Synthesis, Crystal Structures and Properties of Ferrocenyl Bis-Amide Derivatives Yielded via the Ugi Four-Component Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
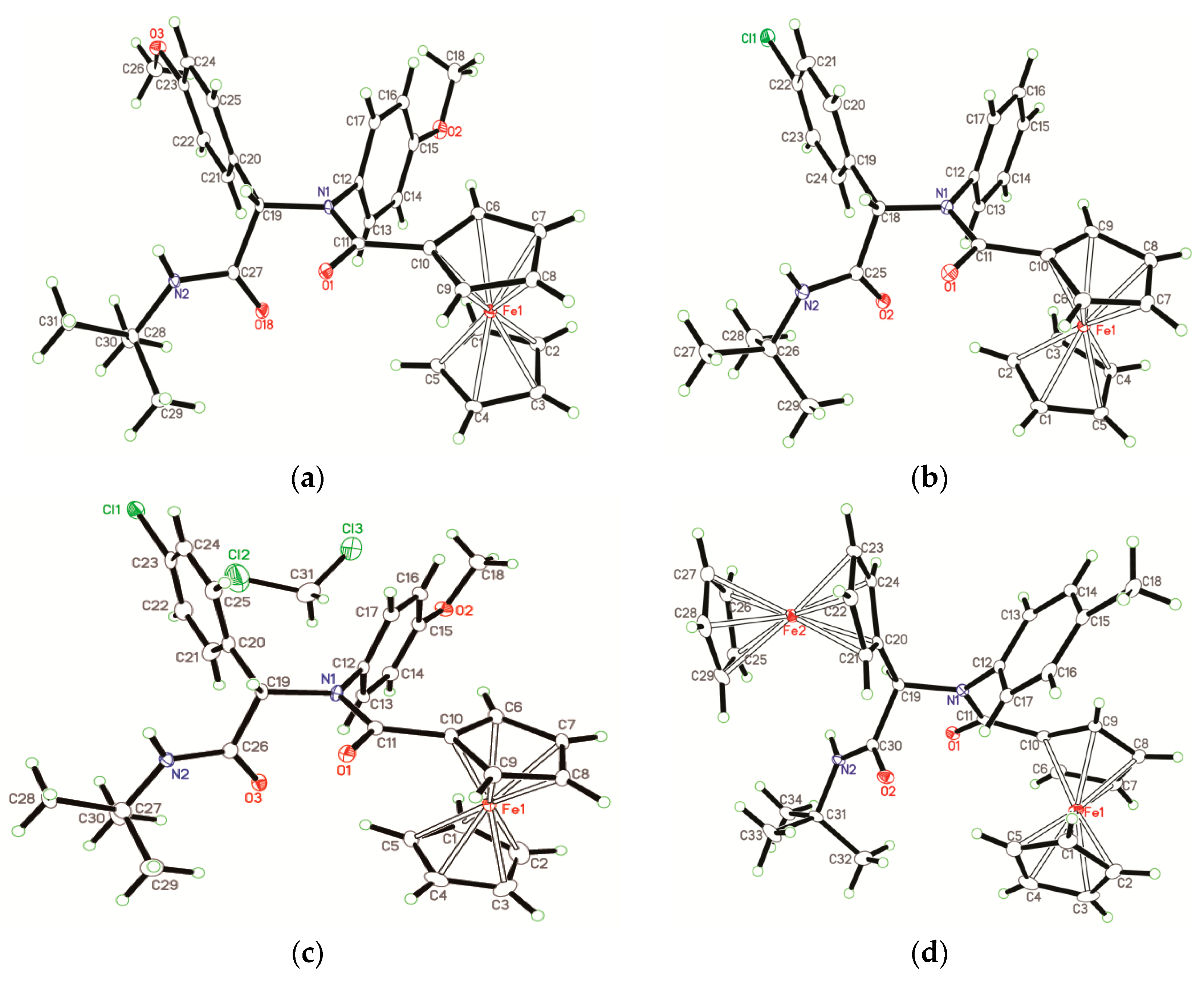
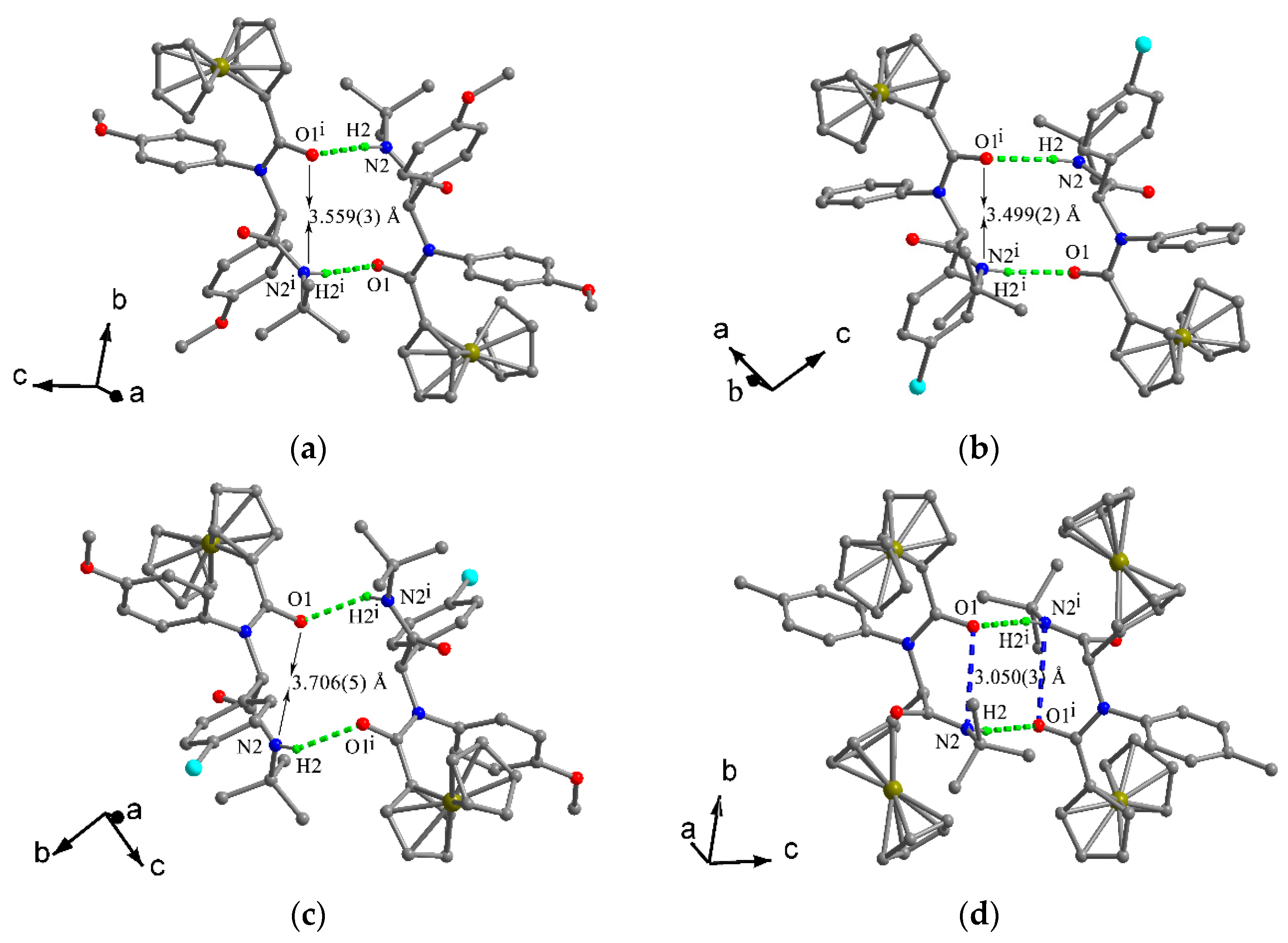
2.2. Crystal Structures
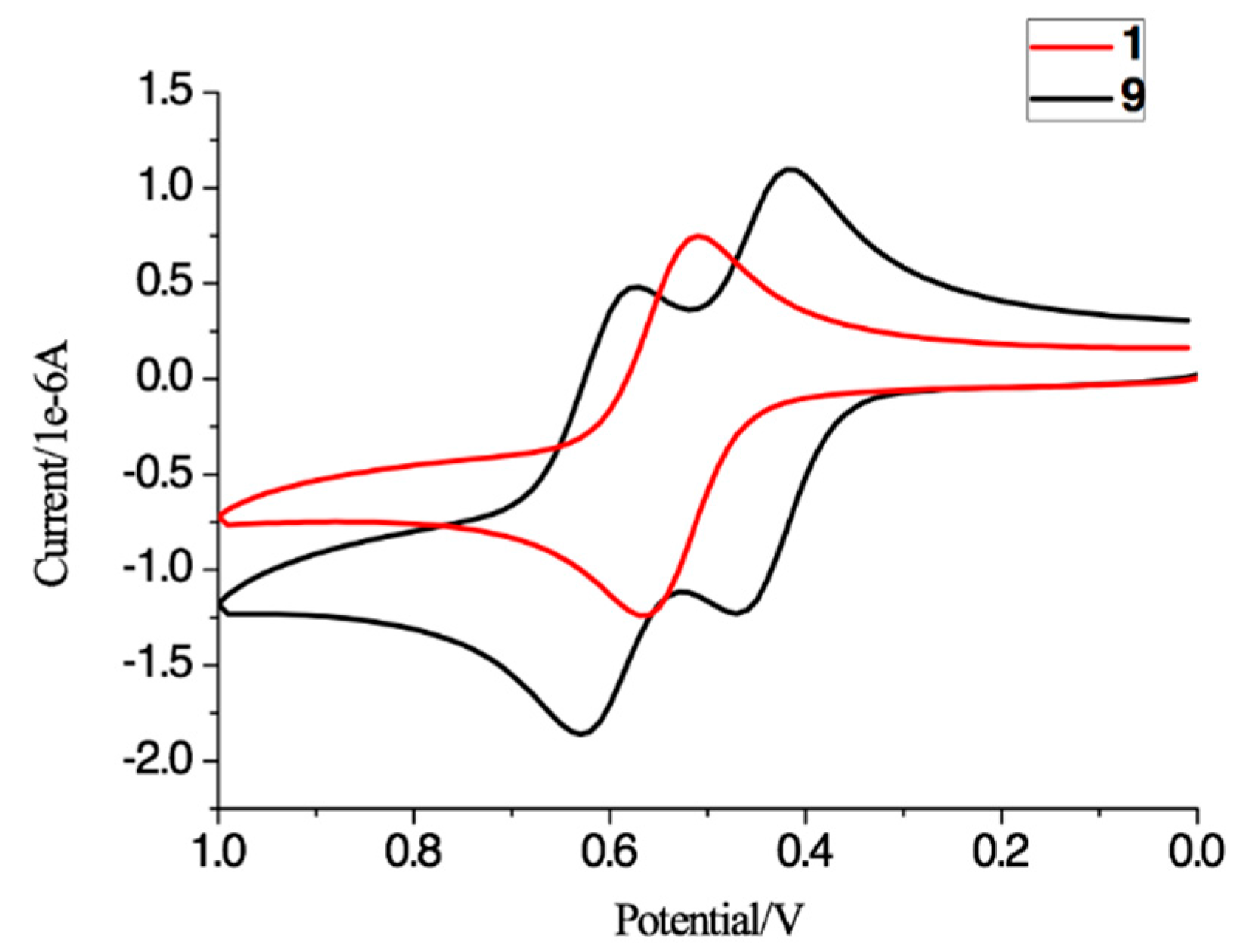
2.3. Electrochemistry
3. Experimental Section
3.1. Materials and Instruments
3.2. Synthesis
3.3. Crystal Structure Determination
3.4. Electrochemistry Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Staveren, D.R. Metzler-Nolte, N. Bioorganometallic chemistry of ferrocene. Chem. Rev. 2004, 104, 5931–5985. [Google Scholar] [CrossRef] [PubMed]
- Albada, B.; Metzler-Nolte, N. Organometallic-peptide bioconjugates: Synthetic strategies and medicinal applications. Chem. Rev. 2016, 116, 11797–11839. [Google Scholar] [CrossRef] [PubMed]
- Arrayás, R.G.; Adrio, J.; Carretero, J.C. Recent applications of chiral ferrocene ligands in asymmetric catalysis. Angew. Chem. Int. Ed. 2006, 45, 7674–7715. [Google Scholar] [CrossRef] [PubMed]
- Chiral Ferrocenes in Asymmetric Catalysis; Dai, L.-X.; Hou, X.-L. (Eds.) Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Beer, P.D.; Gale, P.A.; Chen, G.Z. Mechanisms of electrochemical recognition of cations, anions and neutral guest species by redox-active receptor molecules. Coord. Chem. Rev. 1999, 185–186, 3–36. [Google Scholar] [CrossRef]
- Molina, P.; Tárraga, A.; Caballero, A. Ferrocene-based small molecules for multichannel molecular recognition of cations and anions. Eur. J. Inorg. Chem. 2008, 3401–3417. [Google Scholar] [CrossRef]
- Guo, D.-S.; Liu, Z.-P.; Ma, J.-P.; Huang, R.-Q. A novel ferrocene-based thiacalix[4]arene ditopic receptor for electrochemical sensing of europium(III) and dihydrogen phosphate ions. Tetrahedron Lett. 2007, 48, 1221–1224. [Google Scholar] [CrossRef]
- Moriuchi, T.; Hirao, T. Design of ferrocene-dipeptide bioorganometallic conjugates to induce chirality-organized structures. Acc. Chem. Res. 2010, 43, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Liu, J.; Burgess, K. Minimalist and universal peptidomimetics. Chem. Soc. Rev. 2011, 40, 4411–4421. [Google Scholar] [CrossRef] [PubMed]
- Moriuchi, T.; Nomoto, A.; Yoshida, K.; Ogawa, A.; Hirao, T. Chirality organization of ferrocenes bearing podand dipeptide chains: Synthesis and structural characterization. J. Am. Chem. Soc. 2001, 123, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.-T.; Abu-Irhayem, E.; Kraatz, H.-B. Peptide electron transfer: More questions than answers. Chem. Eur. J. 2005, 11, 5186–5194. [Google Scholar] [CrossRef] [PubMed]
- Kirin, S.I.; Schatzschneider, U.; de Hatten, X.; Weyhermüller, T.; Metzler-Nolte, N. 1,n′-Disubstituted ferrocenoyl amino acids and dipeptides: Conformational analysis by CD spectroscopy, X-ray crystallography, and DFT calculations. J. Organomet. Chem. 2006, 691, 3451–3457. [Google Scholar] [CrossRef]
- Okamoto, S.; Morita, T.; Kimura, S. Electron transfer through a self-assembled monolayer of a double-helix peptide with linking the terminals by ferrocene. Langmuir 2009, 25, 3297–3304. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, R.; Mirzaei, P.; Bazgir, A. A simple synthesis of ferrocenyl bis-amides by a Ugi four-component reaction. J. Organomet. Chem. 2010, 695, 2320–2324. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Amanpour, T.; Mirzaei, P.; Bazgir, A. A simple four-component synthesis of ferrocenyl amidodiesters and ferrocenyl triamides. J. Organomet. Chem. 2011, 696, 3421–3424. [Google Scholar] [CrossRef]
- Shao, G.-K.; Zhao, M.; Wei, Z.; Ma, J.-P.; Guo, D.-S. Two novel ferrocenyl dipeptide-like compounds generated via the Ugi four-component reaction. Acta Cryst. 2015, C71, 667–672. [Google Scholar]
- Saweczko, P.; Enright, G.D.; Kraatz, H.-B. Interaction of ferrocenoyl-dipeptides with 3-aminopyrazole derivatives: β-Sheet models? A synthetic, spectroscopic, structural, and electrochemical study. Inorg. Chem. 2001, 40, 4409–4419. [Google Scholar] [CrossRef] [PubMed]
- Kirin, S.I.; Kraatz, H.-B.; Metzler-Nolte, N. Systematizing structural motifs and nomenclature in 1,n′-disubstituted ferrocene peptides. Chem. Soc. Rev. 2006, 35, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Savage, D.; Alley, S.R.; Hogan, T.; Kelly, P.N.; Draper, S.M.; Fitchett, C.M.; Kenny, P.T.M. The synthesis and structural characterization of N-para-ferrocenyl benzoyl dipeptide esters: The X-ray crystal structure of N-{para-(ferrocenyl)benzoyl}-l-alanine-glycine ethyl ester. J. Organomet. Chem. 2006, 691, 4686–4693. [Google Scholar] [CrossRef]
- Dömling, A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef] [PubMed]
- Ugi, I.; Meyr, R.; Fetzer, U.; Steinbrückner, C. Versuche mit isonitrilen. Angew. Chem. 1959, 71, 386. [Google Scholar]
- Thompson, L.A.; Ellman, J.A. Synthesis and applications of small molecule libraries. Chem. Rev. 1996, 96, 555–600. [Google Scholar] [CrossRef] [PubMed]
- Dömling, A.; Ugi, I. Multicomponent reactions with isocyanides. Angew. Chem. Int. Ed. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Brandl, M.; Weiss, M.S.; Jabs, A.; Sühnel, J.; Hilgenfeld, R. C−H⋅⋅⋅π interactions in proteins. J. Mol. Biol. 2001, 307, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S.; Honda, K.; Uchimaru, T.; Mikami, M.; Tanabe, K. The magnitude of the CH/π interaction between benzene and some model hydrocarbons. J. Am. Chem. Soc. 2000, 122, 3746–3753. [Google Scholar] [CrossRef]
- Umezawa, Y.; Tsuboyama, S.; Takahashi, H.; Uzawa, J.; Nishio, M. CH/π interaction in the conformation of peptides. A database study. Bioorg. Med. Chem. 1999, 7, 2021–2026. [Google Scholar] [CrossRef]
- Uncuța, C.; Tudose, A.; Căproiu, M.T.; Plăveți, M.; Kakou-Yao, R. Beckmann and cyclization reactions of δ-oxo-α,β-unsaturated ketoximes obtained from pyrylium salts and hydroxylamine. Formation of 2-aryl(or alkyl)amino-4,6-disubstituted pyrylium salts. Tetrahedron 1999, 55, 15011–15024. [Google Scholar]
- Liu, H.-Y.; Mou, R.-Q.; Sun, C.-Z.; Zhang, S.-Y.; Guo, D.-S. Synthesis of ferrocene[c]pyridin-2(1H)-one derivatives via Pd(II)-catalyzed C–H activation reaction under air. Tetrahedron Lett. 2016, 57, 4676–4679. [Google Scholar] [CrossRef]
- Tang, J.; Liu, X.-F.; Zhang, L.-Y.; Xu, X.-L.; Zhan, P.-R. A new convenient method for the synthesis of formyl ferrocene with triethyl orthoformate and A1C13. Synth. Commun. 2000, 30, 1657–1660. [Google Scholar] [CrossRef]
- Reeves, P.C. Carboxylation of aromatic compounds: Ferrocenecarboxylic acid. Org. Synth. 1988, 6, 625–627. [Google Scholar]
- Sonoda, A.; Moritani, I. Reactions of ferrocenylcarbene IV. The synthesis of [3]-ferrocenophan-2-one tosylhydrazone and the thermal decomposition of its sodium salt. J. Organomet. Chem. 1971, 26, 133–140. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97; Program for Crystal Structure Solution; University of Göttingen: Lower Saxony, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97; Program for Crystal Structure Refinement; University of Gottingen: Lower Saxony, Germany, 1997. [Google Scholar]
Sample Availability: Not available. |
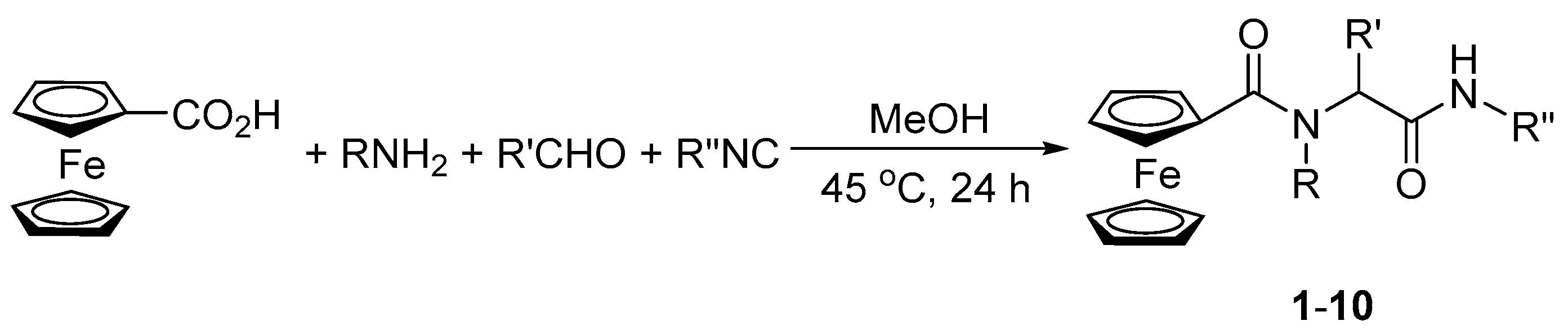

| Product | RNH2 | R′CHO | R″NC | Yield (%) |
|---|---|---|---|---|
| 1 |  |  | t-BuNC | 73 |
| 2 |  |  | t-BuNC | 68 |
| 3 |  |  | t-BuNC | 81 |
| 4 |  |  | t-BuNC | 73 |
| 5 |  |  | t-BuNC | 91 |
| 6 |  |  | t-BuNC | 76 |
| 7 |  |  | CyNC | 87 |
| 8 |  |  | CyNC | 91 |
| 9 |  |  | t-BuNC | 87 |
| 10 |  |  | t-BuNC | 91 |
| 3 | 4 | 6 | 9 | ||
|---|---|---|---|---|---|
| T1 | −5.4(2) | 4.7(1) | −0.3(3) | −1.1(2) | 1.1(2) |
| T2 | −6.0(2) | 4.9(1) | −10.2(4) | −0.8(2) | 0.8(2) |
| T3 | −5.8(2) | 4.5(1) | −10.1(4) | −1.2(2) | 1.2(2) |
| T4 | −5.5(2) | 4.5(1) | −10.3(4) | −0.8(2) | 0.8(2) |
| T5 | −5.4(2) | 4.0(1) | −10.9(3) | −1.7(2) | 1.7(2) |
| D1 | 0.7(1) | 0.9(1) | 0.5(2) | 0.7(1) | |
| D2 | 78.0(2) | 83.2(2) | 80.8(4) | 84.6(3) | |
| D3 | 13.1(2) | 3.0(1) | 1.0(3) | 7.9(2) | |
| D4 | 82.4(2) | 87.4(2) | 89.5(3) | 83.3(2) | |
| Product | D−H⋅⋅⋅A | D−H | H⋅⋅⋅A | D⋅⋅⋅A | D−H⋅⋅⋅A |
|---|---|---|---|---|---|
| 3 | C6−H6⋅⋅⋅Cg3 | 0.93 | 2.69 | 3.454(2) | 138.9 |
| N2−H2⋅⋅⋅O1 a | 0.86 | 2.16 | 3.016(3) | 171.7 | |
| C14−H14⋅⋅⋅O18 b | 0.93 | 2.38 | 3.298(3) | 169.1 | |
| C22−H22⋅⋅⋅Cg1 b | 0.93 | 2.82 | 3.627(3) | 145.3 | |
| 4 | C9−H9⋅⋅⋅Cg3 | 0.93 | 2.60 | 3.392(2) | 142.9 |
| N2−H2⋅⋅⋅O1 a | 0.86 | 2.18 | 3.037(3) | 173.6 | |
| C17−H17⋅⋅⋅ Cg1 b | 0.93 | 2.99 | 3.674(2) | 131.9 | |
| 6 | C6−H6⋅⋅⋅Cg3 | 0.98 | 2.44 | 3.275(5) | 142.5 |
| N2−H2⋅⋅⋅O1 a | 0.86 | 2.22 | 2.968(5) | 145.0 | |
| C19–H19··· O1 a | 0.98 | 2.41 | 3.245(5) | 142.5 | |
| C8–H8···O3 b | 0.98 | 2.23 | 3.198(5) | 169.1 | |
| C4–H4···Cl3 c | 0.98 | 2.59 | 3.529(6) | 160.8 | |
| C31–H31A···Cl3 d | 0.97 | 2.74 | 3.489(8) | 134.7 | |
| 9 | C9–H9···Cg3 | 0.93 | 2.52 | 3.308(2) | 143.0 |
| N2–H2···O1 a | 0.86 | 2.16 | 3.009(3) | 169.8 |
| Product | Epa (mV) | Epc (mV) | Epa − Epc (mV) | E1/2 (mV) | Ipa/Ipc |
|---|---|---|---|---|---|
| 1 | 570 | 510 | 60 | 540 | 1.02 |
| 2 | 560 | 500 | 60 | 530 | 1.04 |
| 3 | 560 | 500 | 60 | 530 | 1.01 |
| 4 | 580 | 520 | 60 | 550 | 1.04 |
| 5 | 580 | 520 | 60 | 550 | 1.05 |
| 6 | 580 | 520 | 60 | 550 | 1.04 |
| 7 | 560 | 500 | 60 | 530 | 1.03 |
| 8 | 560 | 500 | 60 | 530 | 1.00 |
| 9 b | 630, 470 | 570, 410 | 60, 60 | 600, 440 | 1.58, 1.04 |
| 10 b | 630, 470 | 570, 410 | 60, 60 | 600, 440 | 1.51, 1.06 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Shao, G.-K.; Huang, D.-D.; Lv, X.-X.; Guo, D.-S. Synthesis, Crystal Structures and Properties of Ferrocenyl Bis-Amide Derivatives Yielded via the Ugi Four-Component Reaction. Molecules 2017, 22, 737. https://doi.org/10.3390/molecules22050737
Zhao M, Shao G-K, Huang D-D, Lv X-X, Guo D-S. Synthesis, Crystal Structures and Properties of Ferrocenyl Bis-Amide Derivatives Yielded via the Ugi Four-Component Reaction. Molecules. 2017; 22(5):737. https://doi.org/10.3390/molecules22050737
Chicago/Turabian StyleZhao, Mei, Guang-Kui Shao, Dan-Dan Huang, Xue-Xin Lv, and Dian-Shun Guo. 2017. "Synthesis, Crystal Structures and Properties of Ferrocenyl Bis-Amide Derivatives Yielded via the Ugi Four-Component Reaction" Molecules 22, no. 5: 737. https://doi.org/10.3390/molecules22050737





