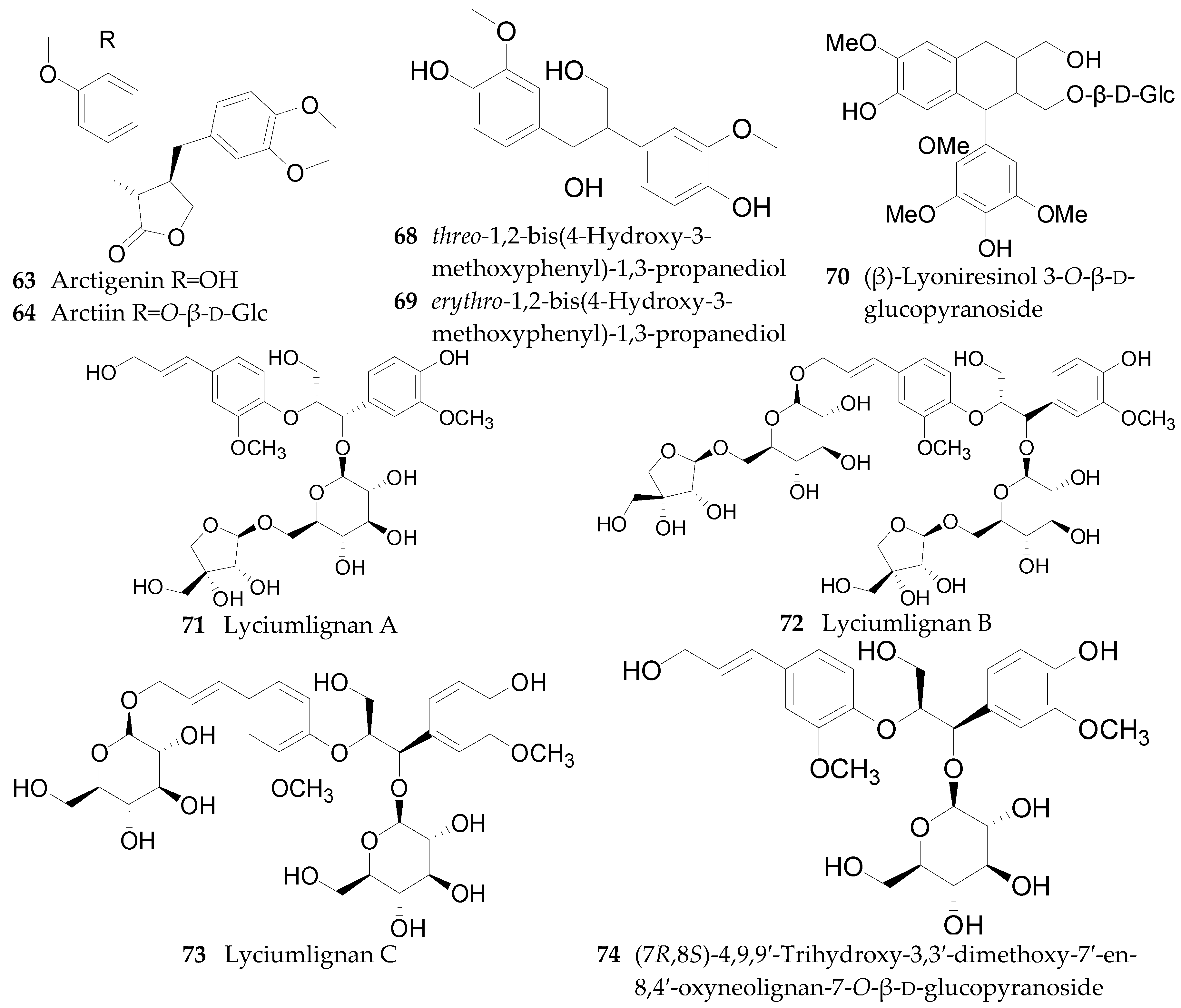
2.2.4. Lignans 62–74
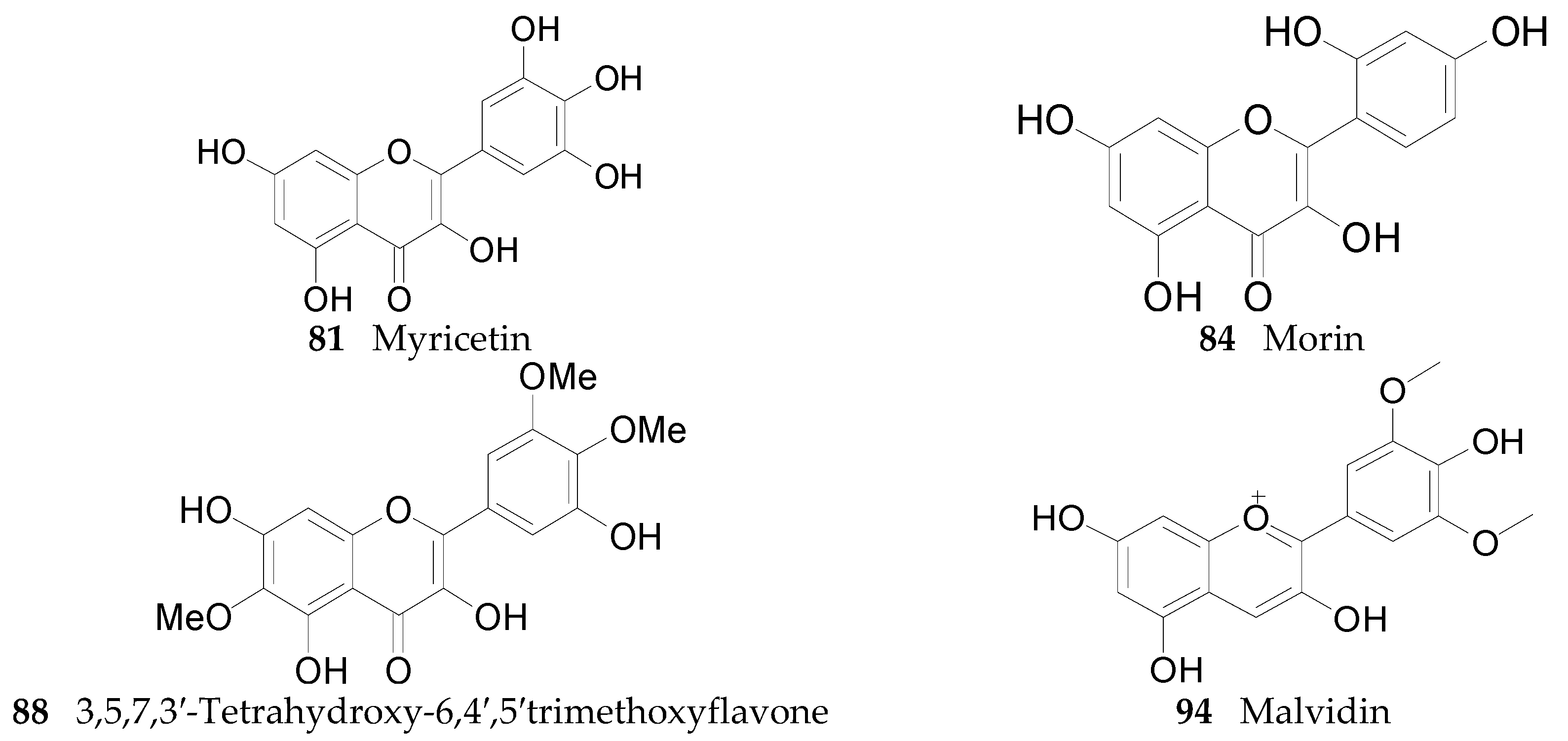
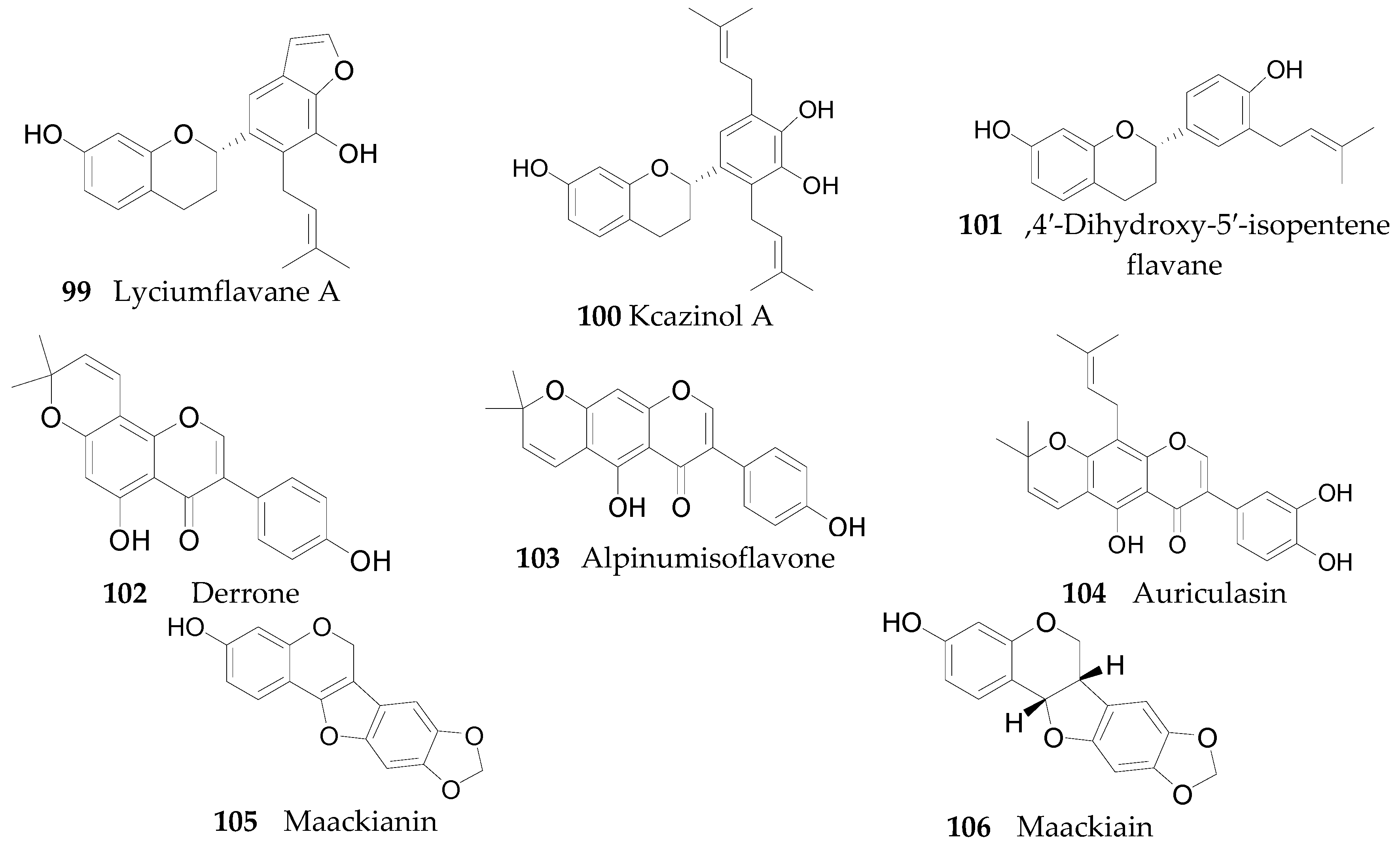
2.2.5. Flavonoids 75–106
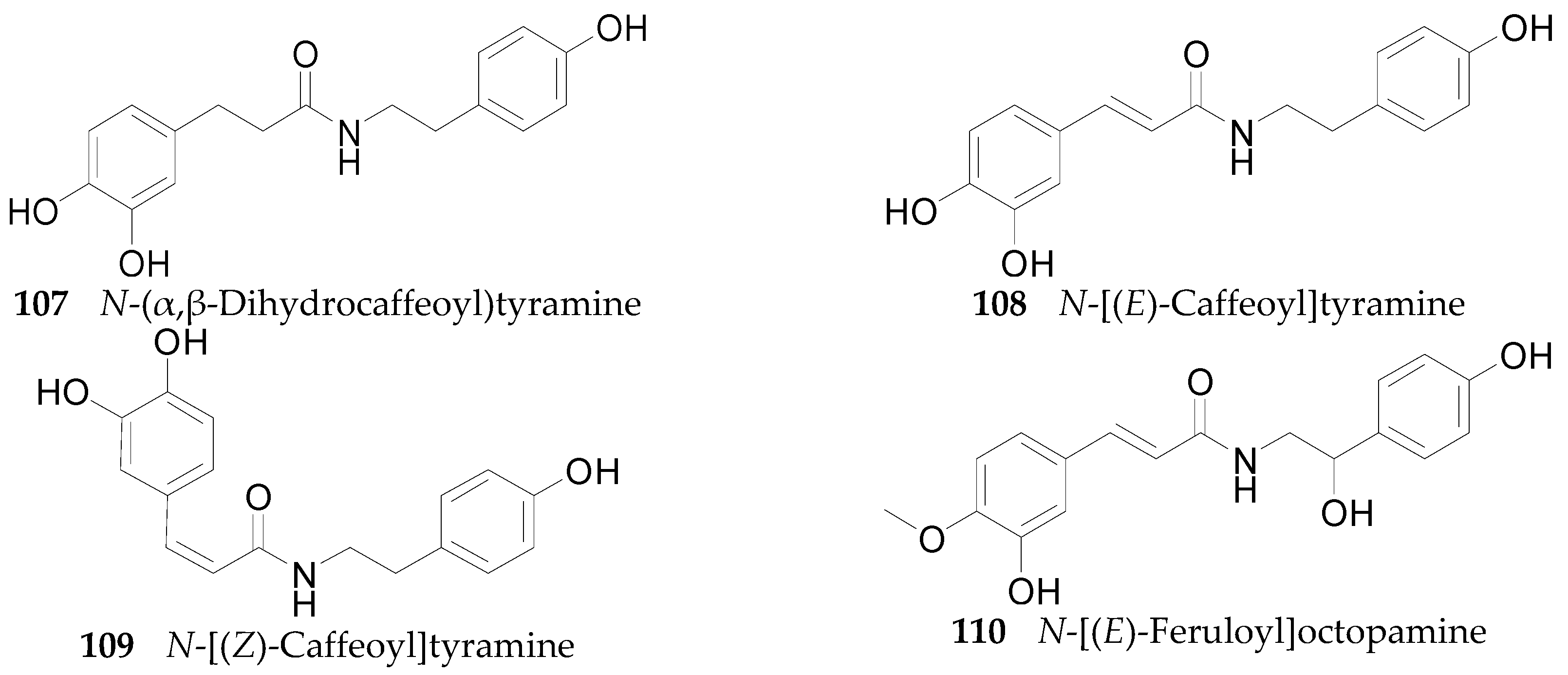
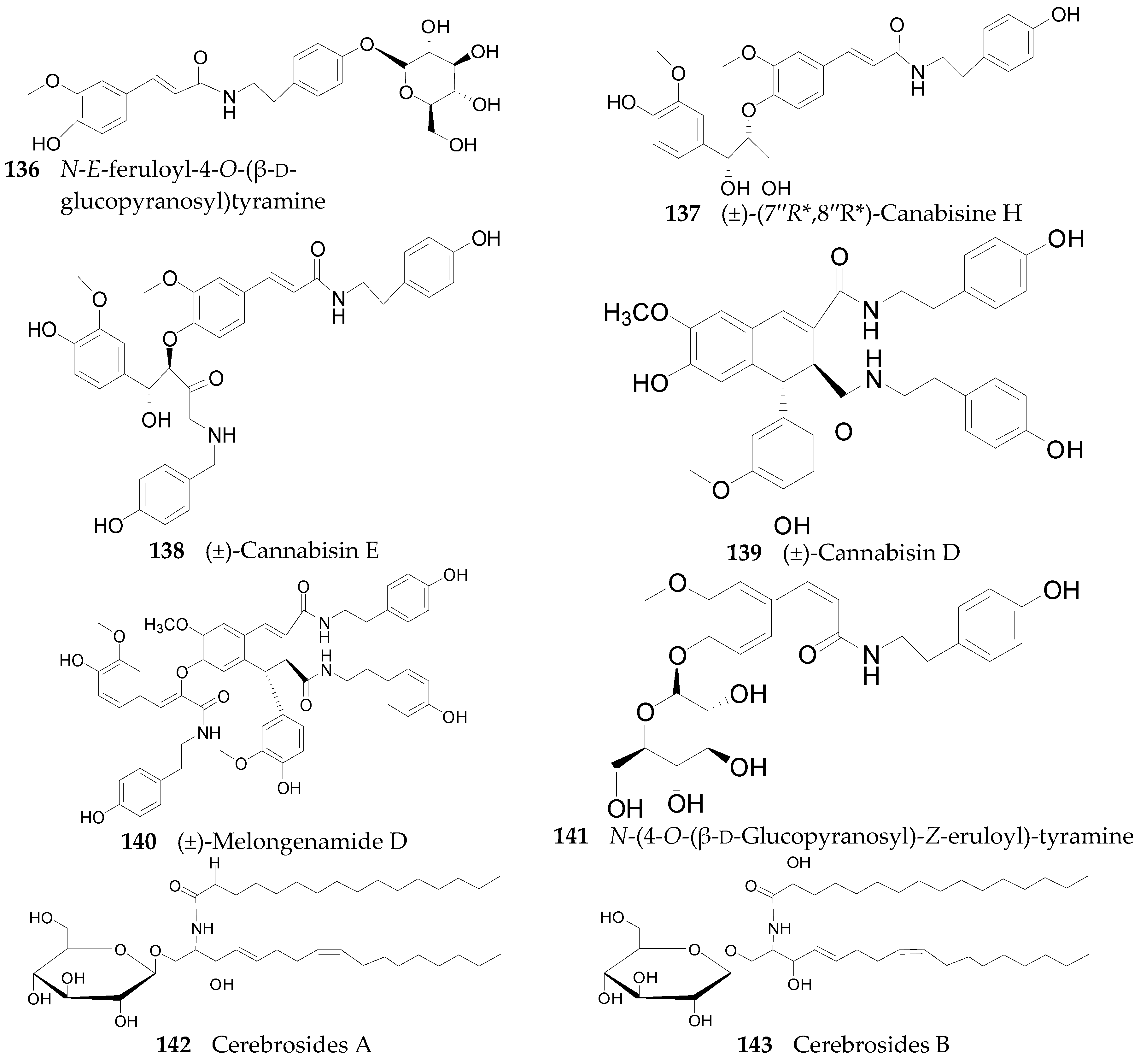
2.2.6. Amides 107–143
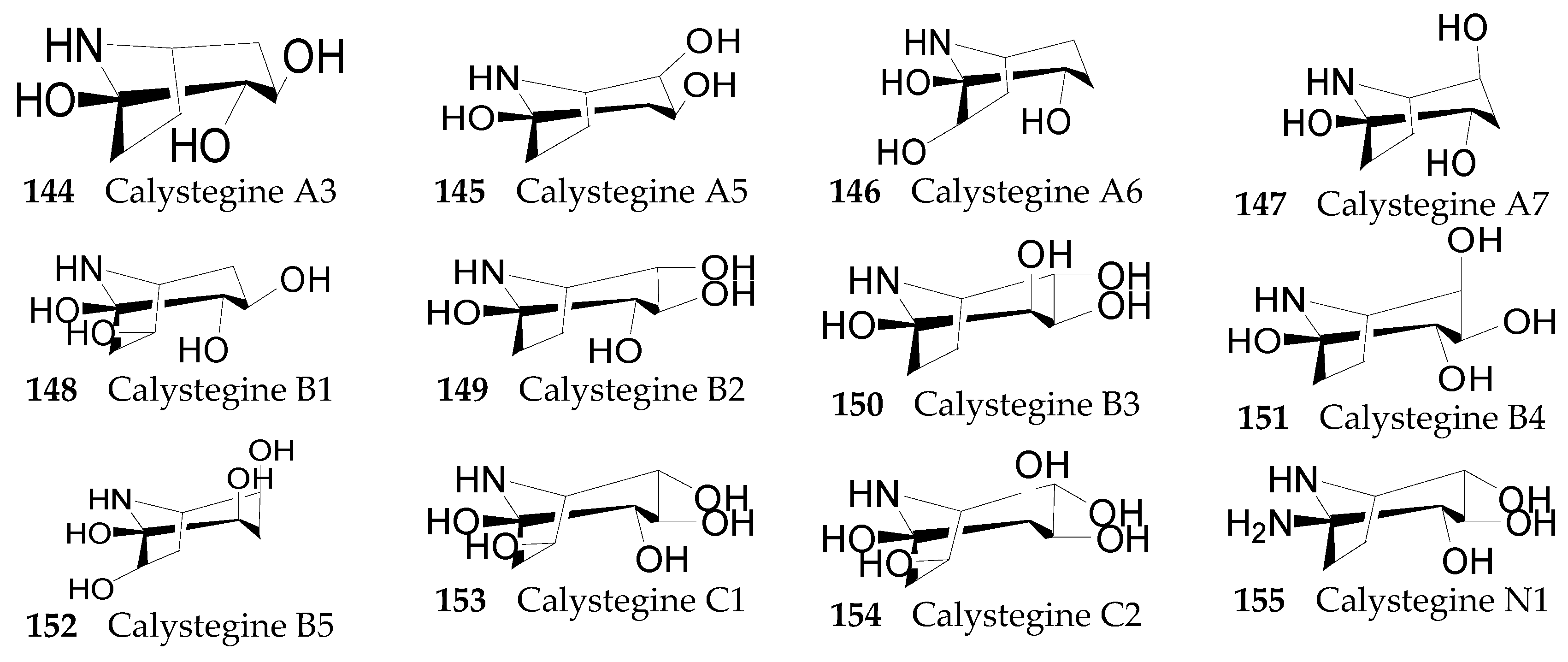
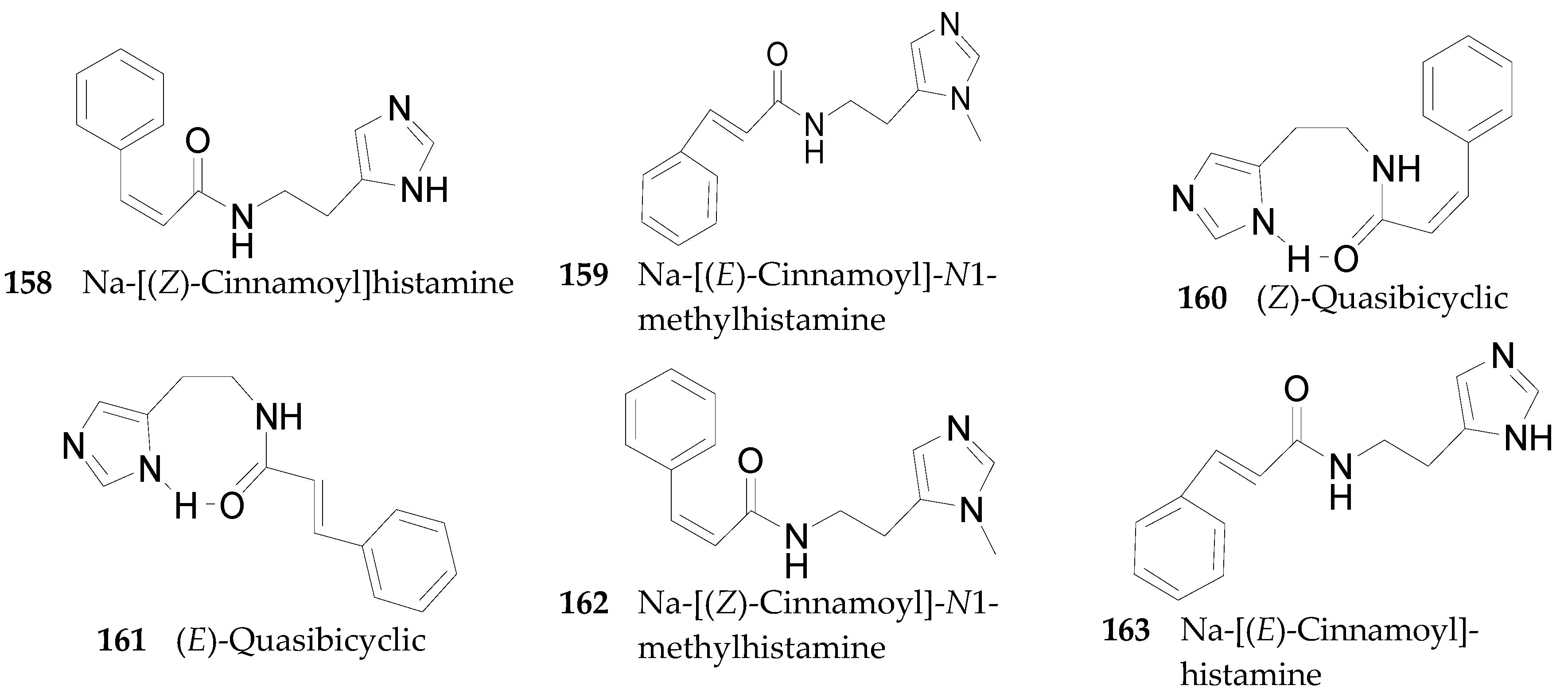
2.2.7. Alkaloids 144–215
Nortropane Alkaloids
Imidazole Alkaloids
Piperidine Alkaloids
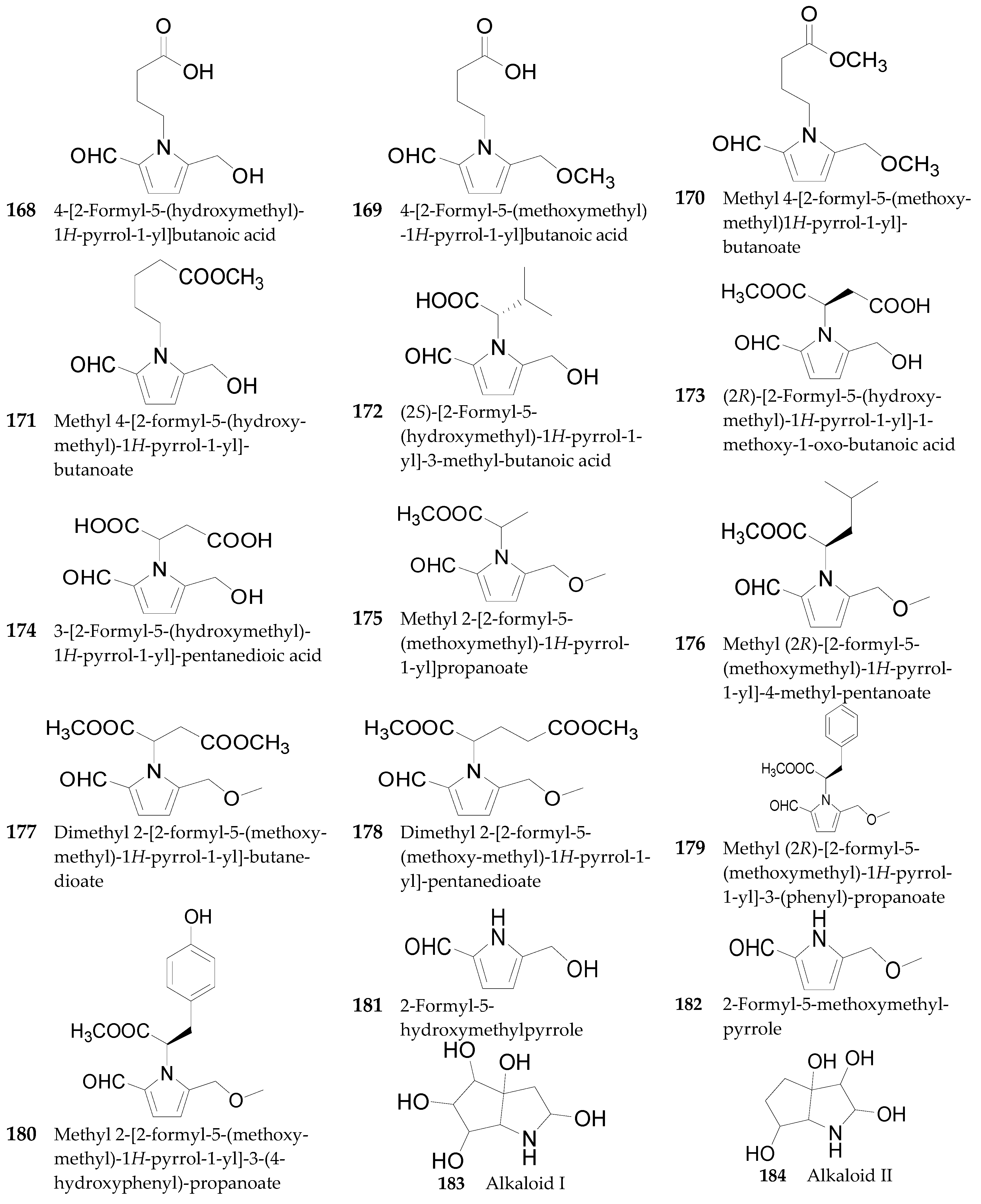
Pyrrole Alkaloids
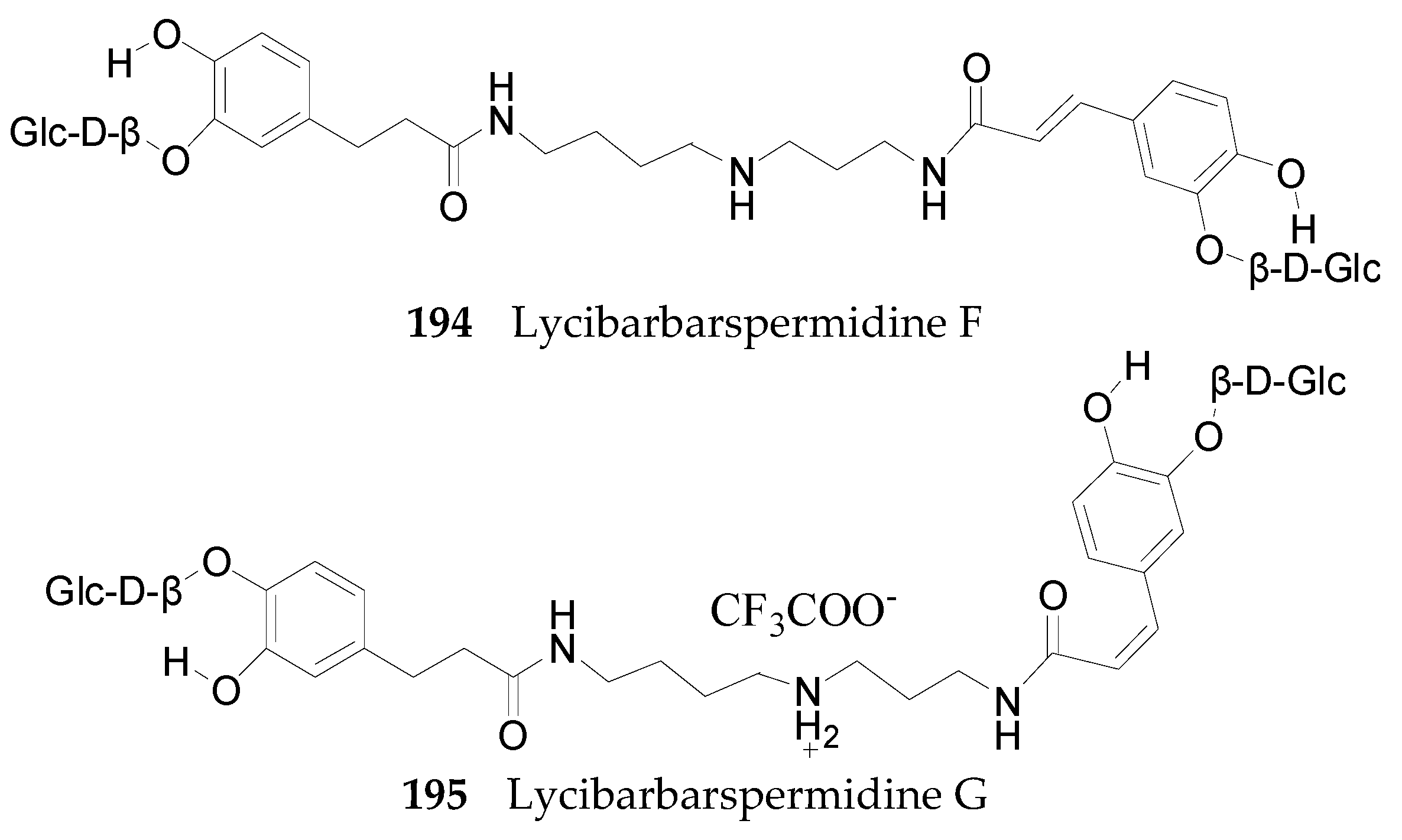
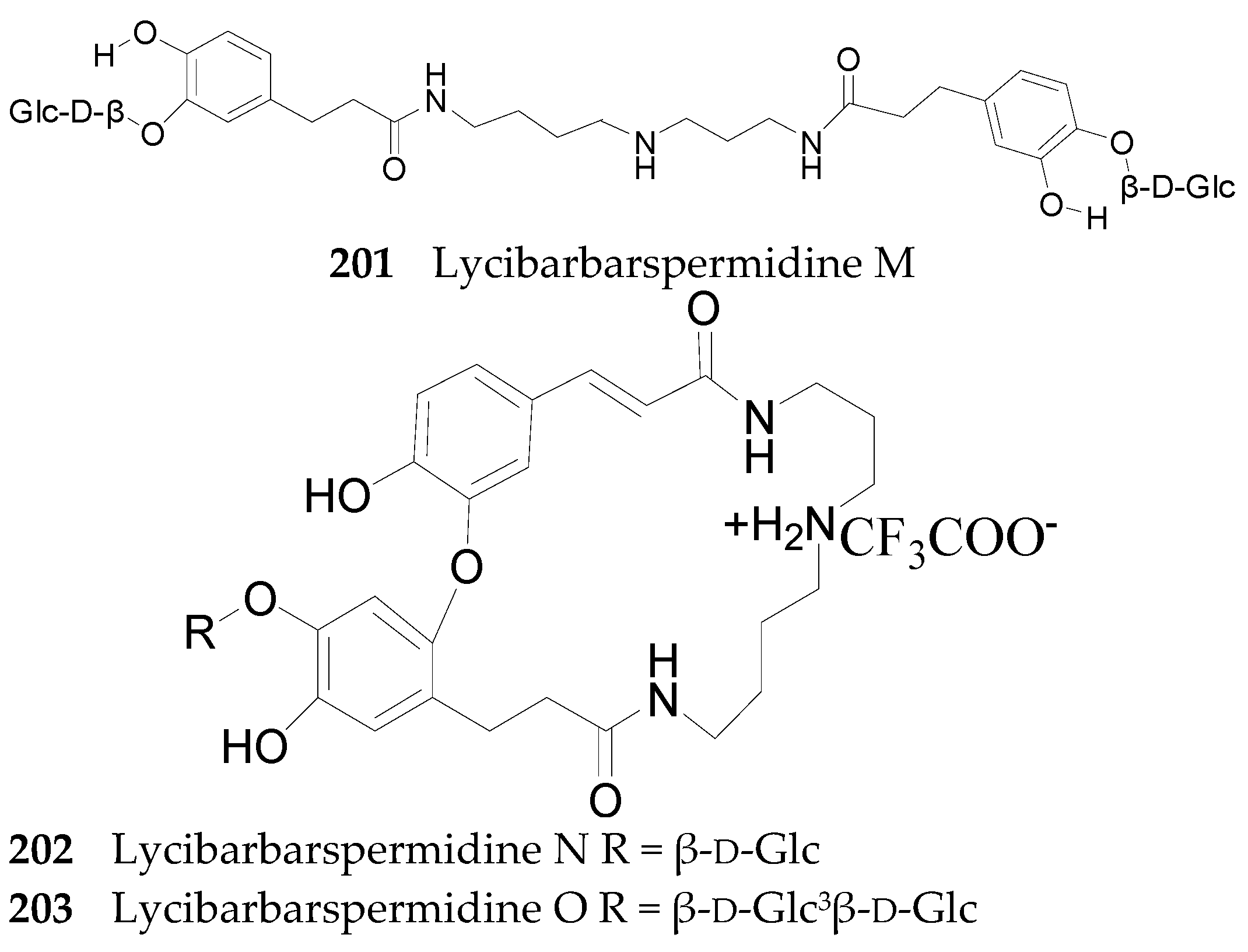
Spermine Alkaloids
Tropane Alkaloids
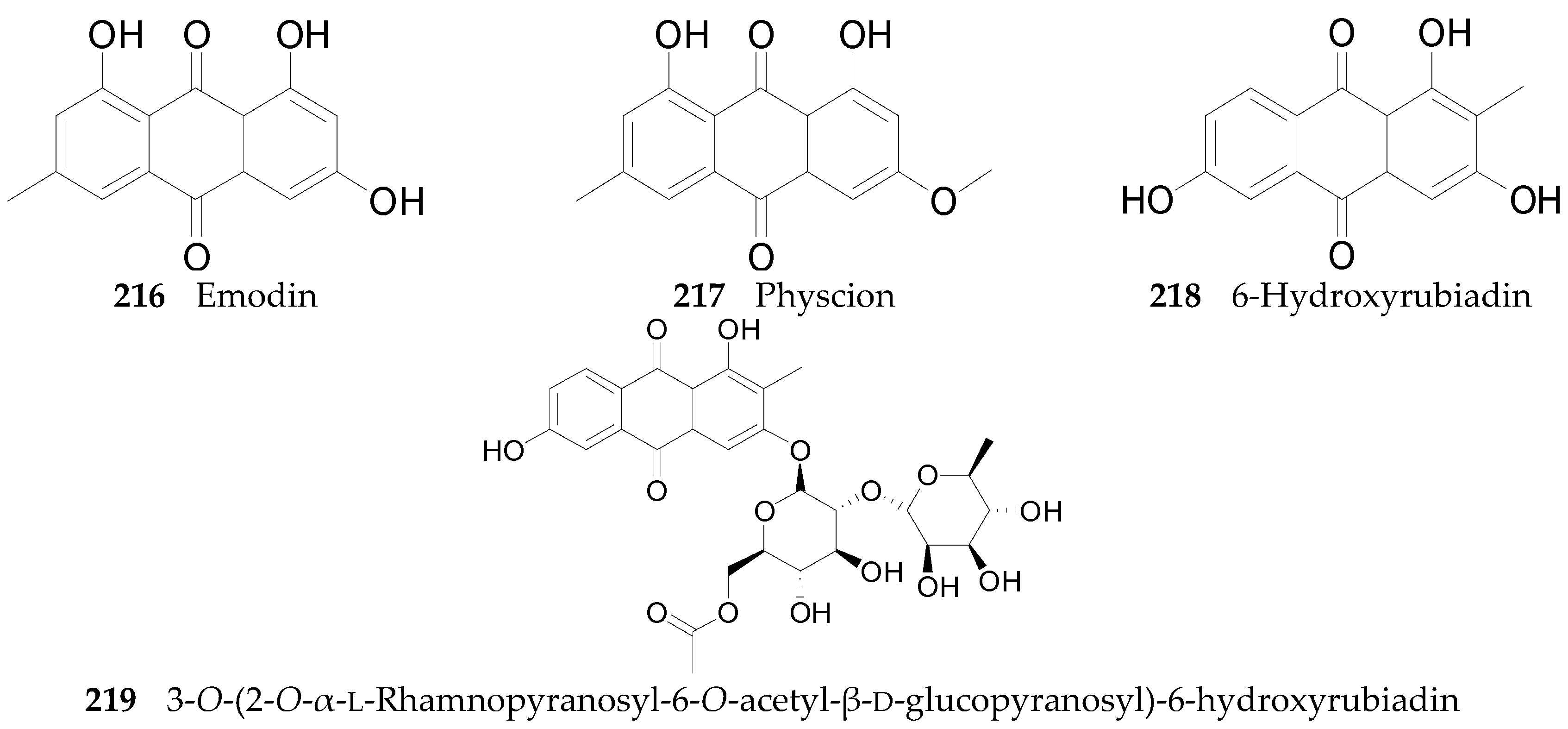
2.2.8. Anthraquinones 216–219
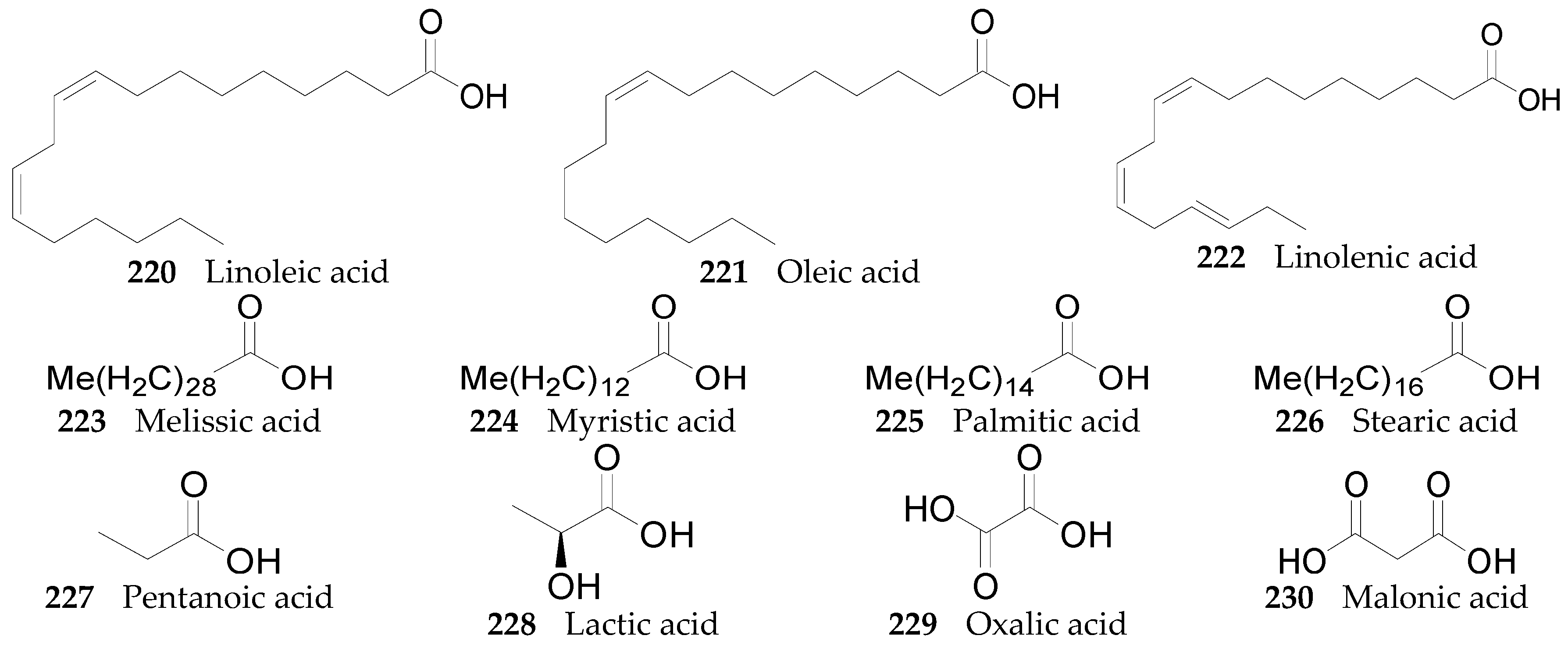
2.2.9. Organic Acids 220–251
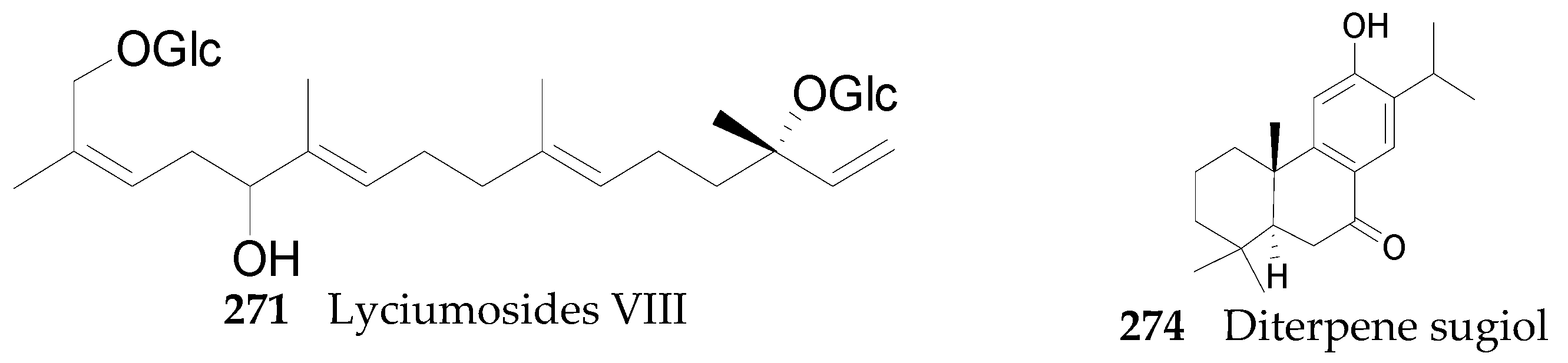
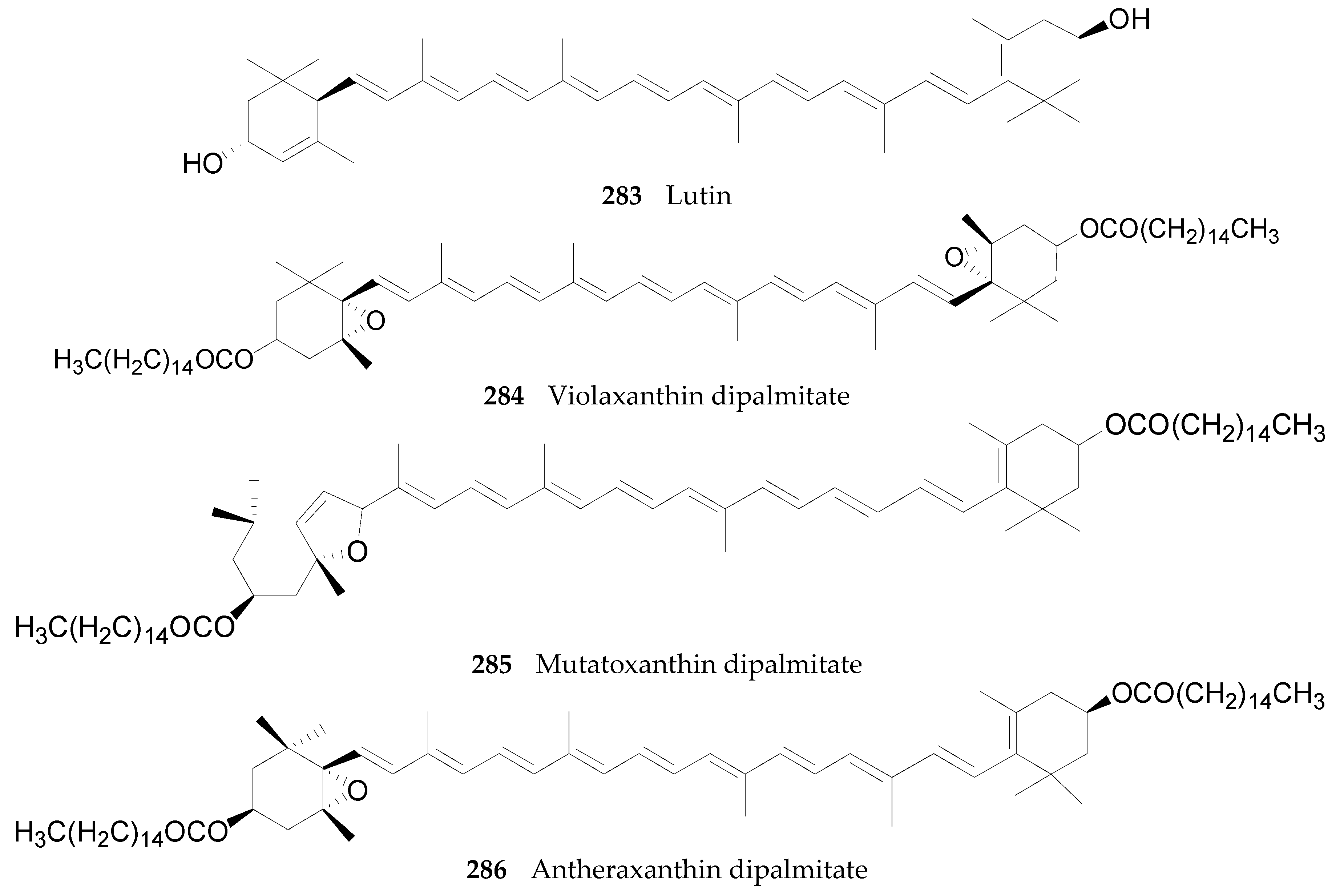
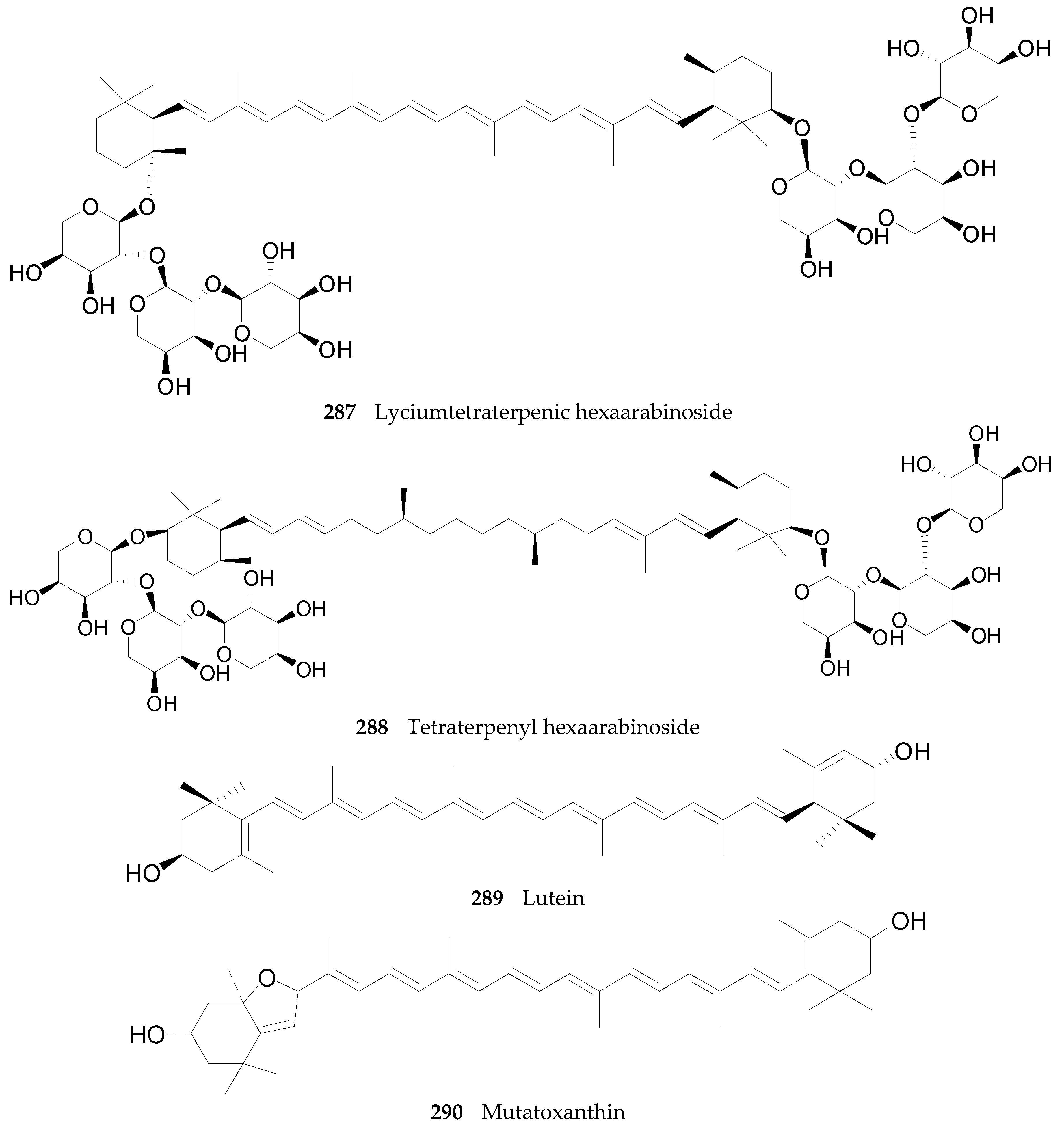
2.2.10. Terpenoids 252–290
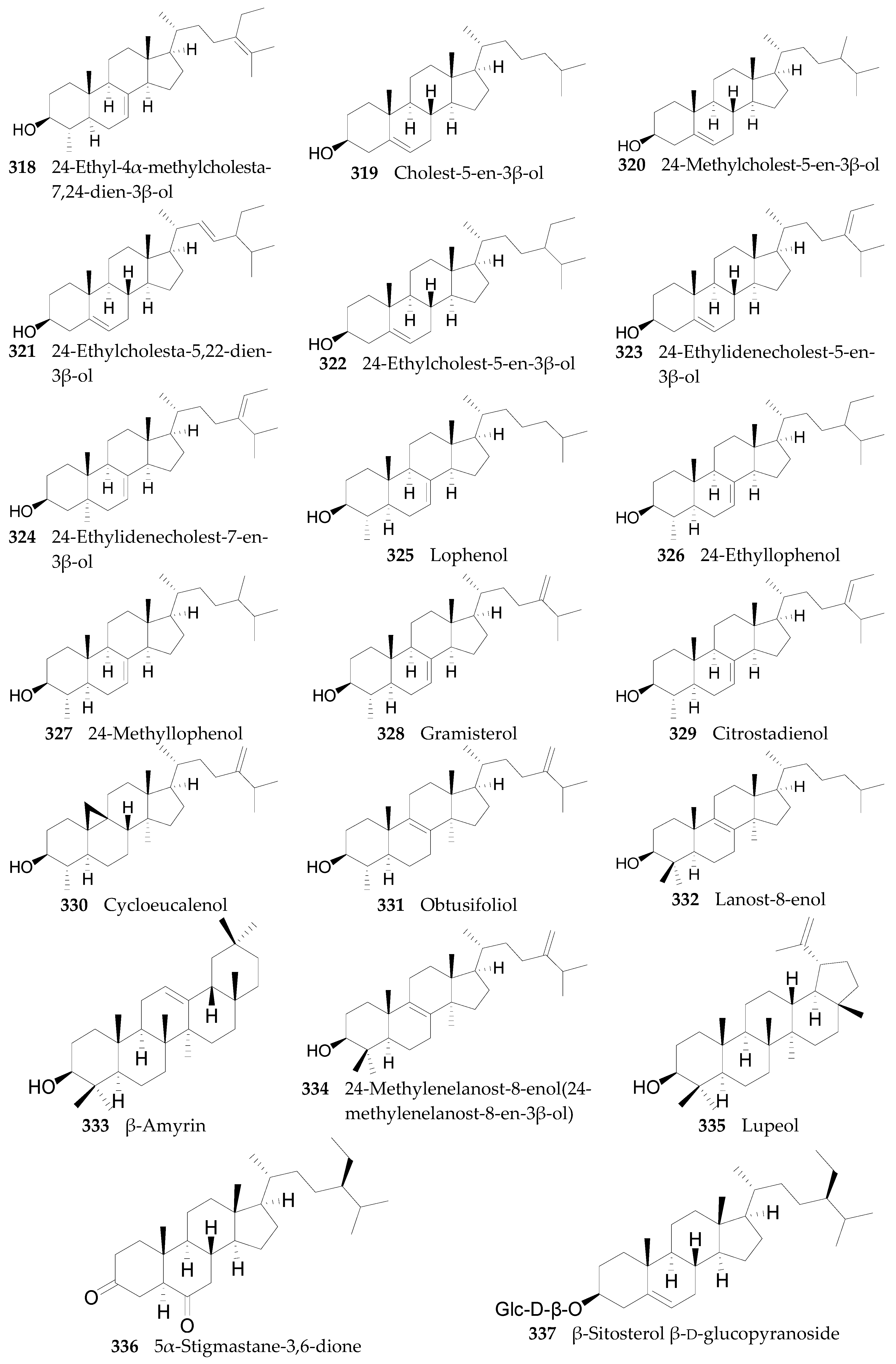
2.2.11. Sterols, Steroids, and Their Derivatives 291–347
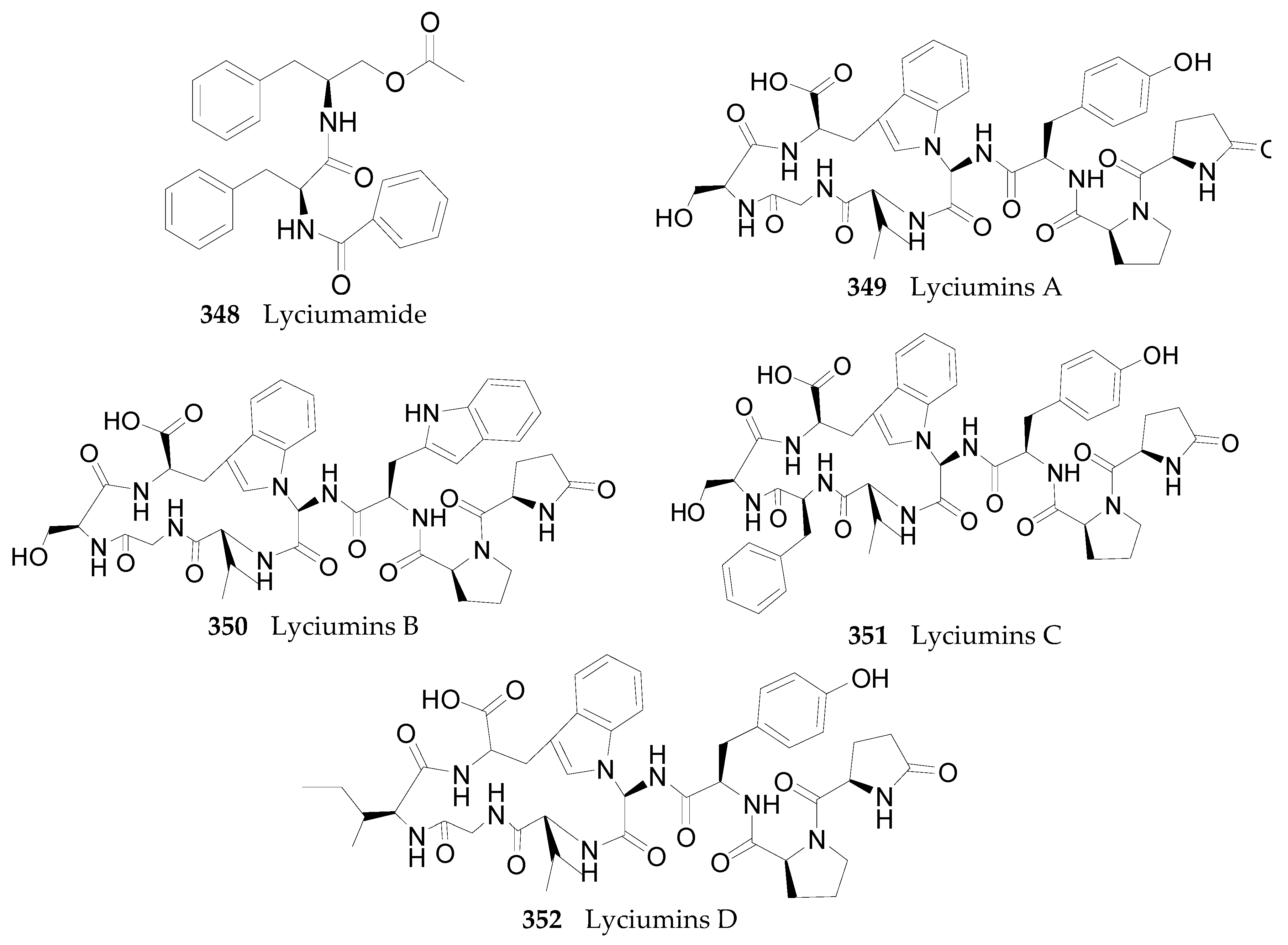
2.2.12. Peptides 348–352
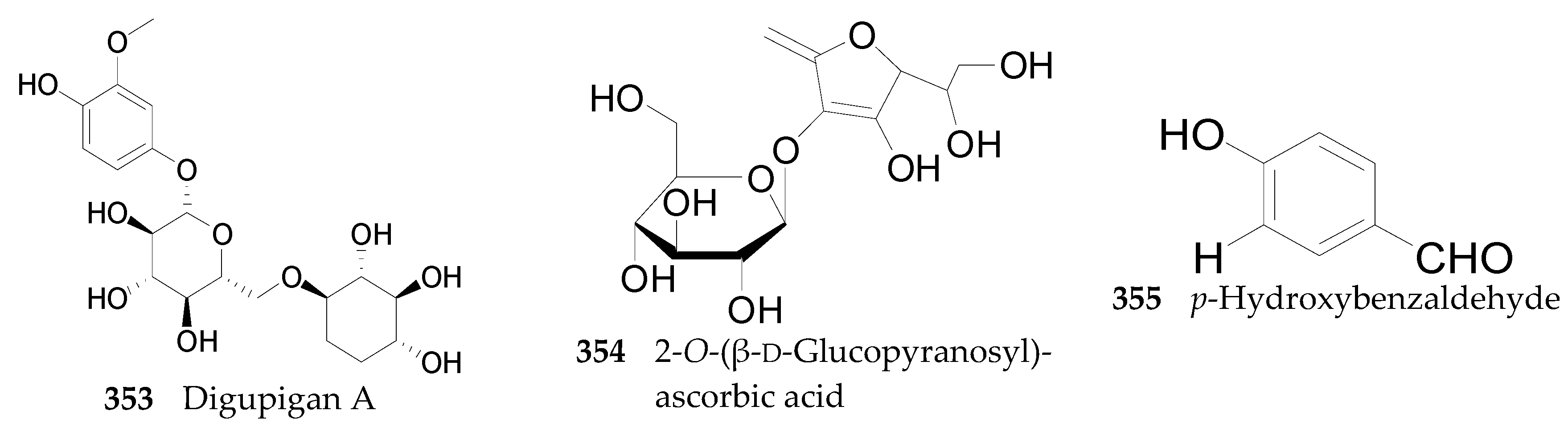
2.2.13. Other Compounds 353–355
3. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Z.Y.; Lu, A.M.; D‘Arcy, W.G. Flora of China. Volume 17: Verbenaceae through Solanaceae; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 1994; pp. 301–304. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China Part I; Chemical Industry Press: Beijing, China, 2015. [Google Scholar]
- Yuenshan, H.; Yu, M.S.; Suetyi, Y.; Kwokfai, S.; Waihung, Y. Polysaccharides from wolfberry antagonizes glutamate excitotoxicity in rat cortical neurons. Cell. Mol. Neurobiol. 2009, 29, 1233–1244. [Google Scholar]
- Yu, M.S.; Wong, Y.Y.; So, K.F.; Fang, J.N.; Yuen, W.H.; Chang, C.C. New polysaccharide from Nerium indicum protects neurons via stress kinase signaling pathway. Brain Res. 2007, 1153, 221. [Google Scholar] [CrossRef] [PubMed]
- Bie, M.; Lv, Y.; Ren, C.; Xing, F.; Cui, Q.; Xiao, J.; So, K.F. Lycium barbarum polysaccharide improves bipolar pulse current-induced microglia cell injury through modulating autophagy. Cell Trans. 2015, 24, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Ruan, Y.W.; Kau, P.W.; Chiu, K.; Chang, R.C.; Chan, H.H.; So, K.F. Effect of Lycium barbarum (Wolfberry) on alleviating axonal degeneration after partial optic nerve transection. Cell Trans. 2015, 24, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Ho, Y.; So, K.; Yuen, W.; Chang, R. Cytoprotective effects of Lycium barbarum on cultured neurons against reducing stress on the endoplasmic reticulum. Int. J. Mol. Med. 2006, 17, 1157–1161. [Google Scholar] [PubMed]
- Ye, Z.; Huang, Q.; Ni, H.X.; Wang, D. Cortex Lycii Radicis extracts improve insulin resistance and lipid metabolism in obese-diabetic rats. Phytotherapy Res. 2008, 22, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.W.; Atanasov, A.G.; Guo, D.A.; Malainer, C.; Zhang, J.X.; Zehl, M.; Guan, S.H.; Heiss, E.H.; Urban, E.; Dirsch, V.M. Activity-guided isolation of NF-κB inhibitors and PPARγ agonists from the root bark of Lycium chinense Miller. J. Ethnopharmacol. 2014, 152, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kotwani, A. Chinese herbal drugs for the treatment of diabetic retinopathy. J. Pharm. Pharmacol. 2016, 69, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Bounous, G. Goji berry fruit (Lycium spp.): Antioxidant compound fingerprint and bioactivity evaluation. J. Funct. Foods 2015, 18, 1070–1085. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Raimondo, E.; Cerutti, A.K.; Prgomet, Z.; Beccaro, G.L. Influence of applied drying methods on phytochemical composition in fresh and dried goji fruits by HPLC fingerprint. Eur. Food Res. Technol. 2016, 242, 1961–1974. [Google Scholar] [CrossRef]
- Horszwald, A.; Andlauer, W. Characterisation of bioactive compounds in berry juices by traditional photometric and modern microplate methods. J. Berry Res. 2011, 1, 189–199. [Google Scholar]
- Yuan, G.; Ren, J.; Ouyang, X.; Wang, L.; Wang, M.; Shen, X.; Zhang, B.; Zhu, B. Effect of Raw Material, Pressing and Glycosidase on the Volatile Compound Composition of Wine Made From Goji Berries. Molecules 2016, 21, 1324. [Google Scholar] [CrossRef] [PubMed]
- Hongli, J.; Yanfang, L.; Zhimou, G.; Fan, Y.; Jixia, W.; Xiaolong, L.; Xiaojun, P.; Xinmiao, L. High-performance liquid chromatography separation of cis-trans anthocyanin isomers from wild Lycium ruthenicum Murr. employing a mixed-mode reversed-phase/strong anion-exchange stationary phase. J. Agric. Chem. 2015, 63, 500. [Google Scholar]
- Jin, H.; Zhao, J.; Zhou, W.; Shen, A.; Yang, F.; Liu, Y.; Guo, Z.; Zhang, X.; Tao, Y.; Peng, X. Preparative separation of a challenging anthocyanin from Lycium ruthenicum Murr. by two-dimensional reversed-phase liquid chromatography/hydrophilic interaction chromatography. RSC Adv. 2015, 5, 62134–62141. [Google Scholar] [CrossRef]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010, 76, 7. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramzamichałowska, A. Goji berry (Lycium barbarum): Composition and health effects—A review. Pol. J. Food Nutr. Sci. 2016, 66, 67–76. [Google Scholar]
- Wang, Q.; Chen, S.Q.; Zhang, Z.H. Determination of polysaccharide content in medlar. J. China Pharm. Univ. 1991, 22, 67–68. [Google Scholar]
- Wu, D.T.; Lam, S.C.; Cheong, K.L.; Feng, W.; Lin, P.C.; Long, Z.R.; Lv, X.J.; Jing, Z.; Ma, S.C.; Li, S.P. Simultaneous determination of molecular weights and contents of water-soluble polysaccharides and their fractions from Lycium barbarum collected in China. J. Pharm. Biomed. Anal. 2016, 129, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Zhang, X.; Yao, W.; Niu, Y.; Gao, X. Structure characterization and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L. Carbohydr. Polym. 2010, 80, 1161–1167. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Curti, D.; Wang, J.; Dobruchowska, J.M.; Gerwig, G.J.; Kamerling, J.P.; Bucheli, P. Cell wall polysaccharides of Chinese Wolfberry (Lycium barbarum): Part 1. Characterisation of soluble and insoluble polymer fractions. Carbohydr. Polym. 2011, 84, 1344–1349. [Google Scholar] [CrossRef]
- Xie, J.H.; Tang, W.; Jin, M.L.; Li, J.E.; Xie, M.Y. Recent advances in bioactive polysaccharides from Lycium barbarum L. Zizyphus jujuba Mill, Plantago spp. and Morus spp.: Structures and functionalities. Food Hydrocoll. 2016, 60, 148–160. [Google Scholar] [CrossRef]
- Wu, D.T.; Cheong, K.L.; Deng, Y.; Lin, P.C.; Wei, F.; Lv, X.J.; Long, Z.R.; Zhao, J.; Ma, S.C.; Li, S.P. Characterization and comparison of polysaccharides from Lycium barbarum in China using saccharide mapping based on PACE and HPTLC. Carbohydr. Polym. 2015, 134, 12. [Google Scholar] [CrossRef] [PubMed]
- Näf, R.; Velluz, A.; Thommen, W. Isolation of a glucosidic precursor of damascenone from Lycium halimifolium mil. Tetrahedron Lett. 1990, 31, 6521–6522. [Google Scholar] [CrossRef]
- Peng, X.; Tian, G. Structural characterization of the glycan part of glycoconjugate LbGp2 from Lycium barbarum L. Carbohydr. Res. 2001, 331, 95. [Google Scholar] [CrossRef]
- Huang, L.; Lin, Y.; Tian, G.; Ji, G. Isolation, purification and physico-chemical properties of immunoactive constituents from the fruit of Lycium barbarum L. Acta Pharm. Sin. 1998, 33, 512–516. [Google Scholar]
- Huang, L.J.; Tian, G.Y.; Ji, G.Z. Structure elucidation of glycan of glycoconjugate LbGp3 isolated from the fruit of Lycium barbarum L. J. Asian Nat. Prod. Res. 1999, 1, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.M.; Huang, L.J.; Qi, C.H.; Zhang, Y.X.; Tian, G.Y. Studies on chemistry and immuno- modulating mechanism of a glycoconjugate from Lycium barbarum L. Chin. J. Chem. 2001, 19, 1190–1197. [Google Scholar] [CrossRef]
- Peng, X.M.; Qi, C.H.; Tian, G.Y.; Zhang, Y.X. Physico-chemical Properties and Bioactivities of a Glycoconjugate LbGp5B from Lycium barbarum L. Chin. J. Chem. 2010, 19, 842–846. [Google Scholar] [CrossRef]
- Gan, L.; Hua, Z.S.; Liang, Y.X.; Bi, X.H. Immunomodulation and antitumor activity by a polysaccharide-protein complex from Lycium barbarum. Int. Immunopharmacol. 2004, 4, 563. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, R.; He, Y.; Guohui, C. Studies on the chemistry of Gouqi polysaccharides. J. Beijing Med. Univ. 1997, 29, 231–232. [Google Scholar]
- Duan, C.L.; Qiao, S.Y.; Wang, N.L.; Zhao, Y.M.; Qi, C.H.; Yao, X.S. Studies on active polysaccharides from Lycium barbarum. Yaoxue Xuebao 2001, 36, 196–199. [Google Scholar]
- Chen, Z.; Tan, K.H.; Tay, S.S.; Chan, S.H. Activation of T lymphocytes by polysaccharide-protein complex from a Chinese medicinal nutrient, Lycium barbarum L. Int. Immunopharmacol. 2008, 8, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Zhang, X.J.; Han, Z.H.; Yu, H.Y.; Lin, Y.; Zhang, W.G.; Sun, F.H.; Wang, T.J. Extraction, purification of Lycium barbarum polysaccharides and bioactivity of purified fraction. Carbohydr. Polym. 2011, 86, 136–141. [Google Scholar] [CrossRef]
- Zhao, C.J.; He, Y.Q.; Li, R.Z. Chemistry and Pharmacological Activity of Peptidoglycan from Lycium barbaruml. Chin. Chem. Lett. 1996, 7, 1009–1010. [Google Scholar]
- Redgwell, R.J.; Curti, D.; Wang, J.; Dobruchowska, J.M.; Gerwig, G.J.; Kamerling, J.P.; Bucheli, P. Cell wall polysaccharides of Chinese Wolfberry (Lycium barbarum): Part 2. Characterisation of arabinogalactan-proteins. Carbohydr. Polym. 2011, 84, 1075–1083. [Google Scholar] [CrossRef]
- Liu, H.; Fan, Y.; Wang, W.; Liu, N.; Zhang, H.; Zhu, Z.; Liu, A. Polysaccharides from Lycium barbarum leaves: Isolation, characterization and splenocyte proliferation activity. Int. J. Biol. Macromol. 2012, 51, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, W.; Guo, J.; Hu, X.; Gong, G.; Huang, L.; Cao, H.; Wang, Z. Sulphation pattern analysis of chemically sulphated polysaccharide LbGp1 from Lycium barbarum by GC-MS. Food Chem. 2015, 170, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, W.; Yu, J.; Zou, S.; Wang, J.; Yao, W.; Gao, X. Characterization and hypoglycemic effect of a polysaccharide extracted from the fruit of Lycium barbarum L. Carbohydr. Polym. 2013, 98, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liu, Y.; Rui, Z.; Yu, J.; Lu, W.; Pan, C.; Yao, W.; Gao, X. Structure characterization, chemical and enzymatic degradation, and chain conformation of an acidic polysaccharide from Lycium barbarum L. Carbohydr. Polym. 2016, 147, 114–124. [Google Scholar]
- Qin, X.; Yamauchi, R.; Aizawa, K.; Inakuma, T.; Kato, K. Structural features of arabinogalactan-proteins from the fruit of Lycium chinense Mill. Carbohydr. Res. 2001, 333, 79–85. [Google Scholar] [CrossRef]
- Qin, X.E. Isolation and Characteristics of Araban Isolated from the Fruitof Lycium chinense Mill. Food Sci. 2003, 24, 52–56. [Google Scholar]
- Qin, X.; Yamauchi, R.; Aizawa, K.; Inakuma, T.; Kato, K. Isolation and Characterization of Arabinogalactan-protein from the Fruit of Lycium chinense Mill. J. Appl. Glycosci. 2000, 47, 155–161. [Google Scholar] [CrossRef]
- Peng, Q.; Lv, X.; Xu, Q.; Li, Y.; Huang, L.; Du, Y. Isolation and structural characterization of the polysaccharide LRGP1 from Lycium ruthenicum. Carbohydr. Polym. 2012, 90, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, G.; Sun, Y.; Gu, X.; Huang, L.; Wang, Z. Isolation, structural characterization, and immunological activity of a polysaccharide LRLP4-A from the leaves of Lycium ruthenicum. J. Carbohydr. Chem. 2016, 35, 40–56. [Google Scholar] [CrossRef]
- Peng, Q.; Song, J.; Lv, X.; Wang, Z.; Huang, L.; Du, Y. Structural Characterization of an Arabinogalactan-Protein from the Fruits of Lycium ruthenicum. J. Agric. Food Chem. 2012, 60, 9424. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Wang, C.; Cheng, Y.; Huang, L.; Wang, Z. Isolation and structural characterization of a polysaccharide LRP4-A from Lycium ruthenicum Murr. Carbohydr. Res. 2013, 365, 20. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Xu, Q.; Yin, H.; Huang, L.; Du, Y. Characterization of an immunologically active pectin from the fruits of Lycium ruthenicum. Int. J. Biol. Macromol. 2014, 64, 69. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Fan, J.; Sun, Y.; Wu, Y.; Liu, Y.; Sun, W.; Zhang, Y.; Wang, Z. Isolation, structural characterization, and antioxidativity of polysaccharide LBLP5-A from Lycium barbarum leaves. Process Biochem. 2015, 51, 314–324. [Google Scholar] [CrossRef]
- Jung, K.; Chin, Y.W.; Kim, Y.C.; Kim, J. Potentially hepatoprotective glycolipid constituents of Lycium chinense fruits. Arch. Pharm. Res. 2005, 28, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Ali, Z.; Khan, I.A. Glycerogalactolipids from the fruit of Lycium barbarum. Phytochemistry 2008, 69, 2856–2861. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.N.; An, Y.W.; Zhan, Z.L.; Xie, J.; Jiang, J.S.; Feng, Z.M.; Ye, F.; Zhang, P.C. Nine new compounds from the root bark of Lycium chinense and their α-glucosidase inhibitory activity. RSC Adv. 2016, 7, 805–812. [Google Scholar] [CrossRef]
- Msonthi, J.; Toyota, M.; Marston, A.; Hostettmann, K. A Novel Phenolic Glycoside from Mondia whytei. Bull. Chem. Soc. Ethiop. 1989, 5, 618. [Google Scholar]
- Zhou, Z.Q.; Xiao, J.; Fan, H.X.; Yu, Y.; He, R.R.; Feng, X.L.; Kurihara, H.; So, K.F.; Yao, X.S.; Gao, H. Polyphenols from wolfberry and their bioactivities. Food Chem. 2016, 214, 644. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.L.; Wang, S.F.; Zhang, X.X. Chemical constituents in fruits of Lycium barbarum. Chin. Tradit. Herb. Drugs 2013, 44, 265–268. [Google Scholar]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Gheldiu, A.M.; Oprean, R.; Crișan, G. Antioxidant, Antimicrobial Effects and Phenolic Profile of Lycium barbarum L. Flowers. Molecules 2015, 20, 15060–15071. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-N.; Chu, C.; Tong, S.-Q.; Cheng, D.-P.; Yan, J.-Z. A new furolactone-type lignan from Lycium chinense. Nat. Prod. Res. 2013, 27, 750–752. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Lee, H.H.; Lee, I.S.; Moon, Y.H.; Woo, E.R. A new phenolic amide from Lycium chinense Miller. Arch. Pharm. Res. 2002, 25, 433. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.L.; Liang, J.Y. Chemical Study on the Root Barks of Mill. J. China Pharm. Univ. 2002, 33, 271–273. [Google Scholar]
- Zhao, J.; Xu, F.; Ji, T.; Li, J. A New Spermidine from the Fruits of Lycium ruthenicum. Chem. Nat. Compd. 2014, 50, 880–883. [Google Scholar] [CrossRef]
- Xie, C.; Xu, L.Z.; Li, X.M.; Li, K.M.; Zhao, B.H.; Yang, S.L. Studieds on Chemical Constituents in Fruit of Lycium barbarum L. China J. Chin. Mater. Med. 2001, 26, 323. [Google Scholar]
- Ma, J.; Tan, C.; Zhu, D. Glycosidic constituents from Carpesium cernuum L. J. Asian Nat. Prod. Res. 2008, 10, 565. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Lu, H.T. Simultaneous determination of phenolic acids and flavonoids in Lycium barbarum Linnaeus by HPLC-DAD-ESI-MS. J. Pharm. Biomed. Anal. 2009, 51, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Peng, Y.; Xu, L.J.; Li, L.; Wu, Q.L.; Xiao, P.G. ChemInform Abstract: Phytochemical and Biological Studies of Lycium Medicinal Plants. Chem. Biodiv. 2011, 8, 976–1010. [Google Scholar] [CrossRef] [PubMed]
- Le, K.; Chiu, F.; Ng, K. Identification and quantification of antioxidants in Fructus lycii. Food Chem. 2007, 105, 353–363. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Xu, L.Z.; Yang, X.J. Advances in chemical constituents of Lycium. Int. J. Tradit. Chin. Med. 1994, 9–13. [Google Scholar]
- Qian, J. The efficiency of flavonoids in polar extracts of Lycium chinense Mill fruits as free radical scavenger. Food Chem. 2004, 87, 283–288. [Google Scholar] [CrossRef]
- Zou, Y.H. Flavones in Leaves of Lycium chinense. J. Instrum. Anal. 2002, 21, 76–78. [Google Scholar]
- Christen, P.; Kapetanidis, I. Flavonoids from Lycium halimifolium1. Planta Med. 1987, 53, 571. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Hano, Y. Compounds of Broussonetia papyrifera (L.) Vent. 2. Structures of Two New Isoprenylated Flavans, Kazinols A and B. Heterocycles 1985, 23, 2835–2842. [Google Scholar] [CrossRef]
- Lee, D.; Bhat, K.P.L.; Fong, H.H.S.; Farnsworth, N.R.; Pezzuto, J.M.; Kinghorn, A.D. Aromatase Inhibitors from Broussonetia papyrifera. J. Nat. Prod. 2001, 64, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.B.; O‘Neil-Johnson, M.; Williams, A.J.; Wheeler, P.; Pol, R.; Moser, A. Dereplication of natural products using minimal NMR data inputs. Organ. Biomol. Chem. 2015, 13, 9957–9962. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M.; Ward, D. Isoflavone precursors of the pterocarpan phytoalexin maackiain in Trifolium pratense. Phytochemistry 1978, 17, 1751–1754. [Google Scholar] [CrossRef]
- Lee, D.G.; Park, Y.; Kim, M.R.; Jung, H.J.; Seu, Y.B.; Hahm, K.S.; Woo, E.R. Anti-fungal effects of phenolic amides isolated from the root bark of Lycium chinense. Biotechnol. Lett. 2004, 26, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Niitsu, K.; Ikeya, Y.; Sato, T.; Katayama, N.; Fukuyama, K.; CHIN, M.; Taguchi, H.; Mitsuhashi, H. Studies on the Crude Drug Containing the Angiotensin I Converting Enzyme Inhibitors (II): On the Active Principles of Fritillaria verticillata WILLDENOW var. thumber gii BAKER. Jpn. J. Pharmacogn. 1987, 41, 174–179. [Google Scholar]
- Zhang, J.X.; Guan, S.H.; Feng, R.H.; Wang, Y.; Wu, Z.Y.; Zhang, Y.B.; Chen, X.H.; Bi, K.S.; Guo, D.A. Neolignanamides, lignanamides, and other phenolic compounds from the root bark of Lycium chinense. J. Nat. Prod. 2013, 76, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Yahara, S.; Shigeyama, C.; Ura, T.; Wakamatsu, K.; Yasuhara, T.; Nohara, T. Cyclic Peptides, Acyclic Diterpene Glycosides and Other Compounds from Lycium chinense Mill. Chem. Pharm. Bull. 1993, 41, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Zhao, Q.; Chen, C.X. The Structure of Lyciumide A. Acta Bot. Yunnanica 1999, 21, 378–380. [Google Scholar]
- Zhou, Z.Q. Studies on Bioactive Constituents of Lycium Barbarum for the Treatment of Alzheimer’s Disease; Jinan University: Guangzhou, China, 2016. [Google Scholar]
- Kim, D.K.; Lim, J.P.; Jin, W.K.; Park, H.W.; Eun, J.S. Antitumor and antiinflammatory constituents from celtis sinensis. Arch. Pharm. Res. 2005, 28, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Kai, G.; Ma, D.; Yan, C.; Tian, X.; Lu, Y.; Du, X.; Tang, H.; Chen, J. Three New Dimers and Two Monomers of Phenolic Amides from the Fruits of Lycium barbarum and Their Antioxidant Activities. J. Agric. Food Chem. 2015, 63, 1067–1075. [Google Scholar]
- Ma, J.; Jones, S.H.; Hecht, S.M. Phenolic acid amides: A new type of DNA strand scission agent from Piper caninum. Bioorgan. Med. Chem. Lett. 2004, 12, 3885–3889. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Hui, Y.; Rupprecht, J.K.; Mclaughlin, J.L.; Wood, K.V. Additional bioactive compounds and trilobacin, a novel highly cytotoxic acetogenin, from the bark of Asimina triloba. J. Nat. Prod. 1992, 55, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.H.; Huang, X.S.; Ye, W.C.; Zhou, G.X. Study on Chemical Constituents of Alocasia macrorrhiza (L.) Schott. Chin. Pharm. J. 2012, 47, 1029–1031. [Google Scholar]
- Li, Y.Z.; Tong, A.P.; Huang, J. Two New Norlignans and a New Lignanamide from Peperomia tetraphylla. Chem. Biodiv. 2012, 9, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Choi, Y.H.; Huh, H.; Kim, J.; Kim, Y.C.; Lee, H.S. New antihepatotoxic cerebroside from Lycium chinense fruits. J. Nat. Prod. 1997, 60, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Asano, N.; Kato, A.; Miyauchi, M.; Kizu, H.; Tomimori, T.; Matsui, K.; Nash, R.J.; Molyneux, R.J. Specific alpha-galactosidase inhibitors, N-methylcalystegines—Structure/activity relationships of calystegines from Lycium chinense. Eur. J. Biochem. 1997, 248, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Chiale, C.A.; Cabrera, J.; Juliani, H.R. NαCinnamoylhistamine derivates from Lycium cestroides. Phytochemistry 1990, 29, 688–689. [Google Scholar] [CrossRef]
- Li, J.; Pan, L.; Naman, C.B.; Deng, Y.; Chai, H.; Keller, W.J.; Kinghorn, A.D. Pyrrole Alkaloids with Potential Cancer Chemopreventive Activity Isolated from a Goji Berry-Contaminated Commercial Sample of African Mango. J. Agric. Food Chem. 2014, 62, 5054–5060. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.W.; Lim, S.W.; Kim, S.H.; Shin, D.Y.; Suh, Y.G.; Kim, Y.B.; Kim, Y.C.; Kim, J. Hepatoprotective pyrrole derivatives of Lycium chinense fruits. Bioorgan. Med. Chem. Lett. 2003, 34, 79–81. [Google Scholar] [CrossRef]
- Youn, U.J.; Lee, J.Y.; Kil, Y.-S.; Han, A.-R.; Chae, C.H.; Ryu, S.Y.; Seo, E.-K. Identification of new pyrrole alkaloids from the fruits of Lycium chinense. Arch. Pharm. Res. 2015, 39, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Joung Youn, U.; Kil, Y.S.; Nam, J.W.; Jin Lee, Y.; Kim, J.; Lee, D.; Lee, J.H.; Seo, E.K. New Pyrrole Alkaloids with Bulky N-Alkyl Side Chains Containing Stereogenic Centers from Lycium chinense. Helv. Chim. Acta 2013, 96, 1482–1487. [Google Scholar] [CrossRef]
- Yamada, H.; Nagai, T.; Abayomi, S.A.; Otani, I. New Alkaloid and Glucosidase Inhibitor Comprising the Same Alkaloid as Active Ingredient. 04-208264. 1992, 7, 29. [Google Scholar]
- Funayama, S.; Yoshida, K.; Konno, C.; Hikino, H. Structure of kukoamine A, a hypotensive principle of Lycium chinense root barks1. Tetrahedron Lett. 1980, 21, 1355–1356. [Google Scholar] [CrossRef]
- Funayama, S.; Zhang, G.R.; Nozoe, S. Kukoamine B, a spermine alkaloid from Lycium chinense. Phytochemistry 1995, 38, 1529–1531. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, S.; Sun, J.; Liu, T.; Chen, P.; Feng, R.; Chen, X.; Wu, W.; Yang, M.; Guo, D.A. Characterization and profiling of phenolic amides from Cortex Lycii by ultra-high performance liquid chromatography coupled with LTQ-Orbitrap mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-Q.; Fan, H.-X.; He, R.-R.; Xiao, J.; Tsoi, B.; Lan, K.-H.; Kurihara, H.; So, K.-F.; Yao, X.-S.; Gao, H. Lycibarbarspermidines A–O, new dicaffeoylspermidine derivatives from wolfberry, with activities against Alzheimer’s disease and oxidation. J. Agric. Food Chem. 2016, 64, 2223–2237. [Google Scholar] [CrossRef] [PubMed]
- Harsh, M.L. Tropane alkaloids from Lycium barbarum Linn. in vivo and in vitro. Curr. Sci. 1989, 58, 817–818. [Google Scholar]
- Adams, M.; Wiedenmann, M.; Tittel, G.; Bauer, R. HPLC-MS trace analysis of atropine in Lycium barbarum berries. Phytochem. Anal. 2006, 17, 279. [Google Scholar] [CrossRef] [PubMed]
- Kokotkiewicz, A.; Migas, P.; Stefanowicz, J.; Luczkiewicz, M.; Krauze-Baranowska, M. Densitometric TLC analysis for the control of tropane and steroidal alkaloids in Lycium barbarum. Food Chem. 2016, 221, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Yong, P. Pharmacognostical Study of Lycium Species. Ph.D. Thesis, Hong Kong Baptist University, Hong Kong, China, 2005. [Google Scholar]
- Han, B.H.; Park, J.H.; Park, M.H.; Han, Y.N. Studies on the alkaloidal components of the fruits of Lycium chinense. Arch. Pharm. Res. 1985, 8, 249–252. [Google Scholar] [CrossRef]
- Noma, M.; Noguchi, M. Occurrence of nicotianamine in higher plants. Phytochemistry 1976, 15, 1701–1702. [Google Scholar] [CrossRef]
- He, J.; Yan, C.T.; Liang, Y.X. A Survey of Chemical Constituents of Lycium barbarum Fruit. Chin. Wild Plant Resour. 1997, 1, 8–11. [Google Scholar]
- Chen, G.; Huo, Y.; Tan, D.-X.; Liang, Z.; Zhang, W.; Zhang, Y. Melatonin in Chinese medicinal herbs. Life Sci. 2003, 73, 19–26. [Google Scholar] [CrossRef]
- Li, Y.B.; Li, P.; Tu, T.F.; Chang, H.T. Isolation and identification of chemical constituents Digupi. Chin. Tradit. Herb. Drugs 2004, 35, 1100–1101. [Google Scholar]
- Wang, Q.; Yue, Y.; He, S.P.; Chen, Y.Y. Chemical Constituents of the Fruit of Licium barbarum L. J. Chin. Pharm. Sci. 1998, 7, 218–220. [Google Scholar]
- Altintas, A.; Kosar, M.; Kirimer, N.; Baser, K.H. C.; Demirci, B. Composition of the essential oils of Lycium barbarum and L. ruthenicum fruits. Chem. Nat. Compd. 2006, 42, 24–25. [Google Scholar] [CrossRef]
- Zhu, X.; Dong, X.; Wang, Y.; Peng, J.; Luo, S. Phenolic compounds from Viburnum cylindricum. Helv. Chim. Acta 2005, 88, 339–342. [Google Scholar] [CrossRef]
- Maldoni, B.E.; Dartayet, G. Estudio del extracto de eter de petroleo de la raiz de Lycium chilense. Rev. Latinoam. Quim 1988, 19, 15. [Google Scholar]
- Lee, H.J.; Ahn, H.J.; Kang, C.S.; Choi, J.C.; Choi, H.J.; Lee, K.G.; Kim, J.I.; Kim, H.Y. Naturally occurring propionic acid in foods marketed in South Korea. Food Control 2010, 21, 217–220. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, K.H.; Chang, K.S.; Bock, J.Y.; Jung, M.Y. Taste and flavor compounds in box thorn (Lycium chinense Miller) leaves. Food Chem. 1997, 58, 297–303. [Google Scholar] [CrossRef]
- Noguchi, M.; Mochida, K.; Shingu, T.; Fujitani, K.; Kozuka, M. Sugiol and 5α-Stigmastane-3,6-dione from the Chinese Drug “Ti-ku-p′i” (Lycii radicis cortex). J. Nat. Prod. 1985, 48, 342–343. [Google Scholar] [CrossRef]
- Zhou, X.W.; Xu, G.J.; Wang, Q. Studies on the Chemical Constituents in Roots of Lycium chinense Mill. China J. Chin. Mater. Med. 1996, 21, 675–676. [Google Scholar]
- Aslanov, S.M.; Mamedova, M.É. Fatty-acid composition of the oil of the seeds and flesh with peel of the fruit of Lycium turcomanicum. Chem. Nat. Compd. 1985, 21, 796. [Google Scholar] [CrossRef]
- Aslanov, S.M.; Mamedova, M.É. Fatty-acid composition of the oil of seeds, pulp and peel oil from the fruit of Lycium turcomanicum. Khim. Prir. Soedin. 1985, 8, 835. [Google Scholar]
- Hiserodt, R.D.; Adedeji, J.; John, T.V.; Dewis, M.L. Identification of Monomenthyl Succinate, Monomenthyl Glutarate, and Dimenthyl Glutarate in Nature by High Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Agric. Food Chem. 2004, 52, 3536–3541. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.S. [Chemical constituents of fructus Lycii and folium Lycii (I)—Nutrients in fructus Lycii and folium Lycii]. Zhong Yao Tong Bao 1986, 11, 41. [Google Scholar] [PubMed]
- Li, Z.; Peng, G.H.; Zhang, S.H. Composition and content of carotenoids in Fructus Lycii. J. Plant Resour. Environ. 1999, 8, 57–58. [Google Scholar]
- Inbaraj, B.S.; Lu, H.; Hung, C.F.; Wu, W.B.; Lin, C.L.; Chen, B.H. Determination of carotenoids and their esters in fruits of Lycium barbarum Linnaeus by HPLC–DAD–APCI–MS. J. Pharm. Biomed. Anal. 2008, 47, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Sannai, A.; Fujimori, T.; Katō, K. Isolation of (−)-1,2-dehydro-α-cyperone and solavetivone from Lycium chinense. Phytochemistry 1982, 21, 2986–2987. [Google Scholar] [CrossRef]
- Chung, I.M.; Ali, M.; Kim, E.H.; Ahmad, A. New tetraterpene glycosides from the fruits of Lycium chinense. J. Asian Nat. Prod. Res. 2013, 15, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.; Murata, Y.; Zhu, B.; Shimoishi, Y.; Tada, M. Changes in Carotenoid Content and its Composition during Maturation of Fructus lycii Fruits. Jpn. J. Food Chem. 2005, 12, 35–39. [Google Scholar]
- Sannai, A.; Fujimori, T.; Uegaki, R.; Akaki, T. Isolation of 3-hydroxy-7,8-dehydro-beta-ionone from Lycium chinense M. Agric. Biol. Chem. 1984, 48, 1629–1630. [Google Scholar] [CrossRef]
- Terauchi, M.; Kanamori, H.; Nobuso, M.; Yahara, S.; Nohara, T. Detection and determination of antioxidative components in Lycium chinense. Shoyakugaku Zashhi 1997, 51, 387–391. [Google Scholar]
- Wang, Y.; Zhao, B.; Ma, H.R.; Aisa, H.A. Two new sesquiterpenoid glycosides from the leaves of Lycium barbarum. J. Asian Nat. Prod. Res. 2016, 18, 871. [Google Scholar] [CrossRef] [PubMed]
- Harsh, M.L.; Nag, T.N. Diosgenin and phytosterols from Lycium barbarium Linn. NAG Actuar. Sci. 1981, 50, 235. [Google Scholar]
- Maldoni, B.E. Sterols in the fruits of Lycium chilense. Fitoterapia 1993, 64, 470. [Google Scholar]
- Hänsel, R.; Huang, J.T. Lycium chinense, II: A semiquantitative assay of the withanolides (author’s transl). Arch. Pharm. 1977, 310, 35–38. [Google Scholar] [CrossRef]
- Hansel, R.; Huang, J.T.; Rosenberg, D. Zwei Withanolide aus Lycium chinense. Arch. Pharm. 1975, 308, 653–654. [Google Scholar] [CrossRef]
- Jeong, T.M.; Yang, M.S.; Nah, H.H. Sterol compositions in three solanaceous seed oils. J. Korean Agric. Chem. Soc. 1978, 21, 51–57. [Google Scholar]
- Itoh, T.; Tamura, T.; Matsumoto, T. 4-Desmethylsgerols in the seeds of Solanaceae. Steroids 1977, 30, 425–433. [Google Scholar] [CrossRef]
- Itoh, T.; Tamura, T.; Matsumoto, T. Triterpene alcohols in the seeds of solanaceae. Phytochemistry 1977, 16, 1723–1726. [Google Scholar] [CrossRef]
- Itoh, T.; Ishii, T.; Tamura, T.; Matsumoto, T. Four new and other 4α-methylsterols in the seeds of Solanaceae. Phytochemistry 1978, 17, 971–977. [Google Scholar] [CrossRef]
- Wang, K.; Sasaki, T.; Li, W.; Li, Q.; Wang, Y.; Asada, Y.; Kato, H.; Koike, K. Two novel steroidal alkaloid glycosides from the seeds of Lycium barbarum. Chem. Biodiv. 2011, 8, 2277. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Mochida, K.; Shingu, T.; Kozuka, M.; Fujitani, K. The constituents of the Chinese drug “ti-ku-’pi”. I. Isolation and constitution of Lyciumamide, a new dipeptide. Chem. Pharm. Bull. 1984, 32, 3584. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Liang, F.; Miao, L.; Ye, J. Study on Bioactive Ingredients inCortex Lyciiby Capillary Zone Electrophoresis with Amperometric Detection. Chin. J. Anal. Chem. 2005, 33, 1611–1614. [Google Scholar]
- Toyodaono, Y.; Maeda, M.; Nakao, M.; Yoshimura, M.; Sugiuratomimori, N.; Fukami, H. 2-O-(beta-d-Glucopyranosyl)ascorbic acid, a novel ascorbic acid analogue isolated from Lycium fruit. J. Agric. Food Chem. 2004, 52, 2092–2096. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Q.; Wang, Q.; Gong, S.L.; Wu, J.K.; Yu, X.S.; Lin, S.W. Analysis of Amino Acids in Chinese Wolfberry. J. China Pharm. Univ. 1991, 1, 53–55. [Google Scholar]
- Cheng, G.Y.; Wei, J.C.; Zou, Y.Z.; Wu, G.R.; Lu, L. Purification of Copper and Zinc Superoxide Dismutase from Lycium barbarum Fruit and Its Properties. J. Nanjing Norm. Univ. (Nat. Sci. Ed.) 1991, 14, 82–92. [Google Scholar]

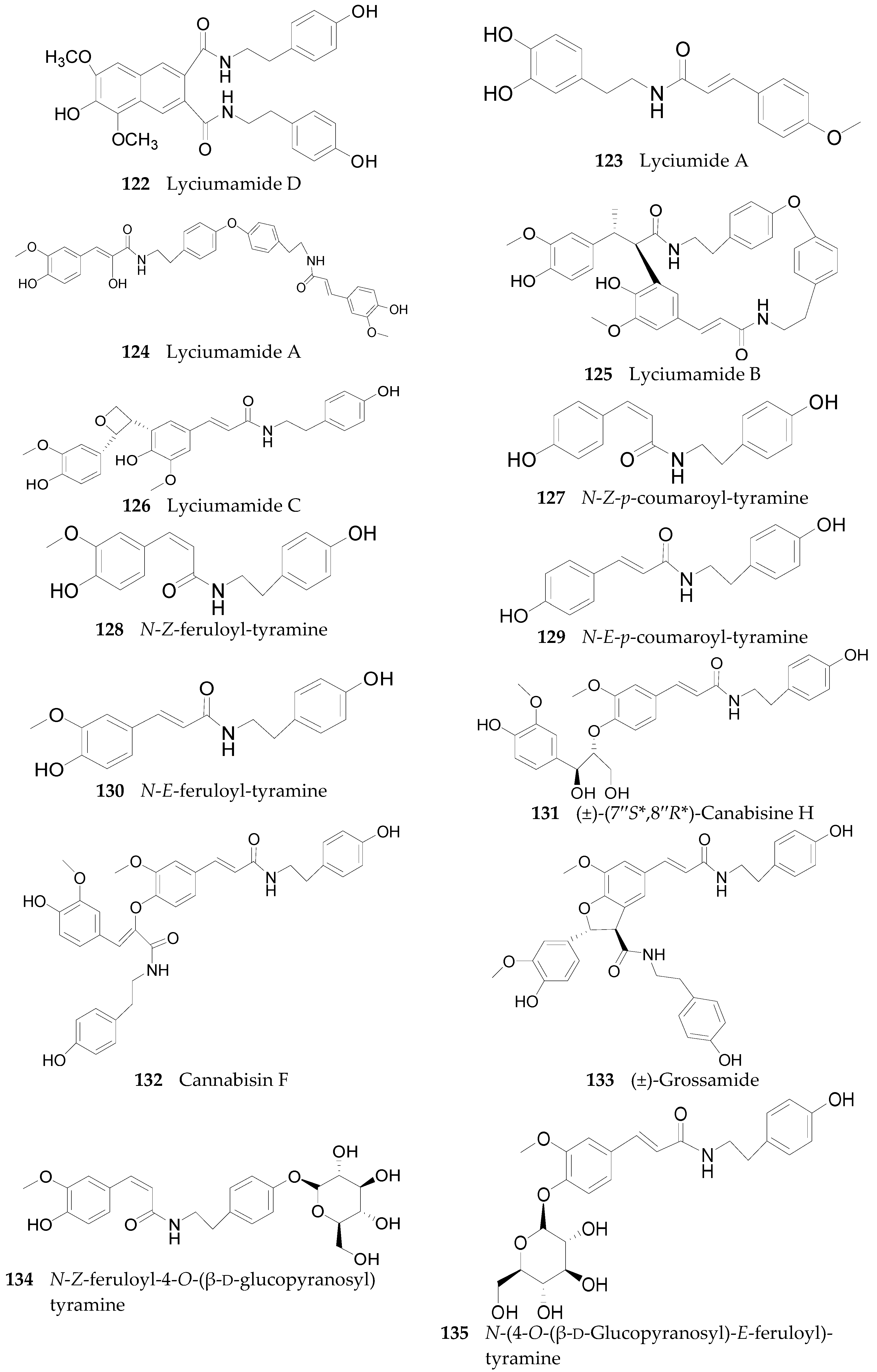






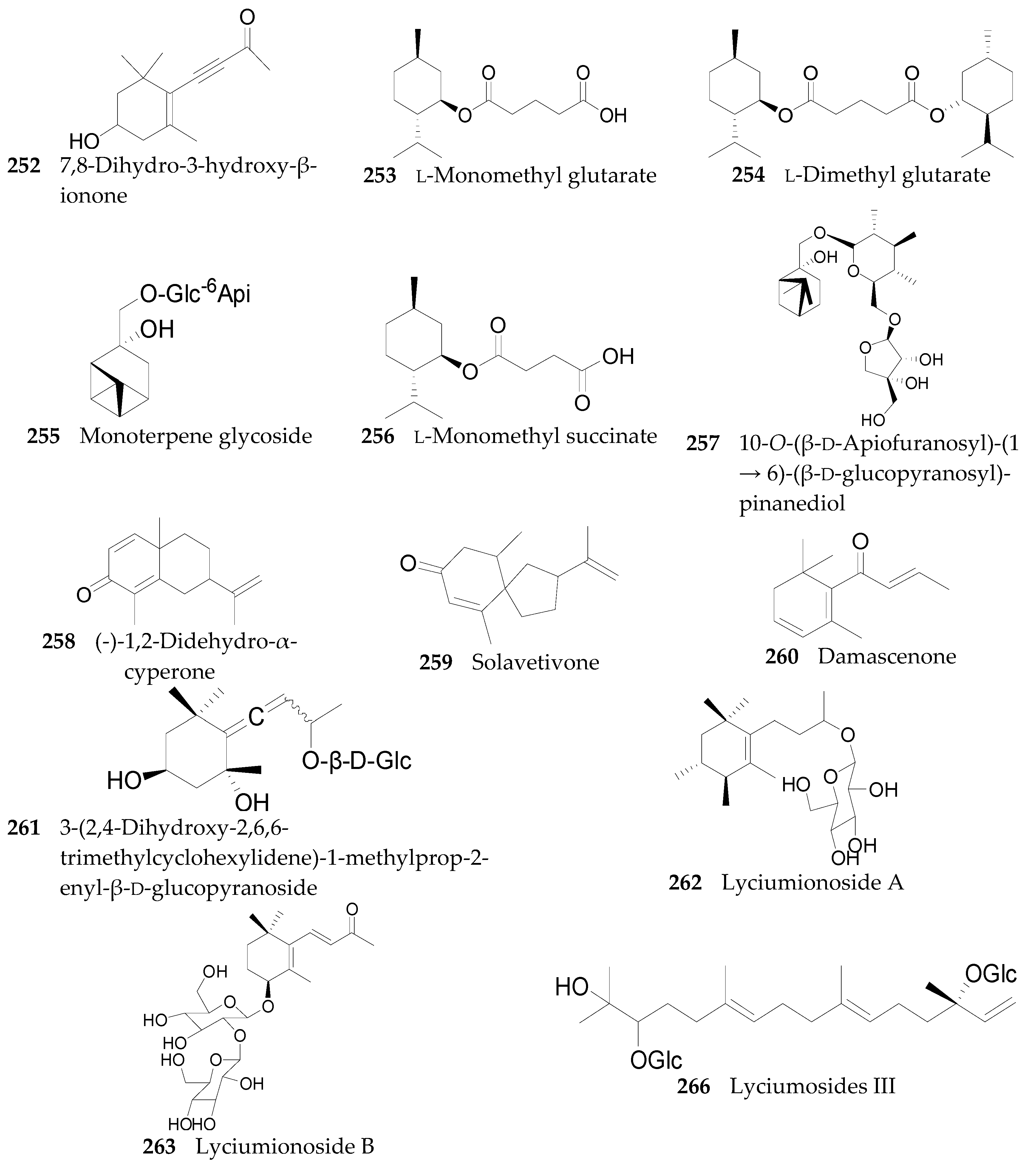

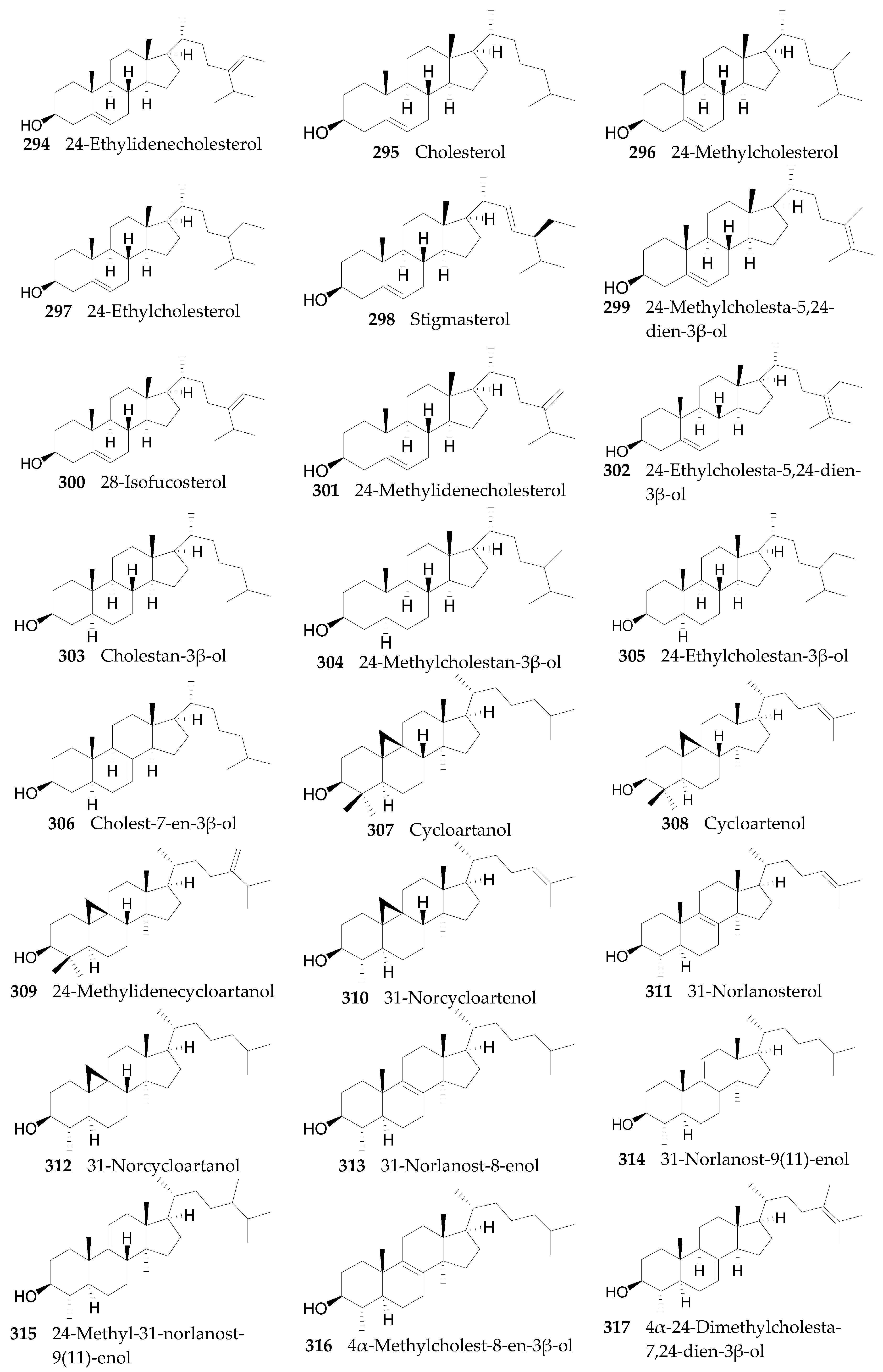

| LBPs | Molar Ratio | Source | Reference |
|---|---|---|---|
| LbGp1 | Ara:Gal:Glc = 2.5:1.0:1.0 | L. barbarum | [26] |
| LbGp2 | Ara:Gal = 4:5 | L. barbarum | [27] |
| LbGp3 | Ara:Gal = 1:1 | L. barbarum | [28,29] |
| LbGp4 | Ara:Gal:Rha:Glc = 1.5:2.5:0.43:0.23 | L. barbarum | [28,30] |
| LbGp5 | Rha:Ara:Xyl:Gal:Man:Glc = 0.33:0.52:0.42:0.94:0.85:1 | L. barbarum | [28] |
| LbGp5B | Rha:Ara:Glc:Gal = 0.1:1:1.2:0.3 | L. barbarum | [31] |
| LBP3p | Rha:Ara:Xyl:Gal:Man:Glc = 1.25:1.10:1.76:1:1.95:2.12 | L. barbarum | [32] |
| LBPC2 | Xyl:Rha:Man = 8.8:2.3:1 | L. barbarum | [33] |
| LBPC4 | Glc | L. barbarum | [33] |
| LBPA1 | heteroglycan | L. barbarum | [33] |
| LBPA3 | heteroglycan | L. barbarum | [33] |
| LBP1a-1 | Glc | L. barbarum | [34] |
| LBP1a-2 | Glc | L. barbarum | [34] |
| LBP3a-1 | GalA | L. barbarum | [34] |
| LBP3a-2 | GalA | L. barbarum | [34] |
| LBPF1 | - | L. barbarum | [35] |
| LBPF2 | - | L. barbarum | [35] |
| LBPF3 | - | L. barbarum | [35] |
| LBPF4 | - | L. barbarum | [35] |
| LBPF5 | Ara, Man, Xyl, Glu, Rha | L. barbarum | [35,36] |
| LBPF6 | - | L. barbarum | [36] |
| LPBC4 | Glc | L. barbarum | [37] |
| LBP-1 | Rha:Ara:Xyl:Gal:Man:GalA = 1:7.85:0.37:0.65:3.01:8.16 | L. barbarum | [22] |
| WSP1 | Rha:Fuc:Ara:Xyl:Man:Gal:Glc = 1.6:0.2:51.4:4.8:1.2:25.9:7.3 | L. barbarum | [23] |
| AGP | Rha:Ara:Xyl:Gal:Glc:GalA:GlcA = 3.3:42.9:0.3:44.3:2.4:7.0 | L. barbarum | [38] |
| LBP-IV | Rha:Ara:Xyl:Glc:Gal = 1.61:3.82:3.44:7.54:1.00 | L. barbarum | [39] |
| LbGp1 | Ara:Gal = 5.6:1 | L. barbarum | [40] |
| LBP-s-1 | Rha:Ara:Xyl:Man:Glu:Gal:Gal A = 1.00:8.34:1.25:1.26:1.91:7.05:15.28 | L. barbarum | [41] |
| p-LBP | Fuc:Rha:Ara:Gal:Glc:Xyl:Gal A:Glc A = 1.00:6.44:54.84:22.98:4.05:2.95:136.98:3.35 | L. barbarum | [42] |
| Cp-2-A | Ara:Gal:Man:Rha:Glu = 6.02:2.71:1.00:0.70:0.67 | L. chinese | [43,44] |
| Cp-2-B | Ara:Gal = 1:0.96 | L. chinese | [43,44] |
| Hp-2-A | Ara:Gal = 5.2:1 | L. chinese | [43,44] |
| Hp-2-B | Ara:Gal = 7.9:1 | L. chinese | [43,44] |
| Hp-2-C | Ara:Gal = 1.2:1 | L. chinese | [43,44] |
| Hp-0-A | Ara:Gal = 14:1 | L. chinese | [43,44] |
| Cp-1-A | Ara:Xyl = 1:1 | L. chinese | [45] |
| Cp-1-B | Ara | L. chinese | [45] |
| Cp-1-C | Ara:Gal = 3:1 | L. chinese | [45] |
| Cp-1-D | Ara:Gal = 1:1 | L. chinese | [45] |
| LRGP1 | Rha:Ara:Xyl:Man:Glu:Gal = 0.65:10.71:0.33:0.67:1:10.41 | L. ruthenicum | [46] |
| LRGP2 | - | L. ruthenicum | [47] |
| LRGP3 | Rha:Ara:Gal = 1.0:14.9:10.4 | L. ruthenicum | [48] |
| LRGP4-A | Rha:Ara:Glu:Gal = 1:7.6:0.5:8.6 | L. ruthenicum | [49] |
| LRGP5 | Rha:Ara:Xyl:Gal:GalA = 1.0:2.2:0.5:1.2:4.7 | L. ruthenicum | [50] |
| LRLP4-A | Rha:Ara:Gal = 1:10.3:5.3 | L. ruthenicum | [47] |
| LBLP5-A | - | L. ruthenicum | [51] |
| No. | Compounds | R1 | R2 | R3 | Source |
|---|---|---|---|---|---|
| 1 | Glycerogalactolipids A | Palmitoyl | Linolenoyl | Linolenoyl | L. barbarum |
| 2 | Glycerogalactolipids B | Palmitoyl | Linolenoyl | Linoleoyl | L. barbarum |
| 3 | Glycerogalactolipids C | Palmitoyl | Linolenoyl | Palmitoyl | L. barbarum |
| 4 | Glycerogalactolipids D | Palmitoyl | Linoleoyl | Palmitoyl | L. barbarum |
| 5 | Glycerogalactolipids E | Palmitoyl | Palmitoyl | Palmitoyl | L. barbarum |
| 6 | Glycerogalactolipids F | Palmitoyl | Palmitoyl | H | L. barbarum |
| 7 | Glycerogalactolipids G | Linolenoyl | Linolenoyl | H | L. barbarum |
| 8 | Glycerogalactolipids H | Linolenoyl | Linoleoyl | H | L. barbarum |
| 9 | Glycerogalactolipids I | Palmitoyl | Linolenoyl | H | L. barbarum |
| 10 | Glycerogalactolipids J | Palmitoyl | Linoleoyl | H | L. barbarum |
| 11 | Glycerogalactolipids K | Palmitoyl | Oleoyl | H | L. barbarum |
| 12 | Glycerogalactolipids L | Stearoyl | Linoleoyl | H | L. barbarum |
| 13 | Glycerogalactolipids M | Palmitoyl | Linolenoyl | – | L. barbarum |
| 14 | Glycerogalactolipids N | Palmitoyl | Linoleoyl | – | L. barbarum |
| 15 | Glycerogalactolipids O | Palmitoyl | Oleoyl | – | L. barbarum |
| 16 | Glycerogalactolipids P | Linolenoyl | Linolenoyl | – | L. chinense |
| 17 | Glycerogalactolipids Q | Linoleoyl | Linolenoyl | – | L. chinense |
| No. | Compounds | R1(R) | R2 | Source |
|---|---|---|---|---|
| 26 | Isoscopoletin | OCH3 | OH | L. barbarum |
| 27 | Scopolin | O-β-d-Glc | OCH3 | L. chinense |
| 28 | Fabiatrin | O-β-d-Glc6-β-d-Xyl | OCH3 | L. chinense |
| No. | Compounds | R1 | R2 | R3 | R4 | Source |
|---|---|---|---|---|---|---|
| 32 | 1-O-E-feruloyl-6-O-β-d-xylopyranosyl-β-d-glucopyranoside | OCH3 | OH | H | COO-β-d-Glc6-β-d-Xyl | L. barbarum |
| 33 | 6-O-E-feruloyl-2-O-β-d-glucopyranosyl-α-d-glucopyranoside | OCH3 | OH | H | COO6-α-d-Glc2-β-d-Glc | L. barbarum |
| 34 | 1-O-E-feruloyl-β-d-glucopyranoside | OCH3 | OH | H | COO-β-d-Glc | L. barbarum |
| 35 | Ethyl-4-O-β-d-glucopyranosyl-E-ferulate | OCH3 | O-β-d-Glc | H | COOCH2CH3 | L. barbarum |
| 36 | Ethyl E-ferulate | OCH3 | OH | H | COOCH2CH3 | L. barbarum |
| 37 | E-sinapinic acid | OCH3 | OH | OCH3 | COOH | L. barbarum |
| 38 | Syringenin | OCH3 | OH | OCH3 | CH2OH | L. barbarum |
| 39 | E-ferulic acid | OCH3 | OH | H | COOH | L. barbarum |
| 40 | Phloretic acid | H | OH | H | COOH | L. barbarum |
| 41 | Dihydroferulic acid | OCH3 | OH | H | COOH | L. barbarum |
| 42 | Ethyl dihydroferulate | OCH3 | OH | H | COOCH2CH3 | L. barbarum |
| 43 | Lycibarbarphenylpropanoids A | H | OH | H | COO-β-d-Glc3-β-d-Glc | L. barbarum |
| 44 | Lycibarbarphenylpropanoids B | H | OH | H | COO-β-d-Glc4-β-d-Glc | L. barbarum |
| 45 | Lycibarbarphenylpropanoids C | OCH3 | OH | H | COO-β-d-Glc3-β-d-Glc | L. barbarum |
| 46 | Lycibarbarphenylpropanoids D | OCH3 | OH | H | COO-β-d-Glc4-β-d-Glc | L. barbarum |
| 47 | Lycibarbarphenylpropanoids E | OCH3 | OH | H | CH2O-β-d-Glc3-β-d-Glc | L. barbarum |
| 48 | Lycibarbarphenylpropanoids F | H | O-β-d-Glc3-β-d-Glc | H | COOCH2CH3 | L. barbarum |
| 49 | Lycibarbarphenylpropanoids G | H | O-β-d-Glc4-β-d-Glc | H | COOCH2CH3 | L. barbarum |
| 50 | Lycibarbarphenylpropanoids H | OCH3 | O-β-d-Glc4-β-d-Glc | H | COOCH2CH3 | L. barbarum |
| 51 | Lycibarbarphenylpropanoids I | O-β-d-Glc | OH | H | COOCH2CH3 | L. barbarum |
| No. | Compounds | R1 | R2 | R3 | R4 | Source |
|---|---|---|---|---|---|---|
| 58 | 6-O-E-p-coumaroyl-2-O-β-d-glucopyranosyl-α-d-glucopyranoside | H | OH | H | COO6-α-d-Glc2-β-d-Glc | L. barbarum |
| 59 | Ethyl-4-O-β-d-glucopyranosyl-E-p-coumarate | H | O-β-d-Glc | H | COOCH2CH3 | L. barbarum |
| 60 | Ethyl E-p-coumarate | H | OH | H | COOCH2CH3 | L. barbarum |
| No. | Compounds | R1 | R2 | R3 | Source |
|---|---|---|---|---|---|
| 62 | Pinoresinol | H | OH | H | L. barbarum |
| 65 | Medioresinol | H | OH | OCH3 | L. barbarum |
| 66 | Syringaresinol | OCH3 | OH | OCH3 | L. barbarum |
| 67 | 4-O-(β-d-glucopyranosyl)syringaresinol | OCH3 | O-β-d-Glc | OCH3 | L. barbarum |
| No. | Compounds | R1 | R2 | R3 | Source |
|---|---|---|---|---|---|
| 75 | Quercitrin | OH | OH | O-α-l-Rha | L. barbarum |
| 76 | Kaempferol | OH | OH | – | L. barbarum |
| 77 | Quercetin | OH | OH | OH | L. barbarum |
| 78 | Rutin | OH | OH | O-β-d-Glc6-α-l-Rha | L. barbarum |
| 79 | Narcissoside | OH | OCH3 | O-β-d-Glc6-α-l-Rha | L. barbarum |
| 80 | 7-O-(β-d-Glucopyranosyl)-rutin | O-β-d-Glc | OH | O-β-d-Glc6-α-l-Rha | L. barbarum |
| 82 | 7-O-(β-d-Glucopyranosyl)-nicotiflorin | O-β-d-Glc | O-β-d-Glc6-α-l-Rha | – | L. barbarum |
| 83 | 7-O-(β-d-Glucopyranosyl)-3-O-[β-d-glucopyranosyl]-(1 → 2)-β-d-galactop | O-β-d-Glc | O-β-d-Glc6-α-l-Glc | – | L. barbarum |
| 85 | Luteolin | OH | OH | OH | L. chinense |
| 86 | Acacetin | OH | H | OCH3 | L. chinense |
| 87 | 7-O-(β-d-Glucopyranosyl)-3-O-[β-d-glucopyranosyl-(1 → 2)-β-d-galactopyranosyl]-quercetin | O-β-d-Glc | OH | O-β-d-Glc2-β-d-Glc | L. chinense |
| 89 | 7-O-[α-l-Rhamno-pyranosyl-(1 → 6)-β-d-glucopyranosyl]-acacetin | O-β-d-Glc6-α-l-Rha | H | OCH3 | L. chinense |
| 90 | 3-O-Sophoroside-quercetin | OH | OH | O-β-d-Glc2-β-d-Glc | L. chinense |
| 91 | Apigenin | OH | H | OH | L. chinense |
| 92 | Isoquercitrin | OH | OH | O-β-d-Glc | L. halimifolium |
| 93 | Nicotiflorin | OH | O-β-d-Glc6-α-l-Rha | – | L. halimifolium |
| No. | Compounds | R1 | R2 | Source |
|---|---|---|---|---|
| 95 | 5-O-(β-d-Glucopyranosyl)-3-O-[4-O-p-E-coumaroyl-α-l-rhamnopyranosyl-(1 → 6)-β-d-glucopyranosyl]-peonidin | H | OH | L. ruthenicum |
| 96 | 5-O-(β-d-Glucopyranosyl)-3-O-[4-O-p-E-coumaroyl-α-l-rhamnopyranosyl-(1 → 6)-β-d-glucopyranosyl]-petunidin | OH | OH | L. ruthenicum |
| 97 | 5-O-(β-d-Glucopyranosyl)-3-O-[4-O-p-Z-coumaroyl-α-l-rhamnopyranosyl-(1 → 6)-β-d-glucopyranosyl]-malvidin | OCH3 | OH | L. ruthenicum |
| 98 | 5-O-(β-d-Glucopyranosyl)-3-O-[4-O-p-E-(β-d-glucopyranoside)-coumaroyl-α-l-rhamnopyranosyl-(1 → 6)-β-d-glucopyranosyl]-petunidin | OH | O-β-d-Glc | L. ruthenicum |
| No. | Compounds | R1 | R2 | R3 | R4 | Source |
|---|---|---|---|---|---|---|
| 189 | Lycibarbarspermidine A | H | β-d-Glc | H | H | L. barbarum |
| 190 | Lycibarbarspermidine B | H | H | β-d-Glc | H | L. barbarum |
| 191 | Lycibarbarspermidine C | β-d-Glc | H | H | H | L. barbarum |
| 192 | Lycibarbarspermidine D | H | H | H | β-d-Glc | L. barbarum |
| 193 | Lycibarbarspermidine E | H | β-d-Glc | β-d-Glc | H | L. barbarum |
| No. | Compounds | R1 | R2 | R3 | R4 | Source |
|---|---|---|---|---|---|---|
| 196 | Lycibarbarspermidine H | H | H | H | β-d-Glc | L. barbarum |
| 197 | Lycibarbarspermidine I | H | β-d-Glc | H | H | L. barbarum |
| 198 | Lycibarbarspermidine J | H | H | β-d-Glc | H | L. barbarum |
| 199 | Lycibarbarspermidine K | β-d-Glc | H | β-d-Glc | H | L. barbarum |
| 200 | Lycibarbarspermidine L | H | β-d-Glc | H | β-d-Glc | L. barbarum |
| No. | Compounds | R1 | R2 | Source |
|---|---|---|---|---|
| 264 | Lyciumosides I | Glc | Glc | L. chinense |
| 265 | Lyciumosides II | Glc2-Glc | Glc | L. chinense |
| 267 | Lyciumosides IV | Glc | Glc4-Rha | L. chinense |
| 268 | Lyciumosides V | Glc6-Rha | Glc | L. chinense |
| 269 | Lyciumosides VI | Glc6-Rha | Glc4-Rha | L. chinense |
| 270 | Lyciumosides VII | Glc2-Rha(6-Glc) | Glc | L. chinense |
| 272 | Lyciumosides IX | Glc | 6-O-malonyl-Glc | L. chinense |
| 273 | Capsianoside II | Rha3-Glc6-Rha | Glc2-Glc | L. chinense |
| No. | Compounds | R1 | R2 | Source |
|---|---|---|---|---|
| 275 | β-Carotene | H | H | L. barbarum |
| 276 | β-Cryptoxanthin | OH | H | L. barbarum |
| 277 | Zeaxanthin | OH | OH | L. barbarum |
| 278 | Zeaxanthin monopalmitate | OCO(CH2)14CH3 | OH | L. barbarum |
| 279 | Zeaxanthin dipalmitate | OCO(CH2)14CH3 | OCO(CH2)14CH3 | L. barbarum |
| 280 | Zeaxanthin monomyristate | OH | OCO(CH2)12CH3 | L. barbarum |
| 281 | Zeaxanthin dimyristate | OCO(CH2)12CH3 | OCO(CH2)12CH3 | L. barbarum |
| 282 | β-Cryptoxanthin palmitate | OCO(CH2)14CH3 | H | L. barbarum |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, D.; Zhao, Y.; Yang, G.; Huang, L. Systematic Review of Chemical Constituents in the Genus Lycium (Solanaceae). Molecules 2017, 22, 911. https://doi.org/10.3390/molecules22060911
Qian D, Zhao Y, Yang G, Huang L. Systematic Review of Chemical Constituents in the Genus Lycium (Solanaceae). Molecules. 2017; 22(6):911. https://doi.org/10.3390/molecules22060911
Chicago/Turabian StyleQian, Dan, Yaxing Zhao, Guang Yang, and Luqi Huang. 2017. "Systematic Review of Chemical Constituents in the Genus Lycium (Solanaceae)" Molecules 22, no. 6: 911. https://doi.org/10.3390/molecules22060911





















