Synthesis and Structural Modification of Marine Natural Products
Abstract
:1. Introduction
2. The Synthesis and Modification of Bioactive NMPs
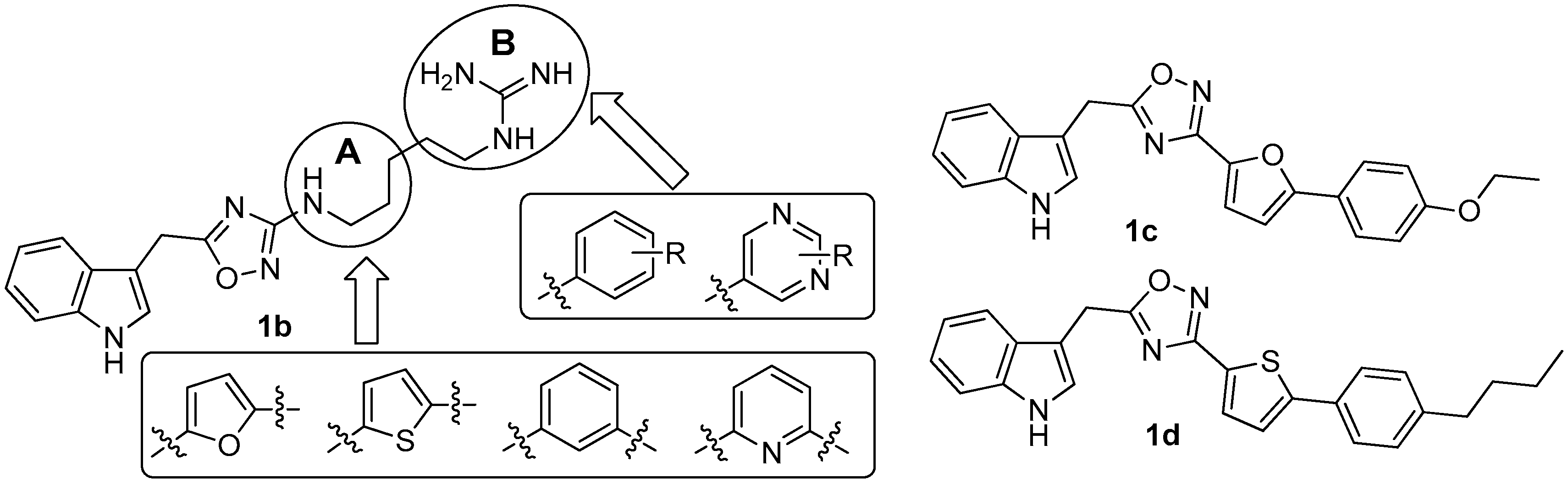
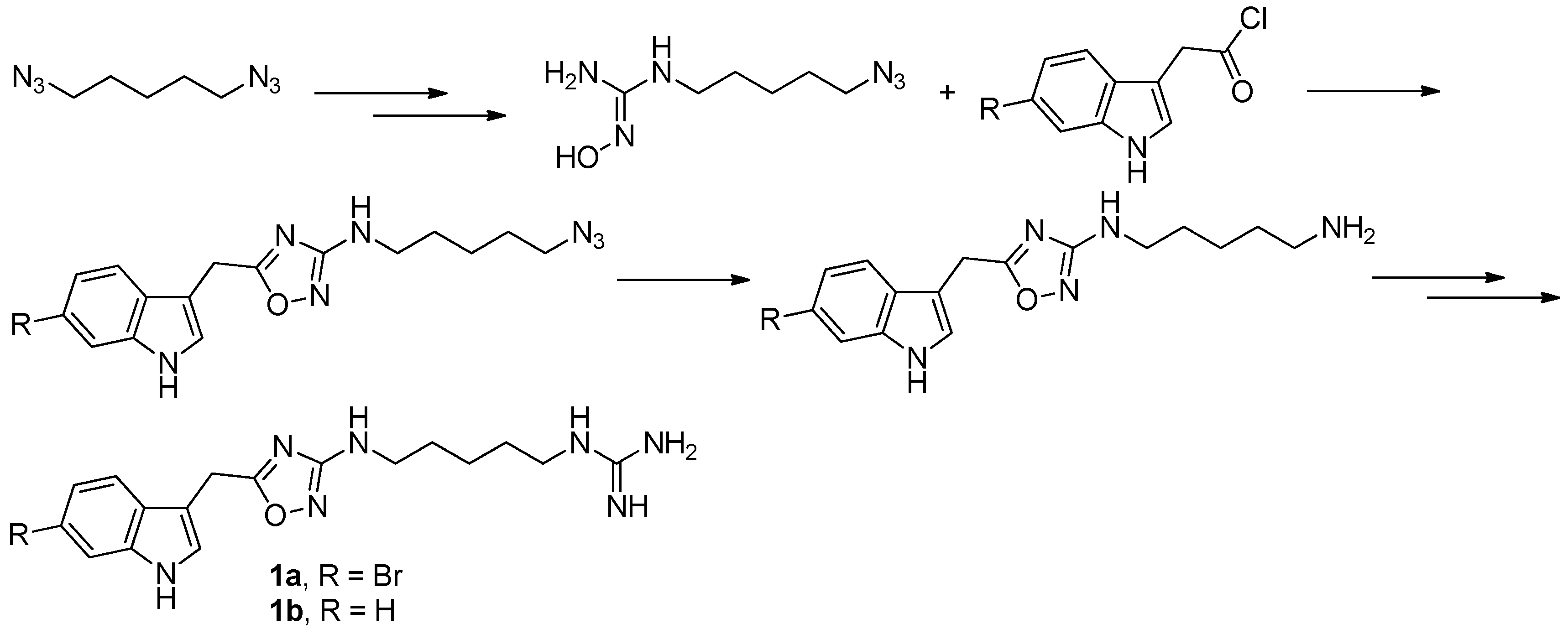
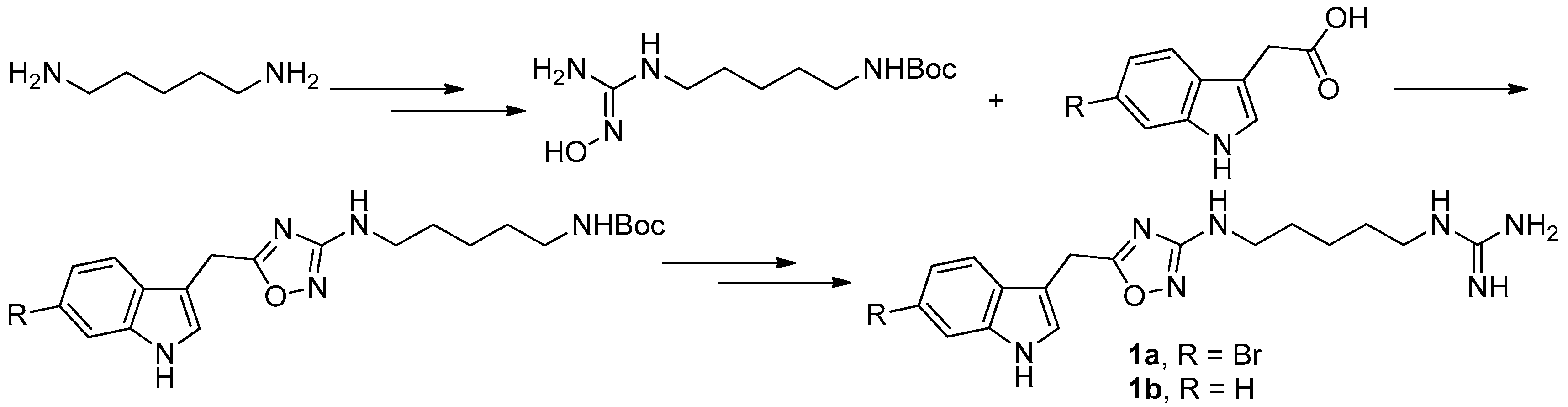
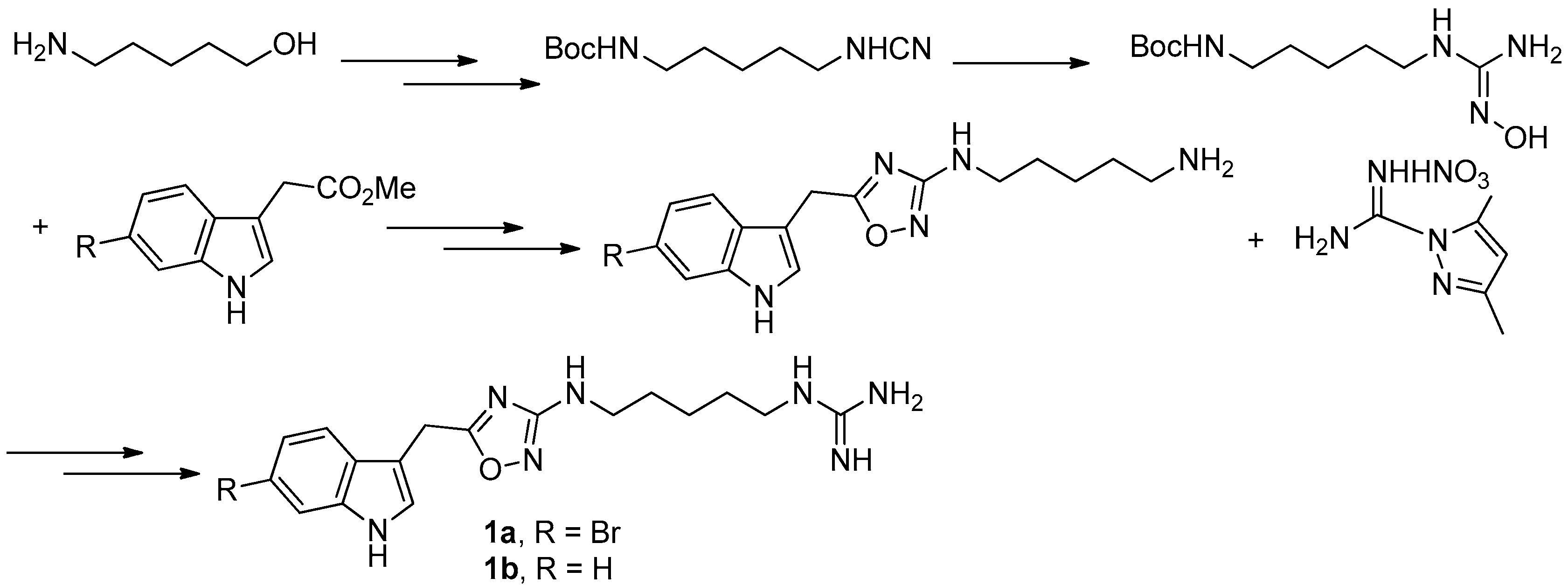
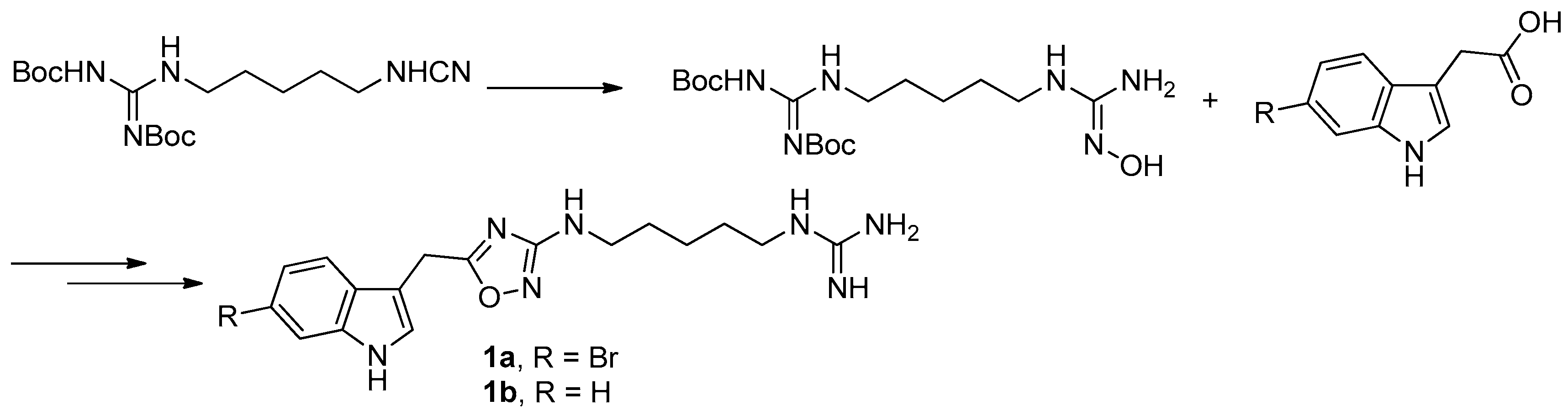
2.1. Phidianidines A (1a) and B (1b)
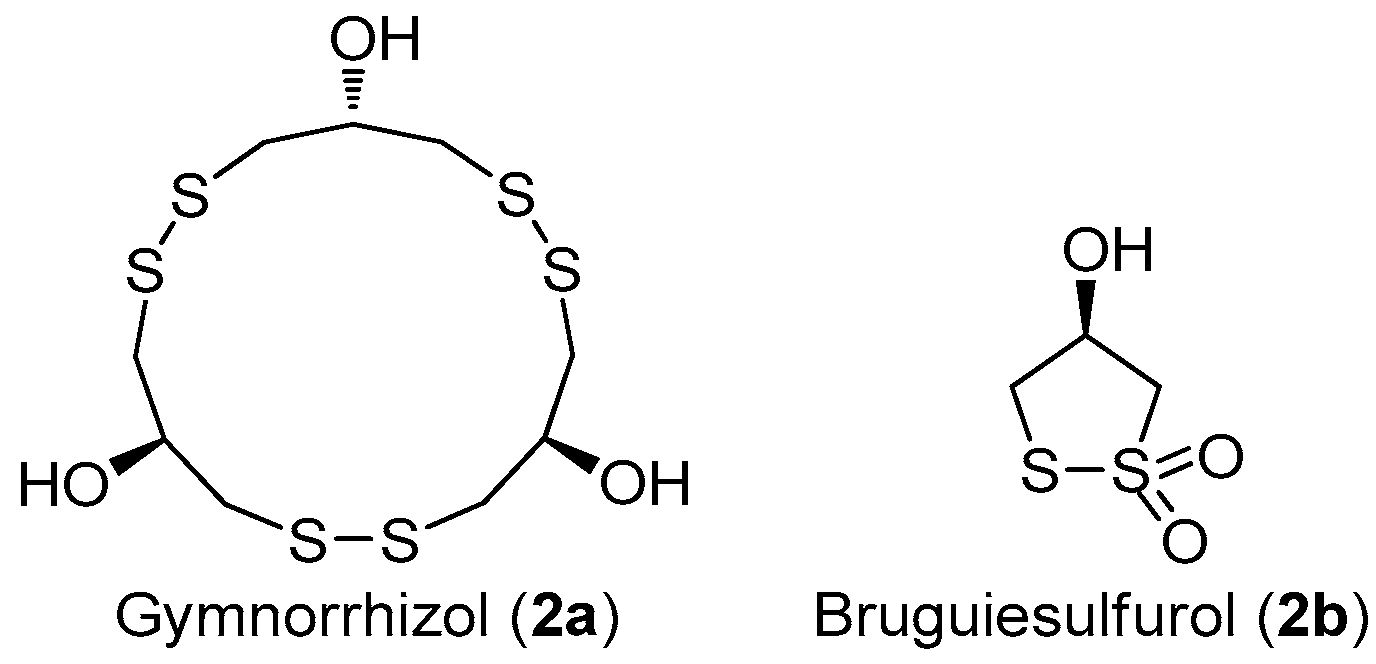
2.2. Gymnorrhizol (2a) and Bruguiesulfurol (2b)
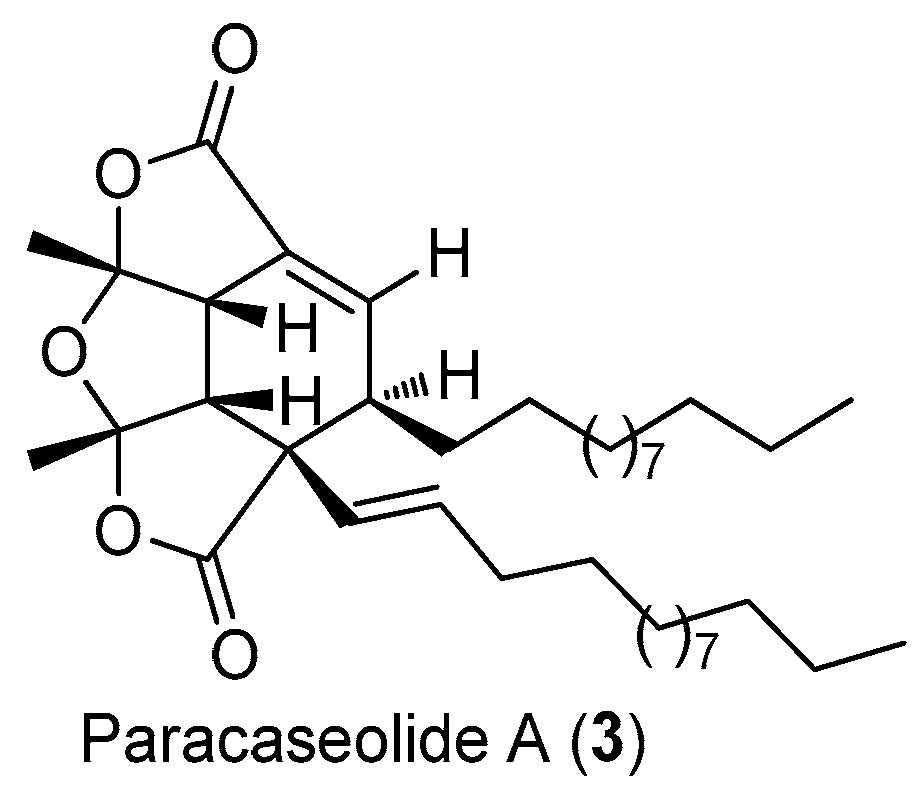
2.3. Paracaseolide A (3)
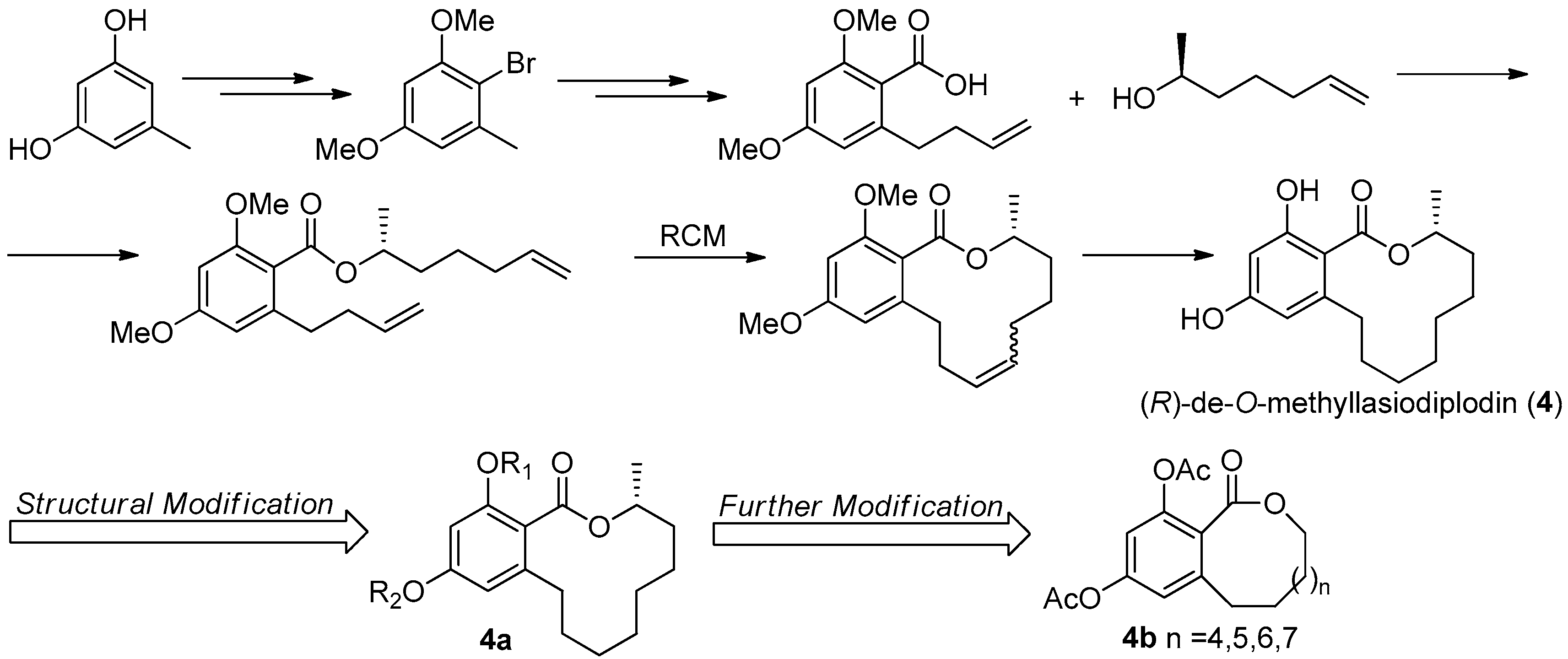
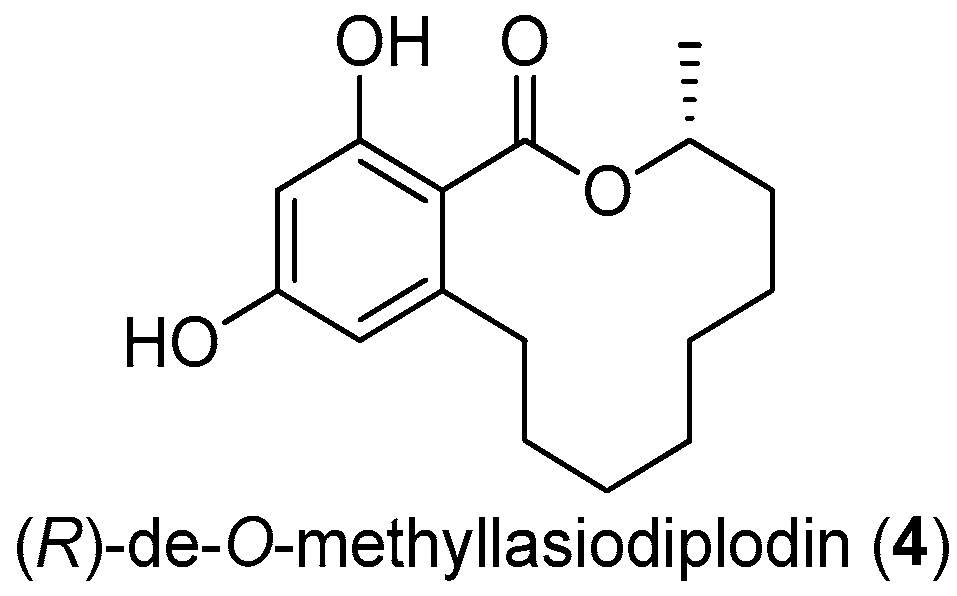
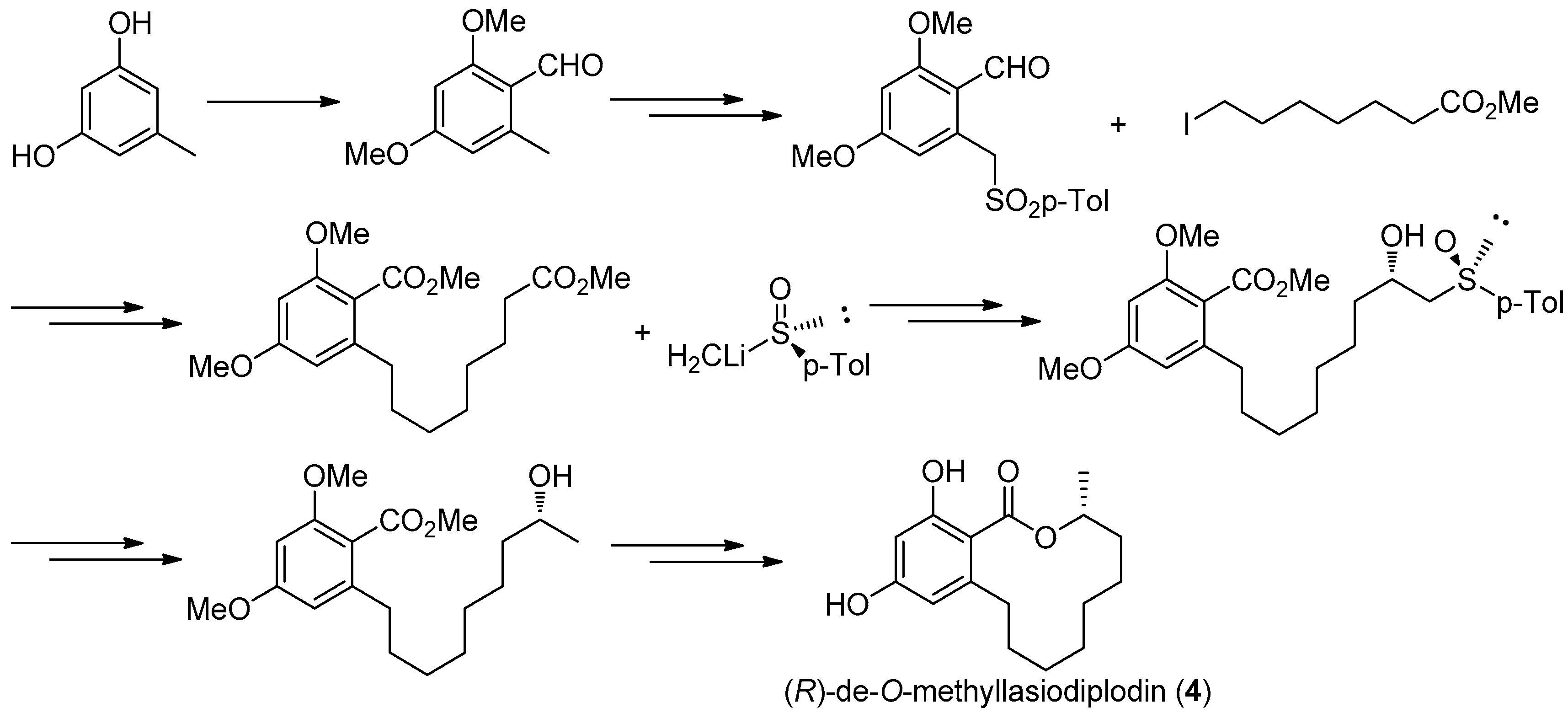
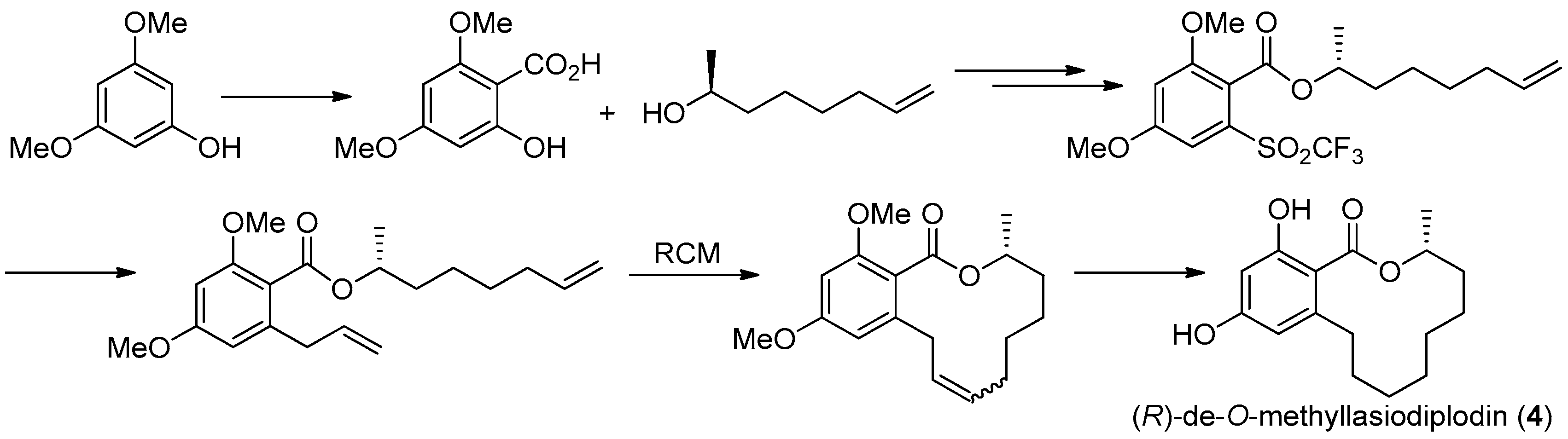
2.4. (R)-de-O-Methyllasiodiplodin (4)
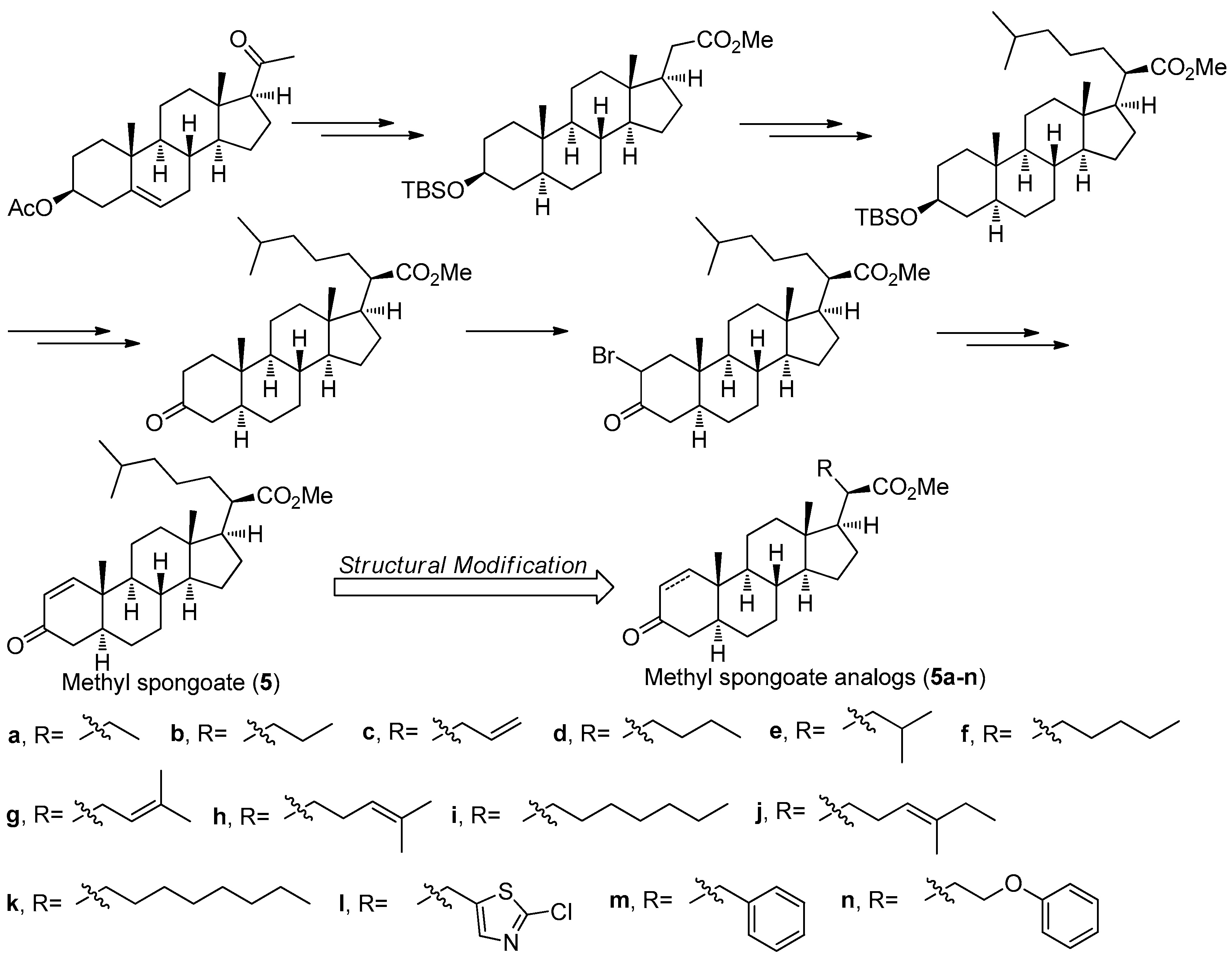
2.5. Methyl Spongoate (5)
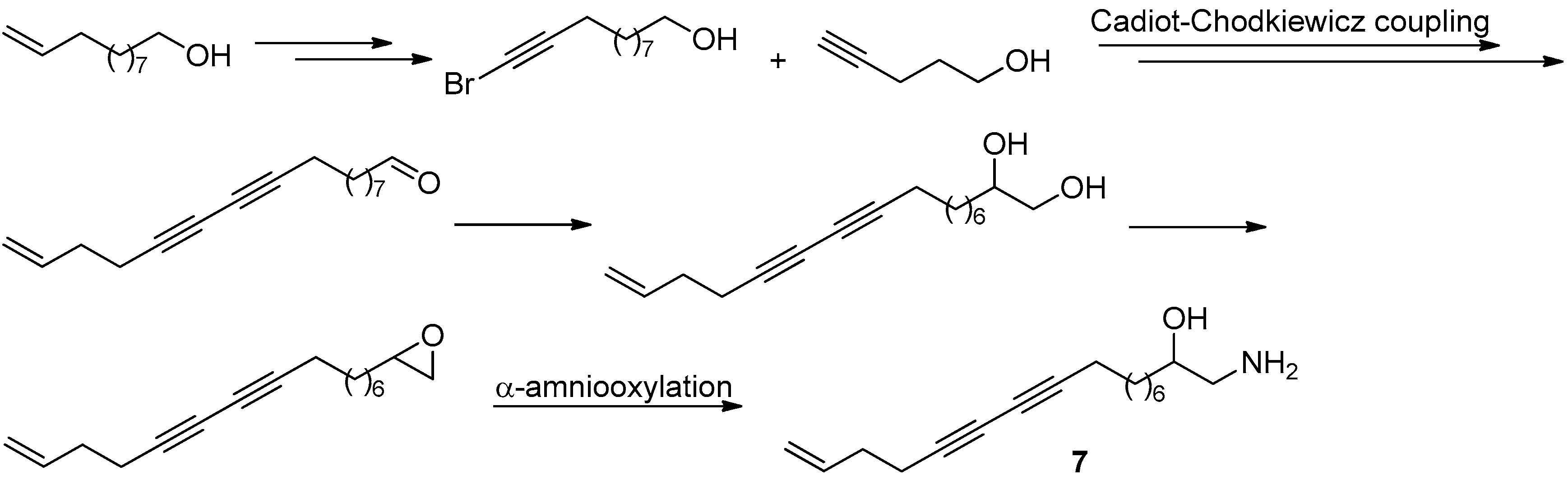
2.6. Methyl Xestospongoate (6) and Distaminolyne A (7)
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prinsep, M.R. Sulfur-containing natural products from marine invertebrates. In Studies in Natural Products Chemistry; Rahman, A.U., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2003; Volume 28, pp. 617–751. [Google Scholar]
- Mayer, A.M.S.; Rodriguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2009–2011: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-inflammatory, Antiprotozoal, Antituberculosis and Antiviral Activities; affecting the Immune and Nervous System, and other Miscellaneous Mechanisms of Action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar] [PubMed]
- Sawadogo, W.R.; Boly, R.; Cerella, C.; Teiten, M.H.; Dicato, M.; Diederich, M. A Survey of Marine Natural Compounds and Their Derivatives with Anti-cancer Activity Reported in 2012. Molecules 2015, 20, 7097–7142. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Li, Y.; Irace, C.; Mollo, E.; Castelluccio, F.; Di Pascale, A.; Cimino, G.; Santamaria, R.; Guo, Y.W.; Gavagnin, M. Structure and cytotoxicity of phidianidines A and B: First finding of 1,2,4-oxadiazole system in a marine natural product. Org. Lett. 2011, 13, 2516–2519. [Google Scholar] [CrossRef] [PubMed]
- Vitale, R.M.; Gatti, M.; Carbone, M.; Barbieri, F.; Felicità, V.; Gavagnin, M.; Florio, T.; Amodeo, P. Minimalist hybrid ligand/receptor-based pharmacophore model for CXCR4 applied to a small-library of marine natural products led to the identification of phidianidine a as a new CXCR4 ligand exhibiting antagonist activity. ACS Chem. Biol. 2013, 8, 2762–2770. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Snider, B.B. Synthesis of phidianidines A and B. J. Org. Chem. 2012, 77, 4832–4836. [Google Scholar] [CrossRef] [PubMed]
- Brogan, J.T.; Stoops, S.L.; Lindsley, C.W. Total synthesis and biological evaluation of phidianidines A and B uncovers unique pharmacological profiles at CNS targets. ACS Chem. Neurosci. 2012, 3, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Manzo, E.; Pagano, D.; Carbone, M.; Ciavatta, M.L.; Gavagnin, M. Synthesis of phidianidine B, a highly cytotoxic 1,2,4-oxadiazole marine metabolite. ARKIVOC 2012, 220–228. [Google Scholar]
- Buchanan, J.C.; Petersen, B.P.; Chamberland, S. Concise total synthesis of phidianidine A and B. Tetrahedron Lett. 2013, 54, 6002–6004. [Google Scholar] [CrossRef]
- Jiang, C.S.; Fu, Y.; Zhang, L.; Gong, J.X.; Wang, Z.Z.; Xiao, W.; Zhang, H.Y.; Guo, Y.W. Synthesis and biological evaluation of novel marine-derived indole-based 1,2,4-oxadiazoles derivatives as multifunctional neuroprotective agents. Bioorg. Med. Chem. Lett. 2015, 25, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, C.S.; Gao, L.X.; Gong, J.X.; Wang, Z.H.; Li, J.Y.; Li, J.; Li, X.W.; Guo, Y.W. Design, synthesis and in vitro activity of phidianidine B derivatives as novel PTP1B inhibitors with specific selectivity. Bioorg. Med. Chem. Lett. 2016, 26, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.S.; Müller, W.E.; Schröder, H.C.; Guo, Y.W. Disulfide- and multisulfide-containing metabolites from marine organisms. Chem. Rev. 2012, 112, 2179–2207. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Q.; Guo, Y.W. Gymnorrhizol, an unusual macrocyclic polydisulfide from the Chinese mangrove Bruguiera gymnorrhiza. Tetrahedron Lett. 2004, 45, 5533–5535. [Google Scholar] [CrossRef]
- Lui, H.L.; Shen, X.; Jiang, H.L.; Guo, Y.W. Structural Studies on an Unusual Novel Macrocyclic Polydisulfide from the Chinese Mangrove Bruguiera gymnorrhiza. Chin. J. Org. Chem. 2008, 28, 246–251. [Google Scholar]
- Huang, X.Y.; Wang, Q.; Liu, H.L.; Zhang, Y.; Xin, G.R.; Shen, X.; Dong, M.L.; Guo, Y.W. Diastereoisomeric macrocyclic polydisulfides from the mangrove Bruguiera gymnorrhiza. Phytochemistry 2009, 70, 2096–2100. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.X.; Shen, X.; Yao, L.G.; Jiang, H.; Krohn, K.; Guo, Y.W. Total synthesis of gymnorrhizol, an unprecedented 15-membered macrocyclic polydisulfide from the Chinese mangrove Bruguiera gymnorrhiza. Org. Lett. 2007, 9, 1715–1716. [Google Scholar] [CrossRef] [PubMed]
- Homhual, S.; Zhang, H.J.; Bunyapraphatsara, N.; Kondratyuk, T.P.; Santarsiero, B.D.; Mesecar, A.D.; Herunsalee, A.; Chaukul, W.; Pezzuto, J.M.; Fong, H.H. Bruguiesulfurol, a new sulfur compound from Bruguiera gymnorrhiza. Planta Med. 2006, 72, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, C.S.; Ma, W.Q.; Gao, L.X.; Gong, J.X.; Li, J.Y.; Li, J.; Guo, Y.W. The first synthesis of natural disulfide bruguiesulfurol and biological evaluation of its derivatives as a novel scaffold for PTP1B inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 5061–5065. [Google Scholar] [CrossRef] [PubMed]
- Iversen, L.F.; Moller, K.B.; Pedersen, A.K.; Peters, G.H.; Petersen, A.S.; Andersen, H.S.; Branner, S.; Mortensen, S.B.; Moller, N.P. Structure determination of T cell protein-tyrosine phosphatase. J. Biol. Chem. 2002, 277, 19982–19990. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, L.X.; Gong, J.X.; Jiang, C.S.; Yao, L.G.; Li, J.Y.; Li, J.; Xiao, W.; Guo, Y.W. Design and synthesis of novel 1,2-dithiolan-4-yl benzoate derivatives as PTP1B inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 2211–2216. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Liu, H.L.; Li, J.; Xin, G.R.; Guo, Y.W. Paracaseolide A, first α-alkylbutenolide dimer with an unusual tetraquinane oxa-cage bislactone skeleton from Chinese mangrove Sonneratia paracaseolaris. Org. Lett. 2011, 13, 5032–5035. [Google Scholar] [CrossRef] [PubMed]
- Noutsias, D.; Vassilikogiannakis, G. First total synthesis of paracaseolide A. Org. Lett. 2012, 14, 3565–3567. [Google Scholar] [CrossRef] [PubMed]
- Guney, T.; Kraus, G.A. Total synthesis of paracaseolide A. Org. Lett. 2013, 15, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Vasamsetty, L.; Khan, F.A.; Mehta, G. Total synthesis of a novel oxa-bowl natural product paracaseolide A via a “putative” biomimetic pathway. Tetrahedron Lett. 2013, 54, 3522–3525. [Google Scholar] [CrossRef]
- Giera, D.S.; Stark, C.B.W. Total synthesis of (±)-paracaseolide A and initial attempts at a Lewis acid mediated dimerization of its putative biosynthetic precursor. RCS Adv. 2013, 3, 21280–21284. [Google Scholar] [CrossRef]
- Boukouvalas, J.; Jean, M.A. Streamlined biomimetic synthesis of paracaseolide A via aerobic oxidation of a 2-silyloxyfuran. Tetrahedron Lett. 2014, 55, 4248–4250. [Google Scholar] [CrossRef]
- Wang, T.; Hoye, T.R. Diels-Alderase-free, bis-pericyclic, [4 + 2] dimerization in the biosynthesis of (±)-paracaseolide A. Nat. Chem. 2015, 7, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.P.; Tang, C.L.; Gao, L.X.; Ma, W.P.; Li, J.Y.; Li, Y.; Li, J.; Nan, F.J. Design and synthesis of paracaseolide A analogues as selective protein tyrosine phosphatase 1B inhibitors. Org. Biomol. Chem. 2014, 12, 3441–3445. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jiang, C.S.; Zhang, Z.L.; Ma, W.Q.; Guo, Y.W. Recent progress regarding the bioactivities, biosynthesis and synthesis of naturally occurring resorcinolic macrolides. Acta Pharmacol. Sin. 2014, 35, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.S.; Zhou, R.; Gong, J.X.; Chen, L.L.; Kurtán, T.; Shen, X.; Guo, Y.W. Synthesis, modification, and evaluation of (R)-de-O-methyllasiodiplodin and analogs as nonsteroidal antagonists of mineralocorticoid receptor. Bioorg. Med. Chem. Lett. 2011, 21, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Solladié, G.; Rubio, A.; Carreño, M.C.; Ruano, J.L.G. Asymmetric synthesis of orsellinic acid type macrolides: The example of lasiodiplodin. Tetrahedron Asymmetry 1990, 1, 187–198. [Google Scholar] [CrossRef]
- Fürstner, A.; Kindler, N. Macrocycle formation by ring-closing-metathesis. 2. An efficient synthesis of enantiomerically pure (R)-(+)-lasiodiplodin. Tetrahedron Lett. 1996, 37, 7005–7008. [Google Scholar]
- Guo, Y.W.; Wang, H.Y.; Gong, J.X.; Zhang, X.D.; Jiang, C.S. A Macrolide Fused with Benzene Derivative with Pancreatic Inhibitory Activity. Patent CN102101854A, 18 December 2009. [Google Scholar]
- Yan, X.H.; Lin, L.P.; Ding, J.; Guo, Y.W. Methyl spongoate, a cytotoxic steroid from the Sanya soft coral Spongodes sp. Bioorg. Med. Chem. Lett. 2007, 17, 2661–2663. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.X.; Miao, Z.H.; Yao, L.G.; Ding, J.; Kurtán, T.; Guo, Y.W. Stereoselective synthesis of methyl spongoate, a new steroid with potent antitumor activities. Synlett 2010, 3, 480–482. [Google Scholar]
- Jiang, C.S.; Huang, C.G.; Feng, B.; Li, J.; Gong, J.X.; Kurtán, T.; Guo, Y.W. Synthesis and antitumor evaluation of methyl spongoate analogs. Steroids 2010, 75, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.F.; Menna, M.; Cai, Y.S.; Guo, Y.W. Polyacetylenes of marine origin: Chemistry and bioactivity. Chem. Rev. 2015, 115, 1543–1596. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.F.; Wang, T.; Cai, Y.S.; He, W.F.; Sun, P.; Li, Y.F.; Huang, Q.; Taglialatela-Scafati, O.; Wang, H.Y.; Guo, Y.W. Brominated polyunsaturated lipids from the Chinese sponge Xestospongia testudinaria as a new class of pancreatic lipase inhibitors. Eur. J. Med. Chem. 2014, 79, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.X.; Wang, H.Y.; He, W.F.; Wang, Z.Z.; Xiao, W.; Guo, Y.W. A concise synthesis of xestospongic acid methyl ester with pancreatic lipase inhibitory activity. J. Asian Nat. Prod. Res. 2013, 15, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.X.; He, W.F.; Liu, H.L.; Jiang, C.S.; Wang, T.; Wang, H.Y.; Guo, Y.W. synthesis and evaluation of pancreatic lipase inhibitory halogenated polyunsaturated lipids from marine natural products: Methyl xestospongoate and analogs. Helv. Chim. Acta 2016, 99, 78–82. [Google Scholar] [CrossRef]
- Wang, J.Y.; Pearce, N.; Chan, S.T.S.; Taylor, R.B.; Page, M.J.; Valentin, A.; Bouguet-Kondracki, M.-L.; Dalton, J.P.; Wiles, S.; Copp, B.R. Biologically Active Acetylenic Amino Alcohol and N-Hydroxylated 1,2,3,4-Tetrahydro-β-carboline Constituents of the New Zealand Ascidian Pseudodistoma opacum. J. Nat. Prod. 2016, 79, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.Y.; Han, G.Y.; Gong, J.X.; Nay, B.; Li, X.W.; Guo, Y.W. Asymmetric total synthesis of distaminolyne A and revision of its absolute configuration. Org. Lett. 2017, 19, 714–717. [Google Scholar] [CrossRef] [PubMed]


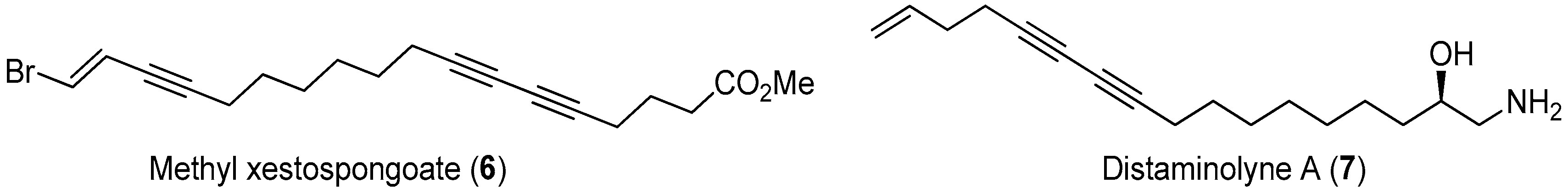

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, H.; Alexandre Muehlmann, L.; Jiang, C.-S.; Guo, Y.-W. Synthesis and Structural Modification of Marine Natural Products. Molecules 2017, 22, 882. https://doi.org/10.3390/molecules22060882
Zhang J, Zhang H, Alexandre Muehlmann L, Jiang C-S, Guo Y-W. Synthesis and Structural Modification of Marine Natural Products. Molecules. 2017; 22(6):882. https://doi.org/10.3390/molecules22060882
Chicago/Turabian StyleZhang, Juan, Hua Zhang, Luis Alexandre Muehlmann, Cheng-Shi Jiang, and Yue-Wei Guo. 2017. "Synthesis and Structural Modification of Marine Natural Products" Molecules 22, no. 6: 882. https://doi.org/10.3390/molecules22060882