Raspberry-Like Bismuth Oxychloride on Mesoporous Siliceous Support for Sensitive Electrochemical Stripping Analysis of Cadmium
Abstract
:1. Introduction
2. Results
2.1. Morphological Characterization
2.2. Electrochemical Characterization
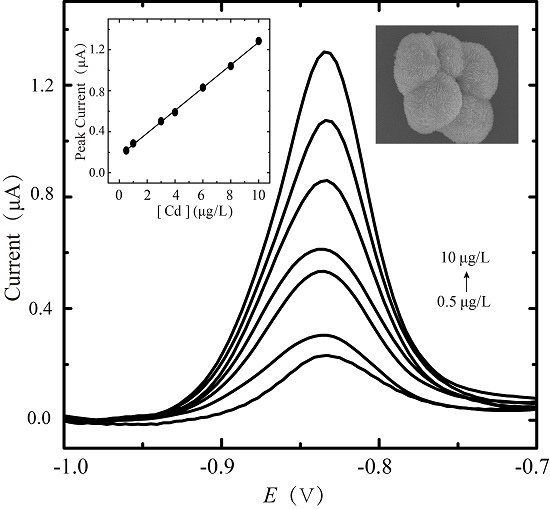
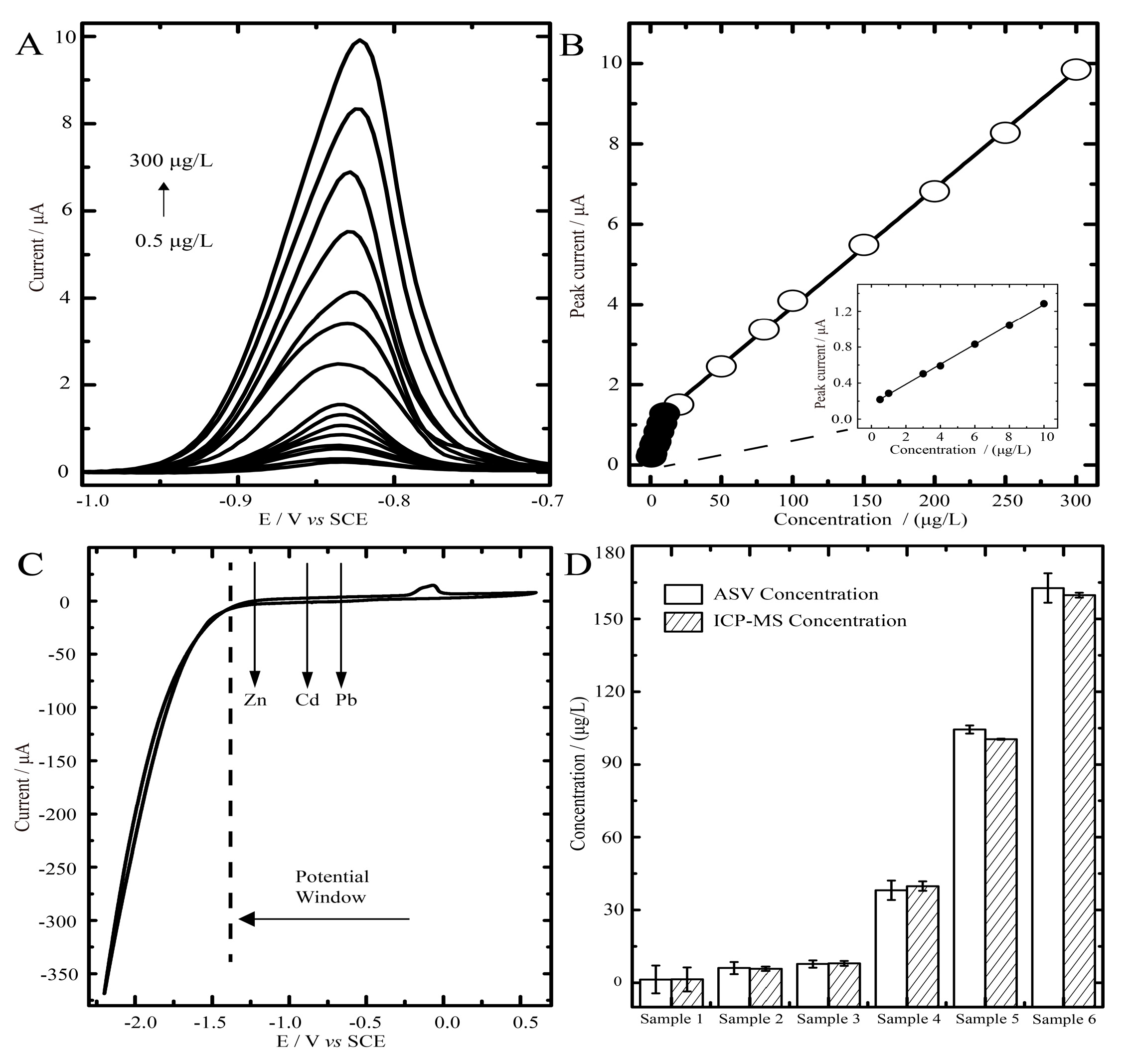
2.3. Anodic Stripping Voltammetric Analysis of Cadmium on BiOCl-SiO2 KIT-6/GCEs
2.4. Blood Samples Determined by ASV on BiOCl—SiO2 KIT-6/GCEs and ICP-MS
3. Materials and Methods
3.1. Chemicals and Solutions
3.2. Instrumentation
3.3. Synthesis of Mesoporous Silica KIT-6
3.4. Synthesis of BiOCl-SiO2 KIT-6 Composites
3.5. Preparation of BiOCl-SiO2 KIT-6 Modified GCE
3.6. Electrochemical Measurements
3.7. Pretreatment of Blood Samples and ICP-MS Measurement
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guttieri, M.J.; Seabourn, B.W.; Liu, C.; Baenziger, P.S.; Waters, B.M. Distribution of cadmium, iron, and zinc in millstreams of hard winter wheat (Triticum aestivum L.). J. Agric. Food Chem. 2015, 63, 10681–10688. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P.M. Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev. Environ. Contam. Toxicol. 2016, 241, 73–137. [Google Scholar]
- Jarup, L. Hazards of heavy metal contamination. Brit. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Shokrollahi, A.; Joybar, S.; Haghighi, H.E.; Niknam, K.; Niknam, E. Application of cloud point preconcentration and flame atomic absorption spectrometry for the determination of cadmium and zinc ions in urine, blood serum and water samples. Quim. Nova 2013, 36, 368–374. [Google Scholar] [CrossRef]
- Rezende, H.C.; Nascentes, C.C.; Coelho, N.M.M. Cloud point extraction for determination of cadmium in soft drinks by thermospray flame furnace atomic absorption spectrometry. Microchem. J. 2011, 97, 118–121. [Google Scholar] [CrossRef]
- Wu, H.; Fang, C.; Du, B.; Zhao, C. Flow injection on-line preconcentration coupled to hydride generation atomic fluorescence spectrometry for ultra-trace amounts of cadmium determination in seawater. Microchim. Acta 2007, 160, 173–178. [Google Scholar] [CrossRef]
- Voica, C.; Dehelean, A.; Kovacs, M.H.; Lazar, M.D. The use of inductively coupled plasma mass spectrometry (ICP-MS) for the determination of toxic and essential elements in different types of food samples. Food Chem. 2012, 110–113. [Google Scholar]
- Lu, H.H.; Jiang, S.J. Organic acids as the modifier to determine Zn, Cd, Tl and Pb in soil by slurry sampling electrothermal vaporization inductively-coupled plasma mass spectrometry. Anal. Chim. Acta 2001, 429, 247–255. [Google Scholar] [CrossRef]
- Beata, K.O.; Krzysztof, D.; Ewa, S.; Jerzy, G. Determination of thallium and other elements (As, Cd, Cu, Mn, Pb, Se, Sb, and Zn) in water and sediment samples from the vicinity of the zinc-lead smelter in Poland. J. Soils Sediments 2005, 5, 71–73. [Google Scholar]
- Chen, J.; Yan, F.; Tan, F.; Ju, H.X. Gold nanoparticles doped three-dimensional sol-gel matrix for amperometric human chorionic gonadotrophin immunosensor. Electroanal. 2006, 18, 1696–1702. [Google Scholar] [CrossRef]
- Chen, J.; Tang, J.H.; Yan, F.; Ju, H.X. A gold nanoparticles/sol-gel composite architecture for encapsulation of immunoconjugate for reagentless electrochemical immunoassay. Biomaterials 2006, 27, 2313–2321. [Google Scholar] [CrossRef] [PubMed]
- Gemma, A.; Josefina, P.; Arben, M. Rencent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar]
- Hocevar, S.B.; Svancara, I.; Ogorevc, B.; Vytras, K. Antimony film electrode for electrochemical stripping analysis. Anal. Chem. 2007, 79, 8639–8643. [Google Scholar] [CrossRef] [PubMed]
- Janegitz, B.C.; Marcolino, L.H.; Campana, S.P.; Faria, R.C.; Fatibello, O. Anodic stripping voltammetric determination of copper (II) using a functionalized carbon nanotubes paate electrode modified with crosslinked chitosan. Sens. Actuators B Chem. 2009, 142, 260–266. [Google Scholar] [CrossRef]
- Hocevar, S.B.; Wang, J.; Deo, R.P.; Ogorevc, B. Potentiometric stripping analysis at bismuth-film electrode. Electroanalysis 2002, 14, 112–115. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.W.; Tahir, H.E.; Zou, X.B.; Wang, P. Rapid and wide-range determination of Cd (II), Pb (II), Cu (II) and Hg (II) in fish tissues using light addressable potentiometric sensor. Food Chem. 2017, 221, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Jiokeng, S.L.Z.; Dongmo, L.M.; Ymele, E.; Ngameni, E.; Tonle, I.K. Sensitive stripping voltammetry detection of Pb (II) at a glassy carbon electrode modified with an amino-functionalized attapulgite. Sens. Actuators B Chem. 2017, 242, 1027–1034. [Google Scholar] [CrossRef]
- Rosolina, S.M.; Chambers, J.Q.; Lee, C.W.; Xue, Z.L. Direct determination of cadmium and lead in pharmaceutical ingredients using anodic stripping voltammetry in aqueous and DMSO/water solutions. Talanta 2015, 893, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Khun, N.W.; Liu, E. Linear sweep anodic stripping voltammetry of heavy metals from nitrogen doped tetrahedral amorphous carbon thin films. Electrochim. Acta 2009, 54, 2890–2898. [Google Scholar] [CrossRef]
- Joanna, K.; Beata, K.O.; Jerzy, G. Electroanalytical methods for determination of the metal content and acetic-acid-available metal fractions in soils. Anal. Bioanal. Chem. 2002, 373, 116–118. [Google Scholar]
- Beata, K.O.; Joanna, P. Determination of lead and cadmium at silver electrode by subtractive anodic stripping voltammetry in plant materials containing Ti. Electroanalysis 2005, 17, 815–818. [Google Scholar]
- Peng, H.; Chan, C.K.; Meister, S.; Zhang, X.F.; Cui, Y. Shape evolution of layer-structured bismuth oxychloride nanostructures via low-temperature chemical vapor transport. Chem. Mater 2009, 21, 247–252. [Google Scholar] [CrossRef]
- Myung, Y.; Wu, F.; Banerjee, S.; Park, J.; Banerjee, P. Electrical conductivity of p-type BiOCl nanosheets. Chem. Commun 2015, 51, 2629–2632. [Google Scholar] [CrossRef] [PubMed]
- Hočevar, S.B.; Ogorevc, B.; Wang, J.; Pihlar, B. A study on operational parameters for advanced use of bismuth film electrode in anodic stripping voltammetry. Electroanalysis 2002, 14, 1707–1712. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Hocevar, S.B.; Farias, P.A.M. Bismuth-coated carbon electrodes for anodic stripping voltammetry. Anal. Chem. 2000, 72, 3218–3222. [Google Scholar] [CrossRef] [PubMed]
- Cerovac, S.; Guzsvany, V.; Konya, Z.; Ashrafi, A.M.; Svancara, I.; Roncevic, S.; Kukovecz, A.; Dalmacija, B.; Vytras, K. Trace level voltammetric determination of lead and cadmium in sediment pore water by a bismuth-oxychloride particle-multiwalled carbon nanotube composite modified glassy carbon electrode. Talanta 2015, 134, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Ananthi, A.; Kumar, S.S.; Phani, K.L. Facile one-step direct electrodeposition of bismuth nanowires on glassy carbon electrode for selective determination of folic acid. Electrochim. Acta 2015, 151, 584–590. [Google Scholar] [CrossRef]
- Tesarova, E.; Baldrianova, L.; Hocevar, S.B.; Svancara, I.; Vytras, K.; Ogorevc, B. Anodic stripping voltammetric measurement of trace heavy metals at antimony film carbon paste electrode. Electrochim. Acta 2009, 54, 1506–1510. [Google Scholar] [CrossRef]
- Kadara, R.O.; Tothill, I.E. Development of disposable bulk-modified screen-printed electrode based on bismuth oxide for stripping chronopotentiometric analysis of lead (II) and cadmium (II) in soil and water samples. Anal. Chim. Acta 2008, 623, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.H.; Han, W.K.; Park, J.S.; Kang, S.G. An electrochemical sensor based on the reduction of screen-printed bismuth oxide for the determination of trace lead and cadmium. Sens. Actuators B Chem. 2008, 135, 309–316. [Google Scholar] [CrossRef]
- Riman, D.; Jirovsky, D.; Hrbac, J.; Prodromidis, M.I. Green and facile electrode modification by spark discharge: Bismuth oxide-screen printed electrodes for the screening of ultra-trace Cd(II) and Pb(II). Electrochem. Commun. 2015, 50, 20–23. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, K.; Xiao, X.; Zhang, L. Synthesis and facet-dependent photoreactivity of BiOClsingle-crystalline nanosheets. J. Am. Chem. Soc. 2012, 134, 4473–4476. [Google Scholar] [CrossRef] [PubMed]
- Pare, B.; Sarwan, B.; Jonnalagadda, S.B. Photocatalytic mineralization study of malachite green on the surface of mn-doped BiOCl activated by visible light under ambient condition. Appl. Surf. Sci. 2011, 258, 247–253. [Google Scholar] [CrossRef]
- Slowing, I.; Trewyn, B.G.; Lin, V.S.Y. Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticleson the endocytosis by human cancer cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef] [PubMed]
- Trewyn, B.G.; Giri, S.; Slowing, I.I.; Lin, V.S.Y. Mesoporous silica nanoparticle based controlled release, drug delivery, and biosensor systems. Chem. Commum. 2007, 38, 3236–3245. [Google Scholar] [CrossRef] [PubMed]
- Walcarius, A. Mesoporous materials and electrochemistry. Chem. Soc. Rev. 2013, 42, 4098–4140. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xiong, C.; Li, Z.; Kong, Y.; Chen, J.; Wang, J. Three-dimensionally controllable synthesis of multichannel silica nanotubes and their application as dual drug carriers. ChemPlusChem 2015, 80, 1615–1623. [Google Scholar] [CrossRef]
- Subhan, F.; Aslam, S.; Yan, Z.; Ikram, M.; Rehman, S. Enhanced desulfurization characteristics of Cu-KIT-6for thiophene. Microporous Mesoporous Mater. 2014, 199, 108–116. [Google Scholar] [CrossRef]
- Vladislavic, N.; Brinic, S.; Grubac, Z.; Buzuk, M. Study of Bi film formation on different carbon based electrodes for possible applicability in electroanalytical determination of cysteine. Int. J. Electrochem. Sci. 2014, 9, 6020. [Google Scholar]
- Zaouak, O.; Authier, L.; Cugnet, C.; Castetbon, A.; Potin-Gautier, M. Bismuth-coated screen-printed microband electrodes for on-field labile cadmium determination. Electroanalysis 2009, 21, 689–695. [Google Scholar] [CrossRef]
- Zhao, G.; Yin, Y.; Wang, H.; Liu, G.; Wang, Z. Sensitive stripping voltammetric determination of Cd(II) and Pb(II) by a bi/multi-walled carbon nanotube-emeraldine base polyaniline-nafion composite modified glassy carbon electrode. Electrochim. Acta 2016, 220, 267–275. [Google Scholar] [CrossRef]
- Soni, K.; Rana, B.S.; Sinha, A.K.; Bhaumik, A.; Nandi, M.; Kumar, M.; Dhar, G.M. 3-d ordered mesoporous kit-6 support for effective hydrodesulfurization catalysts. Appl. Catal. B Environ. 2009, 90, 55–63. [Google Scholar] [CrossRef]
- Hoyer, B.; Florence, T.M.; Batley, G.E. Application of polymer-coated glassy carbon electrodes in anodic stripping voltammetry. Anal. Chem. 1987, 59, 1608–1614. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (BiOCl-SiO2 KIT-6) are available from the author J.C. and Y.Y.S. |
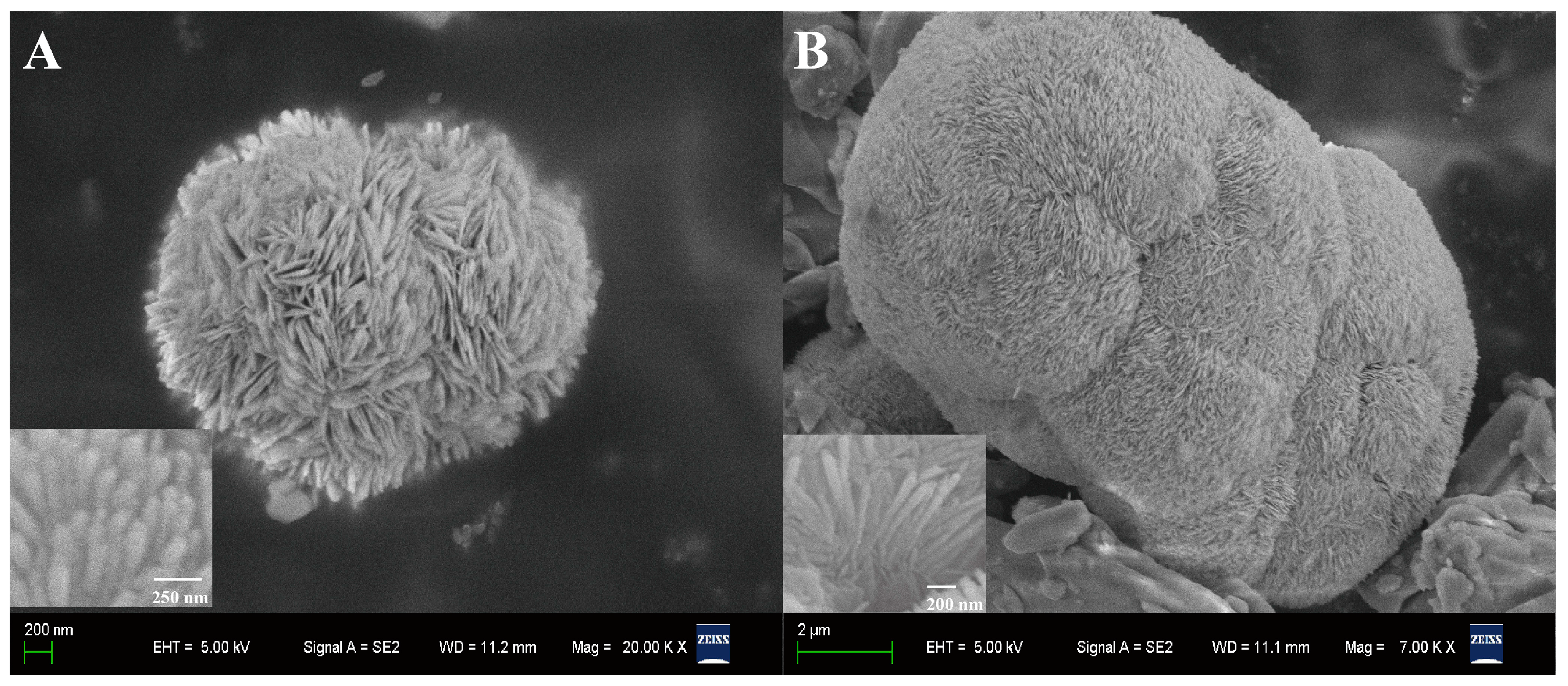
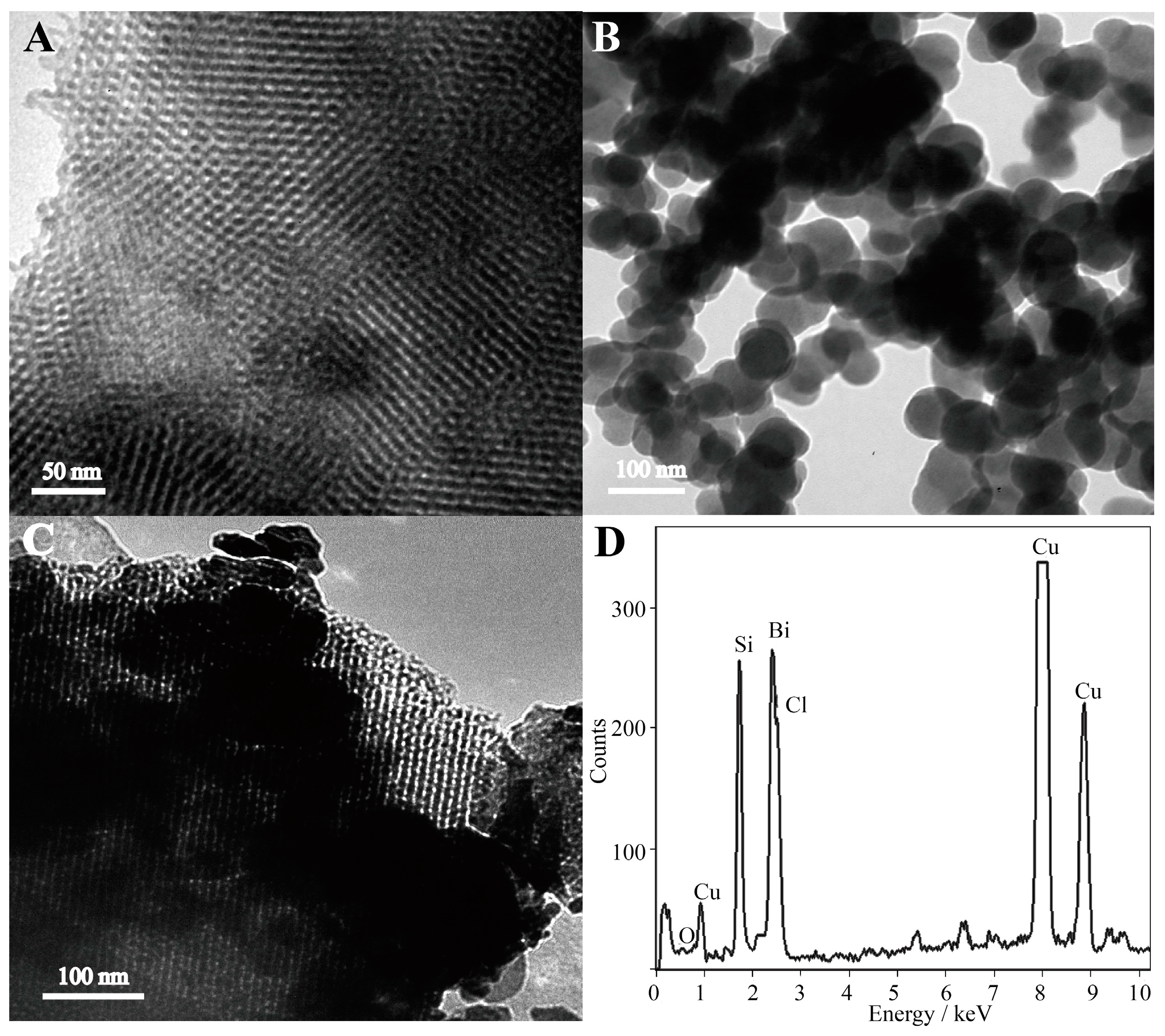
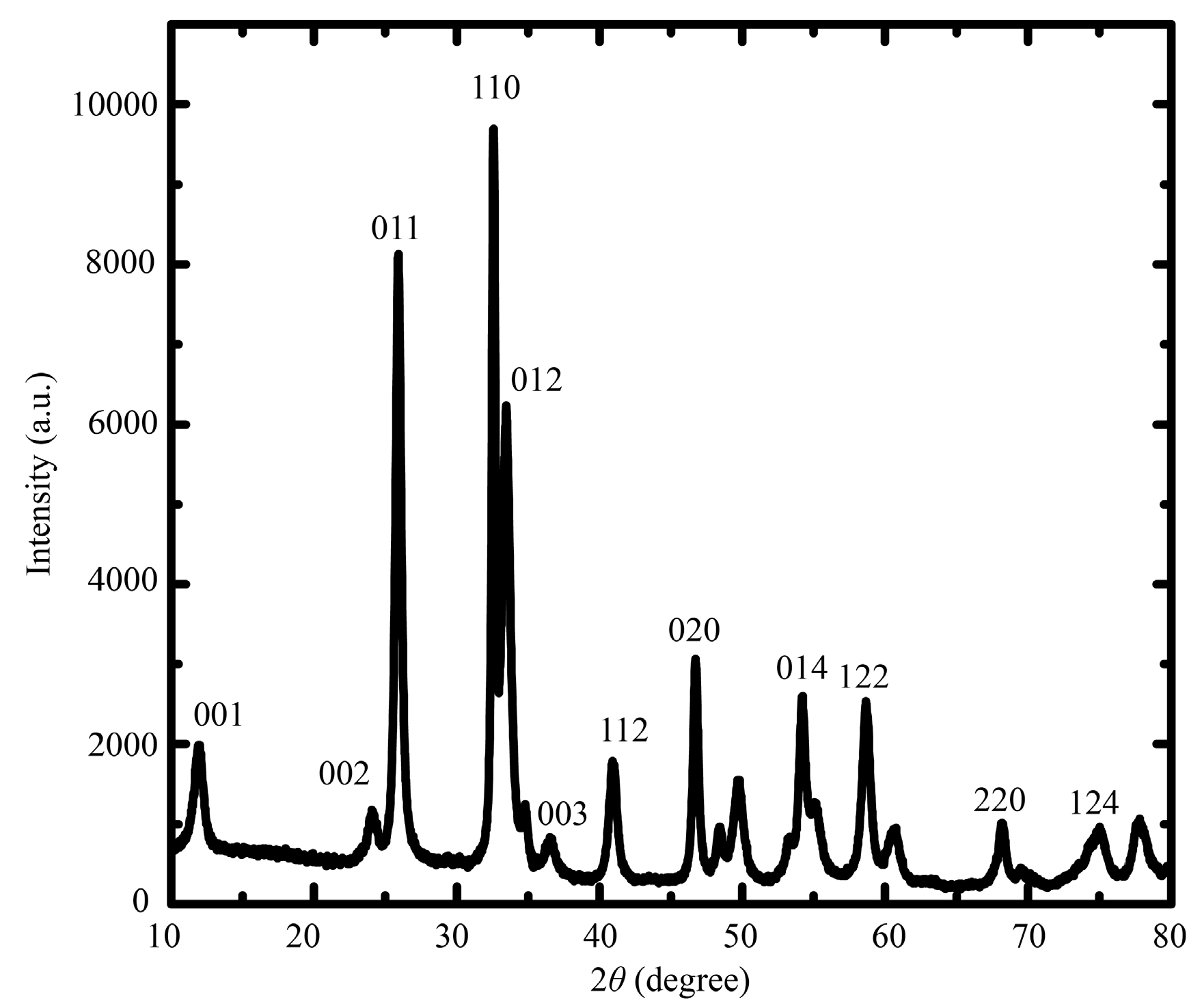
| Electrode | pH | Deposition Potential (V) | Deposition Time (s) | Dropping Volume of Composite (μL) |
|---|---|---|---|---|
| BiOCl-SiO2 KIT-6/GCE | 4.5 | −1.3 | 120 | 10 |
| Electrode | Linear Range (μg/L) | Detection Limit (μg/L) | Reference |
|---|---|---|---|
| Bi/SPME | NR | 1.3 | [40] |
| Bi/MWCNT-EBP-NA/GCE | 1.0–50.0 | 0.06 | [41] |
| BiOCl/MWCNT/GCE | 5–50 | 1.2 | [26] |
| BiFE | NR | 0.2 | [15] |
| BiOPE | 20–100 | 1.5 | [30] |
| BiOCl-SiO2 KIT-6/GCE | 0.5–300 | 0.065 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Xu, Z.; Yu, X.; Shi, X.; Jiang, H.; Li, X.; Kong, Y.; Xu, Q.; Chen, J. Raspberry-Like Bismuth Oxychloride on Mesoporous Siliceous Support for Sensitive Electrochemical Stripping Analysis of Cadmium. Molecules 2017, 22, 797. https://doi.org/10.3390/molecules22050797
Song Y, Xu Z, Yu X, Shi X, Jiang H, Li X, Kong Y, Xu Q, Chen J. Raspberry-Like Bismuth Oxychloride on Mesoporous Siliceous Support for Sensitive Electrochemical Stripping Analysis of Cadmium. Molecules. 2017; 22(5):797. https://doi.org/10.3390/molecules22050797
Chicago/Turabian StyleSong, Yiyan, Zhihui Xu, Xinyu Yu, Xueyan Shi, Huijun Jiang, Xiaoming Li, Yan Kong, Qin Xu, and Jin Chen. 2017. "Raspberry-Like Bismuth Oxychloride on Mesoporous Siliceous Support for Sensitive Electrochemical Stripping Analysis of Cadmium" Molecules 22, no. 5: 797. https://doi.org/10.3390/molecules22050797