Mesoporous Silica Nanoparticles as Carriers for Intracellular Delivery of Nucleic Acids and Subsequent Therapeutic Applications
Abstract
:1. Introduction
2. General Strategies for Preparation of Nucleic Acid-MSN Complexes
3. MSNs as Carriers for Intracellular Delivery of Nucleic Acids and Nucleic Acids-Guided Therapeutic Applications
3.1. DNA-MSN Complexes for DNA Delivery and DNA-Guided Therapeutics
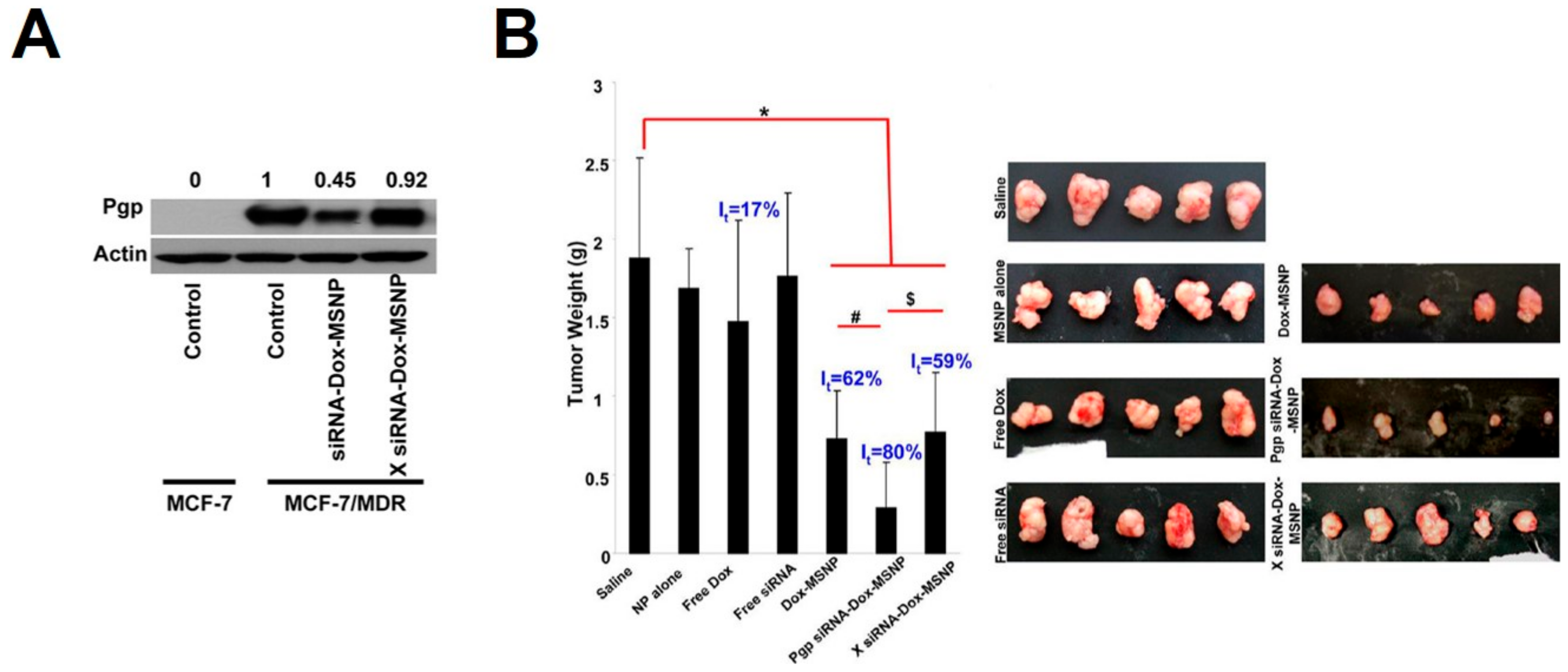
3.2. MiRNA/siRNA-MSN Complexes for miRNA/siRNA Delivery and miRNA/siRNA-Guided Therapeutics
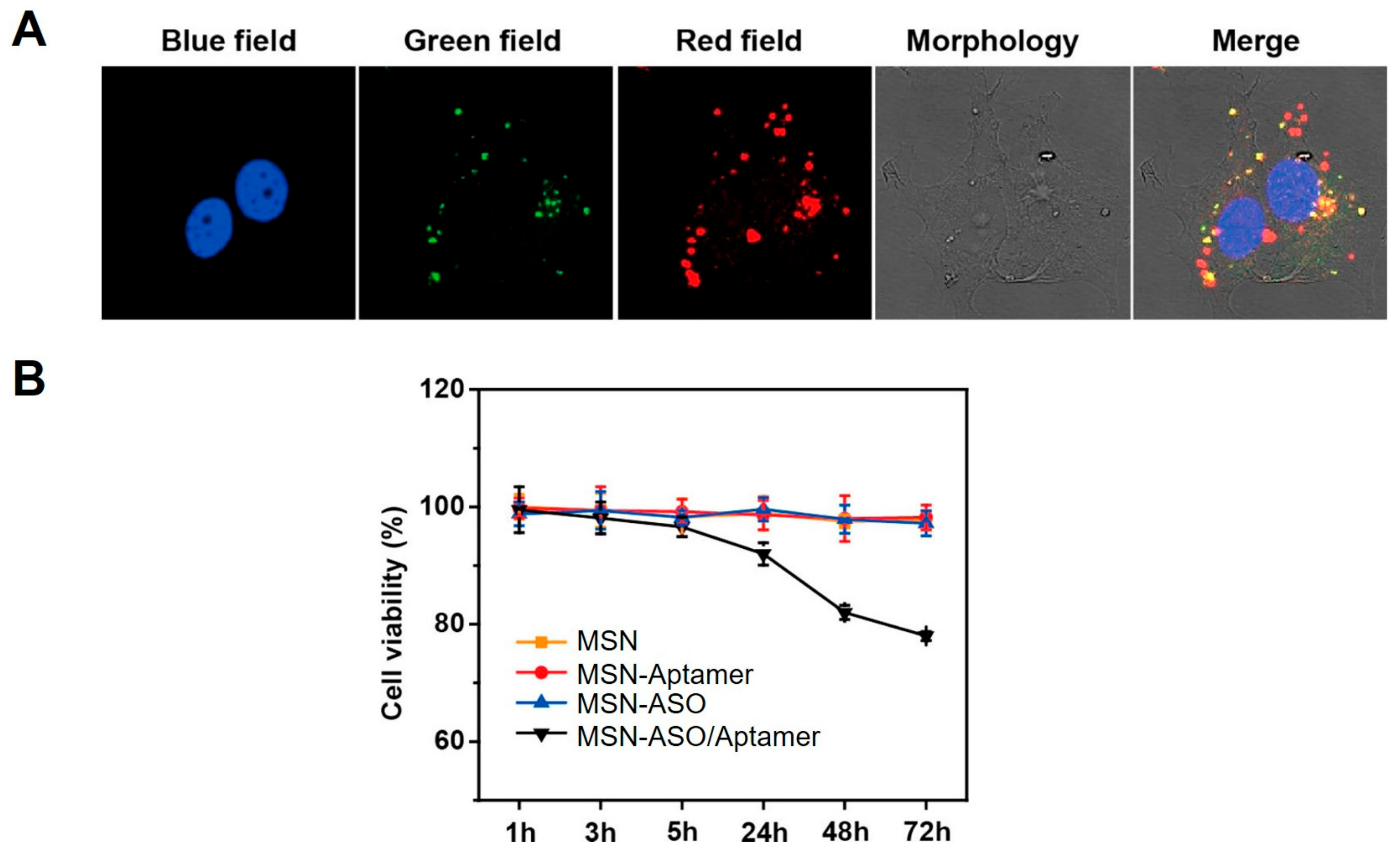
3.3. ASO-MSN Complexes for ASO Delivery and ASO-Guided Therapeutics
4. Perspective
Author Contributions
Conflicts of Interest
References
- Teo, P.Y.; Cheng, W.; Hedrick, J.L.; Yang, Y.Y. Co-delivery of drugs and plasmid DNA for cancer therapy. Adv. Drug Deliv. Rev. 2016, 98, 41–63. [Google Scholar] [CrossRef] [PubMed]
- Storrie, H.; Mooney, D.J. Sustained delivery of plasmid DNA from polymeric scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2006, 58, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Chen, Y.; Leong, K.W. Microrna delivery for regenerative medicine. Adv. Drug Deliv. Rev. 2015, 88, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Mahato, R.I. Mirnas as targets for cancer treatment: Therapeutics design and delivery. Preface. Adv. Drug Deliv. Rev. 2015, 81, v–vi. [Google Scholar] [CrossRef] [PubMed]
- Haussecker, D. Current issues of rnai therapeutics delivery and development. J. Control. Release 2014, 195, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Huang, L. Non-viral nanocarriers for sirna delivery in breast cancer. J. Control. Release 2014, 190, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicki, A.S.; Viljoen, A. Anti-sense oligonucleotide therapies for the treatment of hyperlipidaemia. Expert Opin. Biol. Ther. 2016, 16, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Gatti, R.A. Progress toward therapy with antisense-mediated splicing modulation. Curr. Opin. Mol. Ther. 2009, 11, 116–123. [Google Scholar] [PubMed]
- Preall, J.B.; Sontheimer, E.J. Rnai: Risc gets loaded. Cell 2005, 123, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Croce, C.M. Microrna signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Van Kouwenhove, M.; Kedde, M.; Agami, R. Microrna regulation by rna-binding proteins and its implications for cancer. Nat. Rev. Cancer 2011, 11, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rana, T.M. Therapeutic targeting of micrornas: Current status and future challenges. Nat. Rev. Drug Discov. 2014, 13, 622–638. [Google Scholar] [CrossRef] [PubMed]
- McClorey, G.; Wood, M.J. An overview of the clinical application of antisense oligonucleotides for rna-targeting therapies. Curr. Opin. Pharmacol. 2015, 24, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Felber, B.K.; Valentin, A.; Rosati, M.; Bergamaschi, C.; Pavlakis, G.N. Hiv DNA vaccine: Stepwise improvements make a difference. Vaccines 2014, 2, 354–379. [Google Scholar] [CrossRef] [PubMed]
- Bouchie, A. First microrna mimic enters clinic. Nat. Biotechnol. 2013, 31, 577. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.L.; Reesink, H.W.; Lawitz, E.J.; Zeuzem, S.; Rodriguez-Torres, M.; Patel, K.; van der Meer, A.J.; Patick, A.K.; Chen, A.; Zhou, Y.; et al. Treatment of hcv infection by targeting microrna. N. Engl. J. Med. 2013, 368, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Sullenger, B.A.; Nair, S. From the rna world to the clinic. Science 2016, 352, 1417–1420. [Google Scholar] [CrossRef] [PubMed]
- Giacca, M.; Zacchigna, S. Virus-mediated gene delivery for human gene therapy. J. Control. Release 2012, 161, 377–388. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, M.B.; Zuhorn, I.S. Solid lipid nanoparticles as nucleic acid delivery system: Properties and molecular mechanisms. J. Control. Release 2015, 201, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lehto, T.; Ezzat, K.; Wood, M.J.; El Andaloussi, S. Peptides for nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 106, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Medintz, I.L. Nanoparticles and DNA—A powerful and growing functional combination in bionanotechnology. Nanoscale 2016, 8, 9037–9095. [Google Scholar] [CrossRef] [PubMed]
- Gozuacik, D.; Yagci-Acar, H.F.; Akkoc, Y.; Kosar, A.; Dogan-Ekici, A.I.; Ekici, S. Anticancer use of nanoparticles as nucleic acid carriers. J. Biomed. Nanotechnol. 2014, 10, 1751–1783. [Google Scholar] [CrossRef] [PubMed]
- Tarn, D.; Ashley, C.E.; Xue, M.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous silica nanoparticle nanocarriers: Biofunctionality and biocompatibility. Acc. Chem. Res. 2013, 46, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lee, N.; Kim, T.; Kim, J.; Hyeon, T. Multifunctional mesoporous silica nanocomposite nanoparticles for theranostic applications. Acc. Chem. Res. 2011, 44, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 2012, 41, 2590–2605. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Popat, A.; Hartono, S.B.; Stahr, F.; Liu, J.; Qiao, S.Z.; Lu, G.Q. Mesoporous silica nanoparticles for bioadsorption, enzyme immobilisation, and delivery carriers. Nanoscale 2011, 3, 2801–2818. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Zhang, J.; Linden, M.; Sahlgren, C. Mesoporous silica nanoparticles in tissue engineering—A perspective. Nanomedicine 2016, 11, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Sahlgren, C.; Linden, M. Multifunctional mesoporous silica nanoparticles for combined therapeutic, diagnostic and targeted action in cancer treatment. Curr. Drug Targets 2011, 12, 1166–1186. [Google Scholar] [CrossRef] [PubMed]
- Pascual, L.; Baroja, I.; Aznar, E.; Sancenon, F.; Marcos, M.D.; Murguia, J.R.; Amoros, P.; Rurack, K.; Martinez-Manez, R. Oligonucleotide-capped mesoporous silica nanoparticles as DNA-responsive dye delivery systems for genomic DNA detection. Chem. Commun. 2015, 51, 1414–1416. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Cheng, S.-H.; Liao, W.-N.; Wei, P.-R.; Sung, P.-J.; Weng, C.-F.; Lee, C.-H. Mesoporous silica nanoparticles for the improved anticancer efficacy of cis-platin. Int. J. Pharm. 2012, 429, 138–147. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Su, Z.; Xue, L.; Xu, H.; Zhang, C. Co-delivery of erlotinib and doxorubicin by ph-sensitive charge conversion nanocarrier for synergistic therapy. J. Control. Release 2016, 229, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Nandiyanto, A.B.D.; Kim, S.G.; Iskandar, F.; Okuyama, K. Synthesis of spherical mesoporous silica nanoparticles with nanometer-size controllable pores and outer diameters. Micropor. Mesopor. Mat. 2009, 120, 447–453. [Google Scholar] [CrossRef]
- Sheng, W.; Wei, W.; Li, J.; Qi, X.; Zuo, G.; Chen, Q.; Pan, X.; Dong, W. Amine-functionalized magnetic mesoporous silica nanoparticles for DNA separation. Appl. Surf. Sci. 2016, 387, 1116–1124. [Google Scholar] [CrossRef]
- Ganguly, A.; Ganguli, A.K. Anisotropic silica mesostructures for DNA encapsulation. Bull. Mater. Sci. 2013, 36, 329–332. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Meng, H.; Kabehie, S.; George, S.; Zink, J.I.; Nel, A.E. Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of sirna and DNA constructs. ACS Nano 2009, 3, 3273–3286. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, Q.R.; Zhang, J.; Xia, W.; Gu, H. The packaging of sirna within the mesoporous structure of silica nanoparticles. Biomaterials 2011, 32, 9546–9556. [Google Scholar] [CrossRef] [PubMed]
- Hartono, S.B.; Gu, W.; Kleitz, F.; Liu, J.; He, L.; Middelberg, A.P.J.; Yu, C.; Lu, G.Q.; Qiao, S.Z. Poly-l-lysine functionalized large pore cubic mesostructured silica nanoparticles as biocompatible carriers for gene delivery. ACS Nano 2012, 6, 2104–2117. [Google Scholar] [CrossRef] [PubMed]
- Kar, M.; Tiwari, N.; Tiwari, M.; Lahiri, M.; Sen Gupta, S. Poly-l-arginine grafted silica mesoporous nanoparticles for enhanced cellular uptake and their application in DNA delivery and controlled drug release. Part. Part. Syst. Charact. 2013, 30, 166–179. [Google Scholar] [CrossRef]
- Argyo, C.; Weiss, V.; Braeuchle, C.; Bein, T. Multifunctional mesoporous silica nanoparticles as a universal platform for drug delivery. Chem. Mater. 2014, 26, 435–451. [Google Scholar] [CrossRef]
- Meka, A.K.; Niu, Y.; Karmakar, S.; Hartono, S.B.; Zhang, J.; Lin, C.X.C.; Zhang, H.; Whittaker, A.; Jack, K.; Yu, M.; et al. Facile synthesis of large-pore bicontinuous cubic mesoporous silica nanoparticles for intracellular gene delivery. ChemNanoMat 2016, 2, 220–225. [Google Scholar] [CrossRef]
- Giret, S.; Man, M.W.C.; Carcel, C. Mesoporous-silica-functionalized nanoparticles for drug delivery. Chem. Eur. J. 2015, 21, 13850–13865. [Google Scholar] [CrossRef] [PubMed]
- Park, I.Y.; Kim, I.Y.; Yoo, M.K.; Choi, Y.J.; Cho, M.-H.; Cho, C.S. Mannosylated polyethylenimine coupled mesoporous silica nanoparticles for receptor-mediated gene delivery. Int. J. Pharm. 2008, 359, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, Y.; Ng, K.W.; Zhao, Y. Integrated hollow mesoporous silica nanoparticles for target drug/sirna co-delivery. Chem. Eur. J. 2013, 19, 15593–15603. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tsai, P.-H.; Hung, Y.; Chiou, S.-H.; Mou, C.-Y. Nonviral cell labeling and differentiation agent for induced pluripotent stem cells based on mesoporous silica nanoparticles. ACS Nano 2013, 7, 8423–8440. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.J.; Whittemore, A.D.; Donaldson, M.C.; Belkin, M.; Conte, M.S.; Polak, J.F.; Orav, E.J.; Ehsan, A.; Dell'Acqua, G.; Dzau, V.J. Ex-vivo gene therapy of human vascular bypass grafts with e2f decoy: The prevent single-centre, randomised, controlled trial. Lancet 1999, 354, 1493–1498. [Google Scholar] [CrossRef]
- Dean, N.M.; McKay, R.; Condon, T.P.; Bennett, C.F. Inhibition of protein kinase c-alpha expression in human a549 cells by antisense oligonucleotides inhibits induction of intercellular adhesion molecule 1 (icam-1) mrna by phorbol esters. J. Biol. Chem. 1994, 269, 16416–16424. [Google Scholar] [PubMed]
- Compagno, D.; Lampe, J.N.; Bourget, C.; Kutyavin, I.V.; Yurchenko, L.; Lukhtanov, E.A.; Gorn, V.V.; Gamper, H.B., Jr.; Toulme, J.J. Antisense oligonucleotides containing modified bases inhibit in vitro translation of leishmania amazonensis mrnas by invading the mini-exon hairpin. J. Biol. Chem. 1999, 274, 8191–8198. [Google Scholar] [CrossRef] [PubMed]
- Opalinska, J.B.; Gewirtz, A.M. Nucleic-acid therapeutics: Basic principles and recent applications. Nat. Rev. Drug Discov. 2002, 1, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.R.; Baeza, A.; Vallet-Regi, M. Recent applications of the combination of mesoporous silica nanoparticles with nucleic acids: Development of bioresponsive devices, carriers and sensors. Biomater. Sci. 2017, 5, 353–377. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Fuentes-Paniagua, E.; Baeza, A.; Sanchez-Nieves, J.; Cicuendez, M.; Gomez, R.; de la Mata, F.J.; Gonzalez, B.; Vallet-Regi, M. Mesoporous silica nanoparticles cecorated with carbosilane dendrons as new non-viral oligonucleotide delivery carriers. Chem. Eur. J. 2015, 21, 15651–15666. [Google Scholar] [CrossRef] [PubMed]
- Brevet, D.; Hocine, O.; Delalande, A.; Raehm, L.; Charnay, C.; Midoux, P.; Durand, J.-O.; Pichon, C. Improved gene transfer with histidine-functionalized mesoporous silica nanoparticles. Int. J. Pharm. 2014, 471, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Niemelae, M.; Westermarck, J.; Rosenholm, J.M. Mesoporous silica nanoparticles with redox-responsive surface linkers for charge-reversible loading and release of short oligonucleotides. Dalton Trans. 2014, 43, 4115–4126. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Na, H.-K.; Kim, Y.-K.; Ryoo, S.-R.; Cho, H.S.; Lee, K.E.; Jeon, H.; Ryoo, R.; Min, D.-H. Facile synthesis of monodispersed mesoporous silica nanoparticles with ultralarge pores and their application in gene delivery. ACS Nano 2011, 5, 3568–3576. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Zhu, Y.; Xu, Y.; Zhu, M.; Morita, H.; Hanagata, N. Mesoporous silica nanoparticles for enhancing the delivery efficiency of immunostimulatory DNA drugs. Dalton Trans. 2014, 43, 5142–5150. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Kim, M.; Eltohamy, M.; Yun, Y.-R.; Jang, J.-H.; Kim, H.-W. Efficacy of mesoporous silica nanoparticles in delivering BMP-2 plasmid DNA for in vitro osteogenic stimulation of mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2013, 101A, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Suwalski, A.; Dabboue, H.; Delalande, A.; Bensamoun, S.F.; Canon, F.; Midoux, P.; Saillant, G.; Klatzmann, D.; Salvetat, J.-P.; Pichon, C. Accelerated achilles tendon healing by pdgf gene delivery with mesoporous silica nanoparticles. Biomaterials 2010, 31, 5237–5245. [Google Scholar] [CrossRef] [PubMed]
- Tivnan, A.; Orr, W.S.; Gubala, V.; Nooney, R.; Williams, D.E.; McDonagh, C.; Prenter, S.; Harvey, H.; Domingo-Fernandez, R.; Bray, I.M.; et al. Inhibition of neuroblastoma tumor growth by targeted delivery of microrna-34a using anti-disialoganglioside gd2 coated nanoparticles. PLoS ONE 2012, 7, e38129. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Shen, H.-H.; Wu, Z.; Wang, B.; Zhao, D.; He, L. Self-assembly of bi-functional peptides on large-pore mesoporous silica nanoparticles for mirna binding and delivery. J. Mater. Chem. B 2015, 3, 7653–7657. [Google Scholar] [CrossRef]
- Yu, M.; Xue, Y.; Ma, P.X.; Mao, C.; Lei, B. Intrinsic ultrahigh drug/mirna loading capacity of biodegradable bioactive glass nanoparticles towards highly efficient pharmaceutical delivery. ACS Appl. Mater. Interfaces 2017, 9, 8460–8470. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Cheng, Q.; Jiang, Q.; Huang, Y.; Yang, Z.; Han, S.; Zhao, Y.; Guo, S.; Liang, Z.; Dong, A. Intracellular cleavable poly(2-dimethylaminoethyl methacrylate) functionalized mesoporous silica nanoparticles for efficient sirna delivery in vitro and in vivo. Nanoscale 2013, 5, 4291–4301. [Google Scholar] [CrossRef] [PubMed]
- Hom, C.; Lu, J.; Liong, M.; Luo, H.; Li, Z.; Zink, J.I.; Tamanoi, F. Mesoporous silica nanoparticles facilitate delivery of sirna to shutdown signaling pathways in mammalian cells. Small 2010, 6, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Hartono, S.B.; Phuoc, N.T.; Yu, M.; Jia, Z.; Monteiro, M.J.; Qiao, S.; Yu, C. Functionalized large pore mesoporous silica nanoparticles for gene delivery featuring controlled release and co-delivery. J. Mater. Chem. B 2014, 2, 718–726. [Google Scholar] [CrossRef]
- Ngamcherdtrakul, W.; Morry, J.; Gu, S.; Castro, D.J.; Goodyear, S.M.; Sangvanich, T.; Reda, M.M.; Lee, R.; Mihelic, S.A.; Beckman, B.L.; et al. Cationic polymer modified mesoporous silica nanoparticles for targeted sirna delivery to HER2+ breast cancer. Adv. Funct. Mater. 2015, 25, 2646–2659. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.; Wang, M.; Ma, Y.; Xia, W.; Gu, H. A mesoporous silica nanoparticle—PEI—fusogenic peptide system for sirna delivery in cancer therapy. Biomaterials 2013, 34, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gu, H.; Zhang, D.S.-Z.; Li, F.; Liu, T.; Xia, W. Highly effective inhibition of lung cancer growth and metastasis by systemic delivery of sirna via multimodal mesoporous silica-based nanocarrier. Biomaterials 2014, 35, 10058–10069. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang Ding, S.-Z.; Gu, H.; Wang, X.; Liu, T.; Wang, Y.; Di, W. Highly effective antiangiogenesis via magnetic mesoporous silica-based sirna vehicle targeting the vegf gene for orthotopic ovarian cancer therapy. Int. J. Nanomedicine 2015, 10, 2579–2594. [Google Scholar] [PubMed]
- Meng, H.; Mai, W.X.; Zhang, H.; Xue, M.; Xia, T.; Lin, S.; Wang, X.; Zhao, Y.; Ji, Z.; Zink, J.I.; et al. Codelivery of an optimal drug/sirna combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano 2013, 7, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Devi, G.; Qu, Q.; Toh, D.F.; Chen, G.; Zhao, Y. Intracellular delivery of antisense peptide nucleic acid by fluorescent mesoporous silica nanoparticles. Bioconjug. Chem. 2014, 25, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mu, Y.; Lu, J.; Wei, W.; Wan, Y.; Liu, S. Target-cell-specific fluorescence silica nanoprobes for imaging and theranostics of cancer cells. Anal. Chem. 2014, 86, 3602–3609. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Qian, L.; Uttamchandani, M.; Li, L.; Yao, S.Q. Single-vehicular delivery of antagomir and small molecules to inhibit miR-122 function in hepatocellular carcinoma cells by using "smart" mesoporous silica nanoparticles. Angew. Chem. Int. Ed. 2015, 54, 10574–10578. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Qian, L.; Ge, J.; Fu, J.; Yuan, P.; Yao, S.C.L.; Yao, S.Q. Cell-penetrating poly(disulfide)-assisted intracellular delivery of mesoporous silica nanoparticles for inhibition of miR-21 function and detection of subsequent therapeutic effects. Angew. Chem. Int. Ed. 2016, 55, 9272–9276. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, S.V.; Emami, F.S.; Berry, R.J.; Jones, S.E.; Naik, R.R.; Deschaume, O.; Heinz, H.; Perry, C.C. Chemistry of aqueous silica nanoparticle surfaces and the mechanism of selective peptide adsorption. J. Am. Chem. Soc. 2012, 134, 6244–6256. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, X.; Ma, Y.; Gu, H. Endosomal escape kinetics of mesoporous silica-based system for efficient sirna delivery. Int. J. Pharm. 2013, 448, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.R.; Muthuswamy, E.; Wani, A.; Brichacek, M.; Castaneda, A.L.; Brock, S.L.; Oupicky, D. Enhanced gene and sirna delivery by polycation-modified mesoporous silica nanoparticles loaded with chloroquine. Pharm. Res. 2010, 27, 2556–2568. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, S.F. Overcoming cellular barriers for rna therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Cheng, F.; Zhou, R.; Cao, J.; Li, J.; Burda, C.; Min, Q.; Zhu, J.J. DNA-hybrid-gated multifunctional mesoporous silica nanocarriers for dual-targeted and microrna-responsive controlled drug delivery. Angew. Chem. Int. Ed. 2014, 53, 2371–2375. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Hao, N.; Chen, H.-Y.; Xu, J.-J. Tumor-marker-mediated "on-demand" drug release and real-time monitoring system based on multifunctional mesoporous silica nanoparticles. Anal. Chem. 2014, 86, 10239–10245. [Google Scholar] [CrossRef] [PubMed]
- Climent, E.; Martinez-Manez, R.; Sancenon, F.; Marcos, M.D.; Soto, J.; Maquieira, A.; Amoros, P. Controlled delivery using oligonucleotide-capped mesoporous silica nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 7281–7283. [Google Scholar] [CrossRef] [PubMed]
- He, D.; He, X.; Wang, K.; Chen, M.; Cao, J.; Zhao, Y. Reversible stimuli-responsive controlled release using mesoporous silica nanoparticles functionalized with a smart DNA molecule-gated switch. J. Mater. Chem. 2012, 22, 14715–14721. [Google Scholar] [CrossRef]
- Meng, H.; Liong, M.; Xia, T.; Li, Z.; Ji, Z.; Zink, J.I.; Nel, A.E. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and p-glycoprotein sirna to overcome drug resistance in a cancer cell line. ACS Nano 2010, 4, 4539–4550. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Tang, C.; Yin, C. Dual-targeting and pH/redox-responsive multi-layered nanocomplexes for smart co-delivery of doxorubicin and sirna. Biomaterials 2015, 60, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yu, D.; Jia, B.; Jin, C.; Liu, X.; Zhao, X.; Zhang, G. Targeting cd133+ laryngeal carcinoma cells with chemotherapeutic drugs and sirna against abcg2 mediated by thermo/pH-sensitive mesoporous silica nanoparticles. Tumor Biol. 2016, 37, 2209–2217. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.M.; Shahin, S.A.; Wen, W.; Finlay, J.B.; Dong, J.; Wang, R.; Dellinger, T.H.; Zink, J.I.; Tamanoi, F.; Glackin, C.A. Nanoparticle delivery of sirna against twist to reduce drug resistance and tumor growth in ovarian cancer models. Nanomedicine 2017, 13, 965–976. [Google Scholar] [CrossRef] [PubMed]
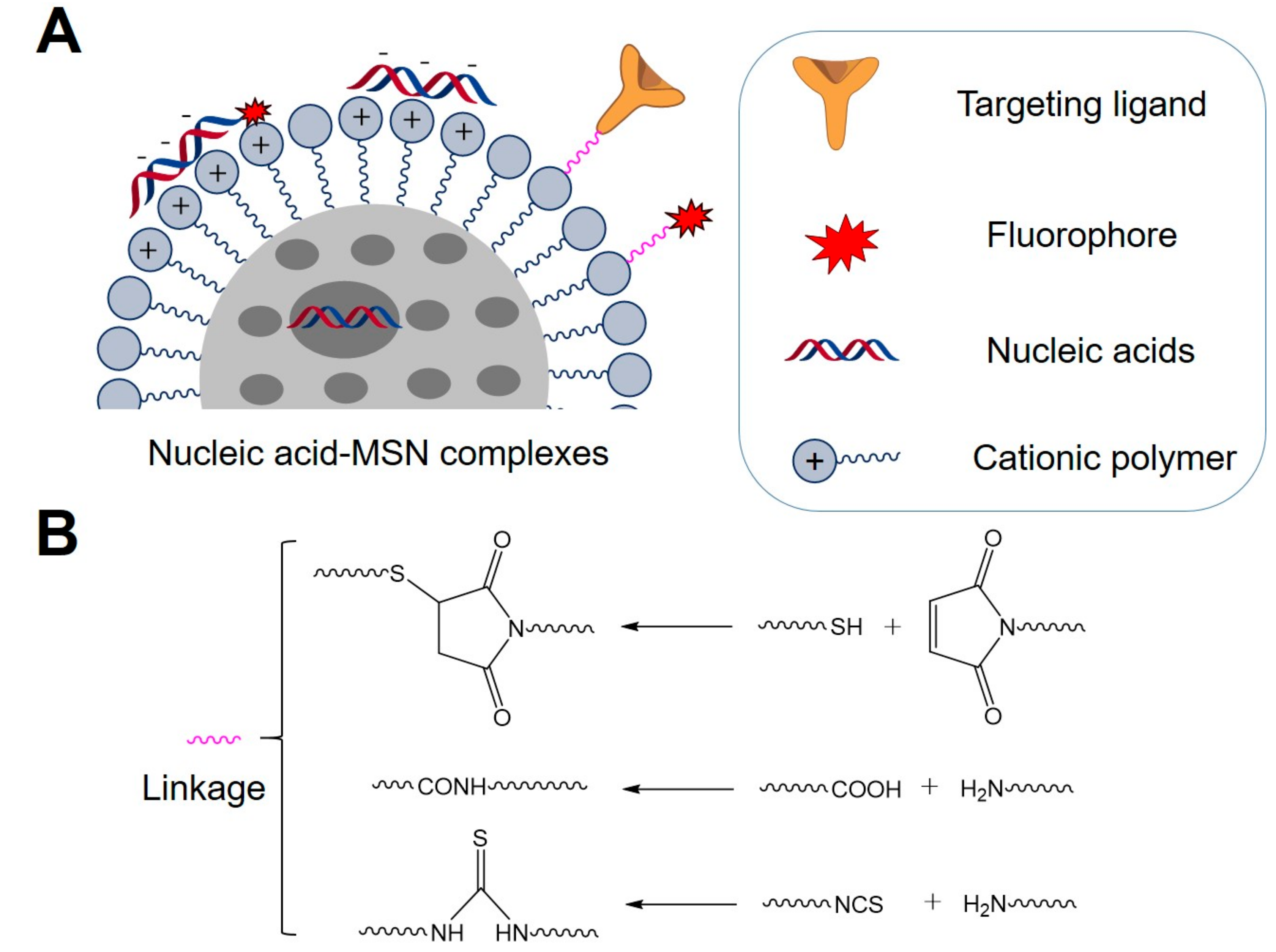
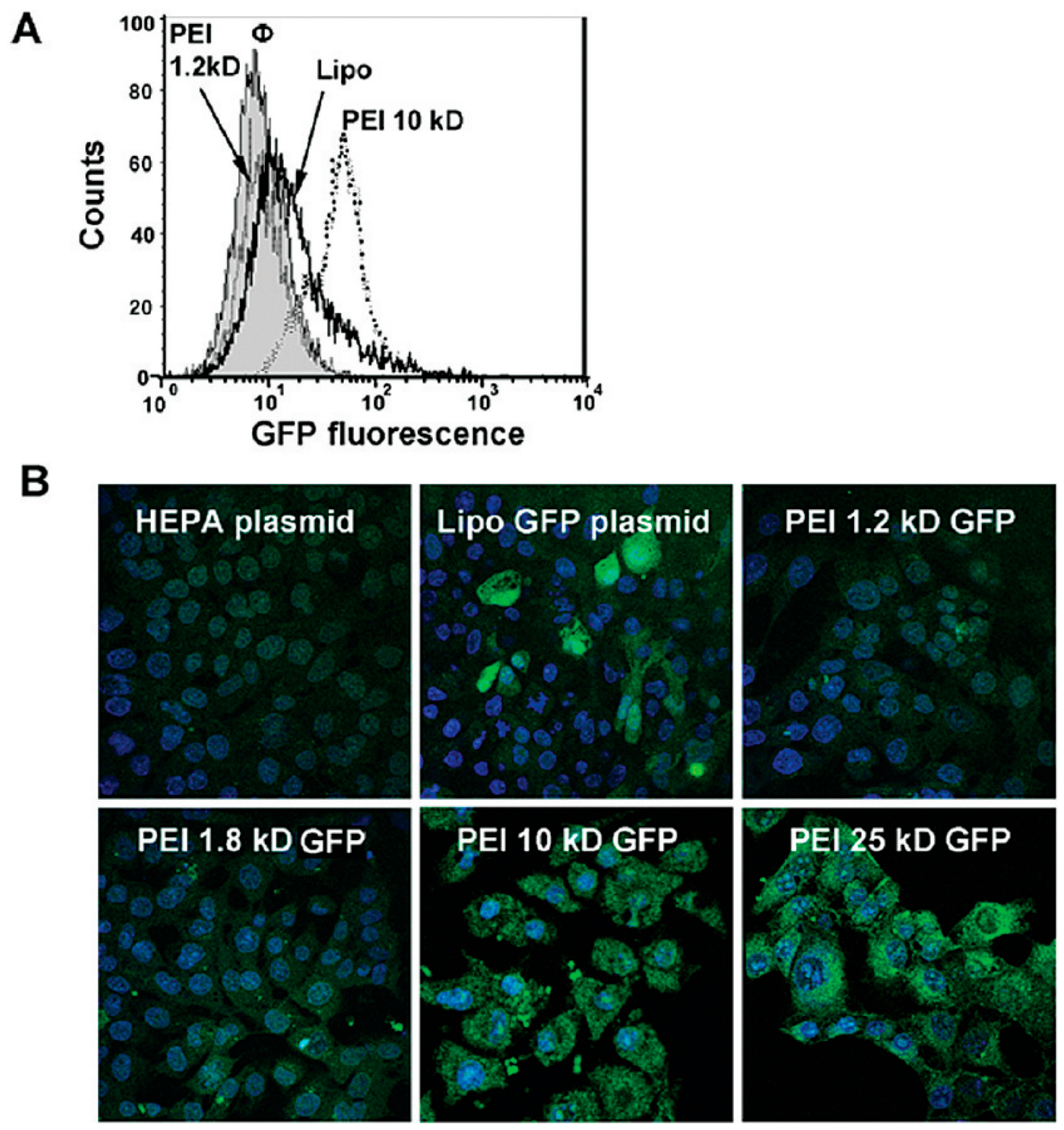

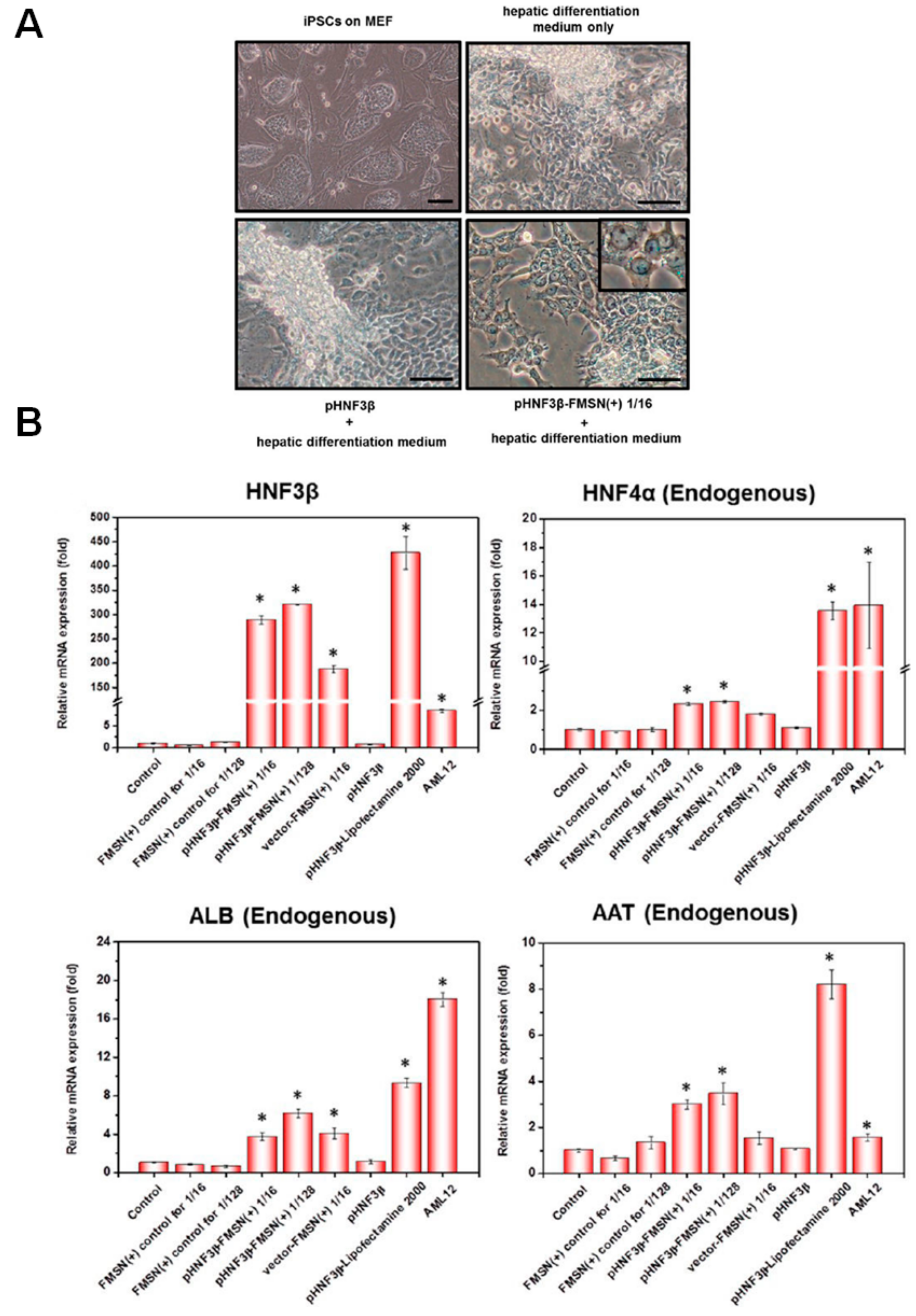


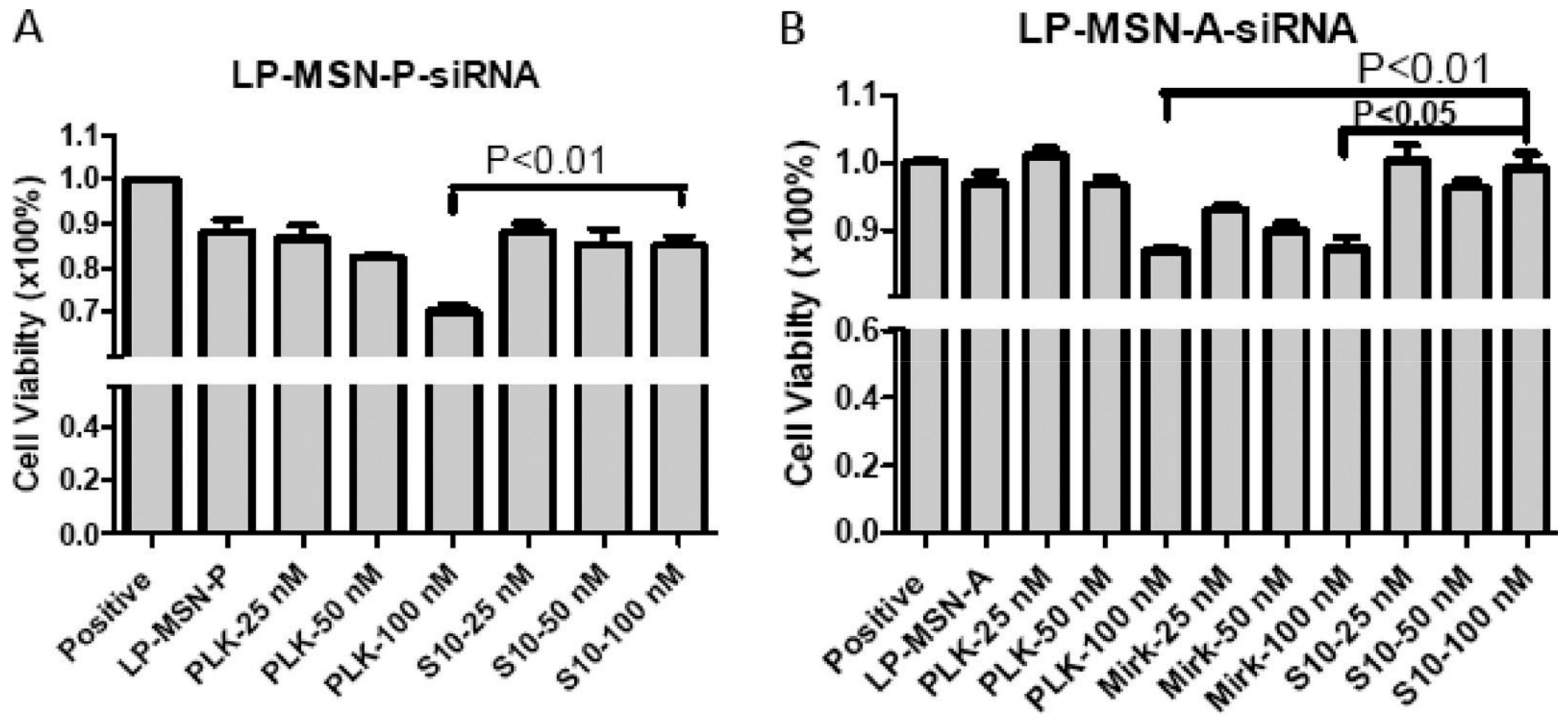


| MSNs | Nucleic Acids/Fluorophore-Labeled Nucleic Acids | Ref. | ||||
|---|---|---|---|---|---|---|
| MSN Size (nm) | Pore Size (nm) | Surface Coatings | Name | Antisense Strand (5’-3’) | Sense Strand (5’-3’) | |
| ~200 | ~2.71 | Carbosilane dendrons | ssDNACy3 | Cy3-TTATCGCTGATTCAAGACTGA | / | [52] |
| ~180 | / | l-histidine | pDNA-Luciferase | / | / | [53] |
| ~120 | ~2.5 | PEI | pDNA-GFP | / | / | [37] |
| ~70 | ~3.5–5 | Amine with disulfide linker | dsDNA | CTGTGGTTGTGTTTGCACTTT | AGTGCAAACACAACCACAGTT | [54] |
| ~80 | ~2.6 | Poly-arginine | pDNA-mCherry | / | / | [40] |
| ~250 | ~23 | Amine | pDNA-GFP | / | / | [55] |
| ~130 | ~12 | Amine | dsDNA | TCAGAGAGTTAGAGAGTTAGAGAGTCAGAGAGTTAGAGAGTTAGAGAGTCAGAGAGTTAGAGAGTTAGAGAG | CTCTCTAACTCTCTAACTCTCTGACTCTC-TAACTCTCTAACTCTCTGACTCTCTAACTCTCTAACTCTCTGA | [56] |
| ~150 | / | Amine | pDNA-HNF3β | / | / | [46] |
| ~205 | ~2.64 | Amine | pDNA-BMP2 | / | / | [57] |
| ~200 | ~3 | Amine | pDNA-PDGF | / | / | [58] |
| ~74 | / | Poly-amidoamine | miR-34a | UGGCAGUGUCUUAGCUGGUUGU | CAAUCAGCAAGUAUACUGCCCU | [59] |
| ~219.5 | ~18.8 | Bifunctional peptide | miR-29b | GCUGGUUUCAUAUGGUGGUUUAGA | GCUGGUUUCAUAUGGUGGUUUAGA | [60] |
| ~250 | ~3.5 | Calcium | miR-5106 | AGGUCUGUAGCUCAGUUGGCAGA-FAM | UCUGCCAACUGAGCUACAGACCU | [61] |
| ~150 | ~10 | PDMAEMA | siRNA-PLK | UUAAGCAGCUCGUUAAUGGTT-FAM or Cy3 | CCAUUAACGAGCUGCUUAATT | [62] |
| ~130 | ~2.5 | PEI | siRNA-Kras | UUUCCUACUAGGACCAUAGGT | CUAUGGUCCUAGUAGGAAATT | [63] |
| ~100–200 | ~28 | Polylysine | siRNA-MirK | UUCCGGAACAUGAAGUGCCGC | GGCACUUCAUGUUCCGGAAUU | [39] |
| ~100–200 | ~28 | Polylysine | siRNA-S10 | ACGUCGCAGUAACUGUUGCUU | ACGUCGCAGUAACUGUUGCUU | [39] |
| ~100–200 | ~11 | PDMAEA | siRNA-PLK | UUAAGCAGCUCGUUAAUGGTT | CCAUUAACGAGCUGCUUAATT | [64] |
| ~100 | / | PEI | siRNA-HER2 | UUGGGCAUGGACUCAAACGUGUU | CACGUUUGAGUCCAUGCCCAAUU | [65] |
| ~50 | ~3.6 | PEI | siRNA-VEGF | GAUCUCAUCAGGGUACUCCTT | GGAGUACCCUGAUGAGAUCTT | [66,67,68] |
| ~51 | / | PEI | siRNA-Pgp | CGGAAGGCCUAAUGCCGAATT | UUGCGCAUUAGGCCUUCCGTT | [69] |
| ~110 | / | Amine with disulfide linker | PNA-Bcl2 | TCTCCCAGCGTGCGCCAT-Cy5 | / | [70] |
| ~60 | / | Amine | Double-stranded ASOs targeting miR-21 | GTCAACATCAGTCTGATAAGCTATGTCGC-FAM | DABCYL-GCGACATAGCT | [71] |
| ~120 | ~3 | Amine | Antagomir-122 | ACAAACACCATTGTCACACTCCA | / | [72] |
| ~105 | ~3.2 | Amines | Antagomir-21 | UCAACAUCAGUCUGAUAAGCUA | / | [73] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, W.; Fan, R.; Miao, Y.; Zhou, Y.; Qin, C.; Shan, X.; Wan, X.; Li, J. Mesoporous Silica Nanoparticles as Carriers for Intracellular Delivery of Nucleic Acids and Subsequent Therapeutic Applications. Molecules 2017, 22, 782. https://doi.org/10.3390/molecules22050782
Cha W, Fan R, Miao Y, Zhou Y, Qin C, Shan X, Wan X, Li J. Mesoporous Silica Nanoparticles as Carriers for Intracellular Delivery of Nucleic Acids and Subsequent Therapeutic Applications. Molecules. 2017; 22(5):782. https://doi.org/10.3390/molecules22050782
Chicago/Turabian StyleCha, Wenzhang, Rengen Fan, Yufeng Miao, Yong Zhou, Chenglin Qin, Xiangxiang Shan, Xinqiang Wan, and Jinbo Li. 2017. "Mesoporous Silica Nanoparticles as Carriers for Intracellular Delivery of Nucleic Acids and Subsequent Therapeutic Applications" Molecules 22, no. 5: 782. https://doi.org/10.3390/molecules22050782





