An Increased Understanding of Enolate Additions under Mechanochemical Conditions
Abstract
:1. Introduction
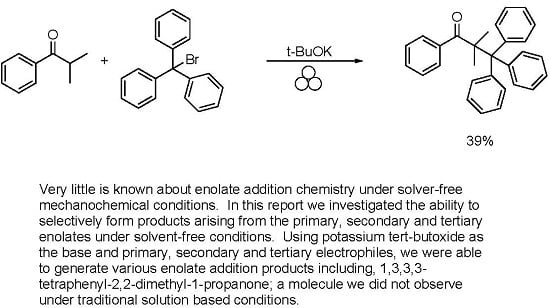
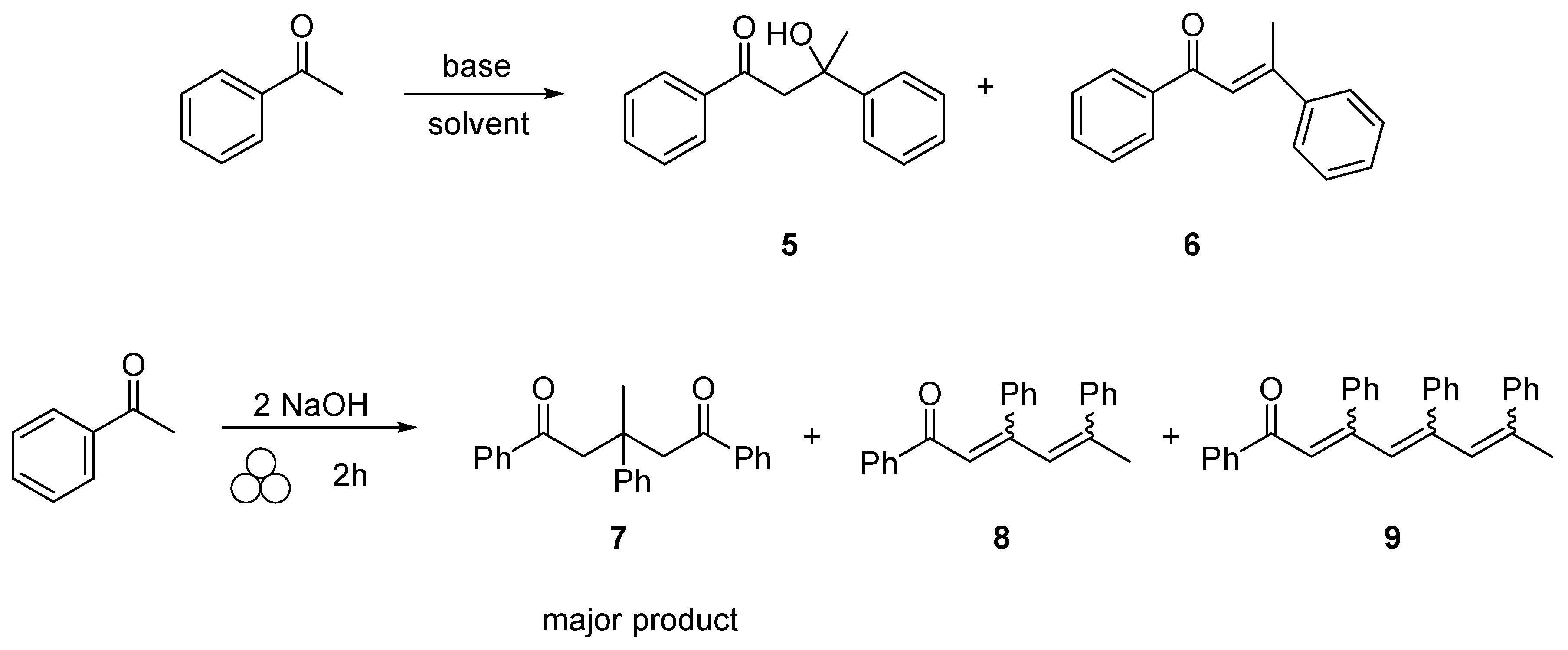
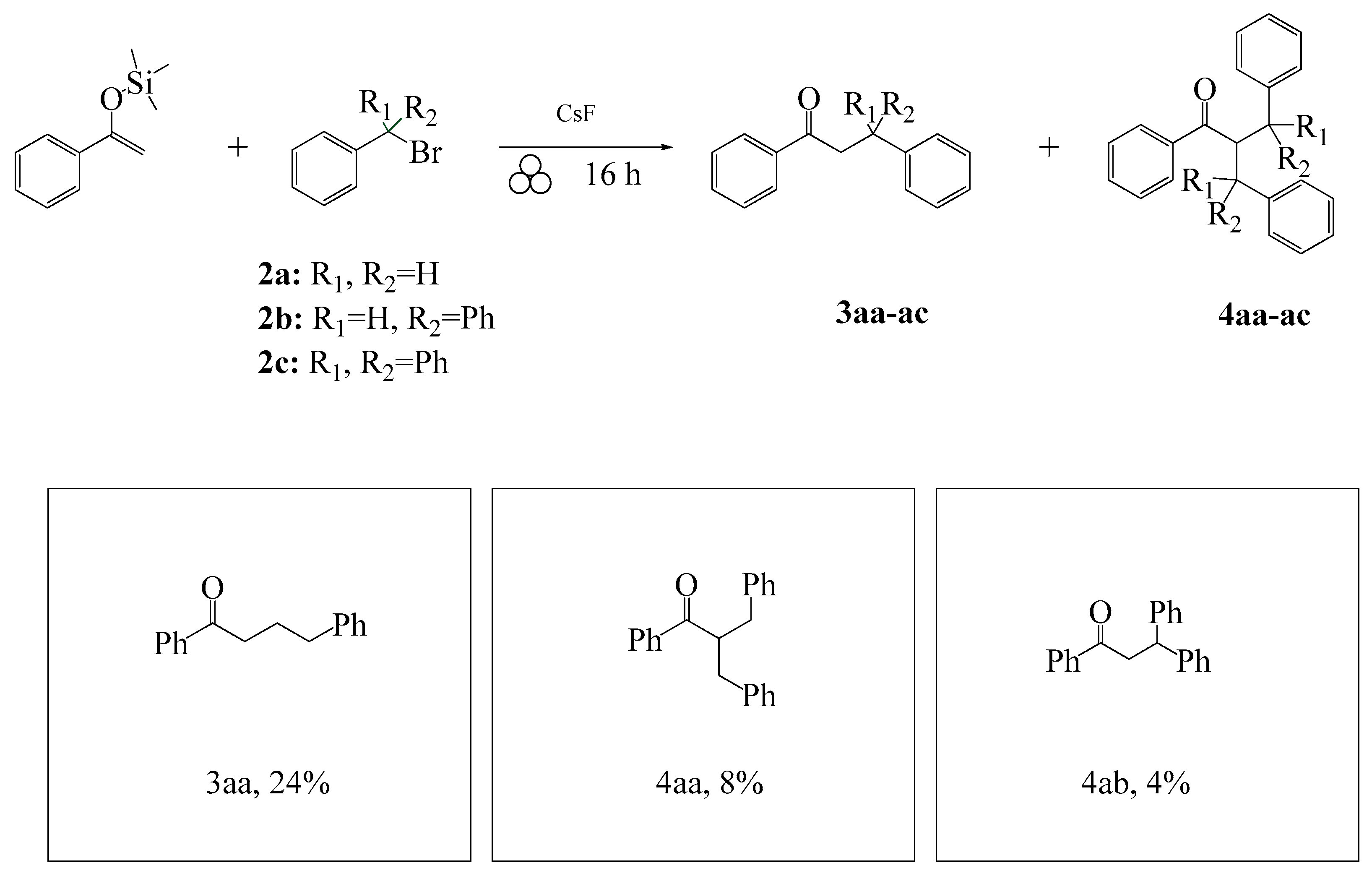
2. Results and Discussion
3. Materials and Methods
General Enolate Reaction Procedure
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Almaşi, D.; Alonso, D.A.; Balaguer, A.-N.; Nájera, C. Water versus solvent-free conditions for the enantioselective inter- and intramolecular aldol reaction employing l-prolinamides and l-prolinethioamides as organocatalysts. Adv. Synth. Catal. 2009, 351, 1123–1131. [Google Scholar] [CrossRef]
- Crawford, D.E.; Miskimmin, C.K.G.; Albadarin, A.B.; Walker, G.; James, S.L. Organic synthesis by twin screw extrusion (TSE): Continuous, scalable and solvent-free. Green Chem. 2017, 19, 1507–1518. [Google Scholar] [CrossRef]
- Gérard, E.M.C.; Sahin, H.; Encinas, A.; Bräse, S. Systematic study of a solvent-free mechanochemically induced domino oxa-michael-aldol reaction in a ball mill. Synlett 2008, 17, 2702–2704. [Google Scholar]
- Kumar, S. An improved one-pot and eco-friendly synthesis of aurones under solvent-free conditions. Green Chem. Lett. Rev. 2014, 7, 95–99. [Google Scholar] [CrossRef]
- Machuca, E.; Juaristi, E. Organocatalytic activity of α,α-dipeptide derivatives of (S)-proline in the asymmetric aldol reaction in absence of solvent. Evidence for non-covalent π-π interactions in the transition state. Tetrahedron Lett. 2015, 56, 1144–1148. [Google Scholar] [CrossRef]
- Rodríguez, B.; Bruckmann, A.; Bolm, C. A highly efficient asymmetric organocatalytic aldol reaction in a ball mill. Chem. Eur. J. 2007, 13, 4710–4722. [Google Scholar] [CrossRef] [PubMed]
- Rappe, C.; Sachs, W.H. Enolization of ketones. Iv. Rate and orientation of base-catalyzed deuteration of some methyl ketones. J. Org. Chem. 1967, 32, 4127–4128. [Google Scholar] [CrossRef]
- Ellis, T.K.; Martin, C.H.; Tsai, G.M.; Ueki, H.; Soloshonok, V.A. Efficient synthesis of sterically constrained symmetrically α,α-disubstituted α-amino acids under operationally convenient conditions. J. Org. Chem. 2003, 68, 6208–6214. [Google Scholar] [CrossRef] [PubMed]
- House, H.O.; Prabhu, A.V.; Phillips, W.V. Chemistry of carbanions. XXVIII. Carbon-13 nuclear magnetic resonance spectra of metal enolates. J. Org. Chem. 1976, 41, 1209–1214. [Google Scholar] [CrossRef]
- Jackman, L.M.; Lange, B.C. Methylation of lithioisobutyrophenone in weakly polar aprotic solvents. The effect of aggregation. J. Am. Chem. Soc. 1981, 103, 4494–4499. [Google Scholar] [CrossRef]
- Damoun, S.; Van de Woude, G.; Choho, K.; Geerlings, P. Influence of alkylating reagent softness on the regioselectivity in enolate ion alkylation: A theoretical local hard and soft acids and bases study. J. Phys. Chem. A 1999, 103, 7861–7866. [Google Scholar] [CrossRef]
- Toda, F.; Tanaka, K.; Hamai, K. Aldol condensations in the absence of solvent: Acceleration of the reaction and enhancement of the stereoselectivity. J. Chem. Soc. Perkin Trans. 1 1990, 3207–3209. [Google Scholar] [CrossRef]
- Jörres, M.; Aceña, J.L.; Soloshonok, V.A.; Bolm, C. Asymmetric carbon-carbon bond formation under solventless conditions in ball mills. ChemCatChem 2015, 7, 1265–1269. [Google Scholar] [CrossRef]
- Waddell, D.; Thiel, I.; Clark, T.; Marcum, S.; Mack, J. Making kinetic and thermodynamic enolates via solvent-free high speed ball milling. Green Chem. 2010, 12, 209–211. [Google Scholar] [CrossRef]
- Díez-Barra, E.; de la Hoz, A.; Loupy, A.; Martínez-González, A.; Martínez-Merino, V.; Merino, S.; Paugam, R.; Sánchez-Verdú, P.; Sansoulet, J.; Torres, J. Unexpected double benzylation of acetophenone under phase transfer catalysis conditions. Acidity or π−π interaction effect? Tetrahedron 1997, 53, 3659–3668. [Google Scholar] [CrossRef]
- McKissic, K.S.; Caruso, J.T.; Blair, R.G.; Mack, J. Comparison of shaking versus baking: Further understanding the energetics of a mechanochemical reaction. Green Chem. 2014, 16, 1628–1632. [Google Scholar] [CrossRef]
- Although we have observed the various aldol self-condensation products under milling conditions, we were unable to isolate and purify these products through various separation methods, therefore we were unable to provide isolated yields. Characterization of these compounds were done through GC-MS and comparison to literature values.
- Koester, R.; Pourzal, A.-A. Condensation products from alkyl phenyl ketones. Synthesis 1973, 674–676. [Google Scholar]
- Wayne, W.; Adkins, H. The condensation of ketones by aluminum t-butoxide to compounds of the mesityl oxide type. J. Am. Chem. Soc. 1940, 62, 3401–3404. [Google Scholar] [CrossRef]
- Liu, W.-Y.; Xu, Q.-H.; Liang, Y.-M.; Chen, B.-H.; Liu, W.-M.; Ma, Y.-X. Preparation of 1,5-diketone derivatives containing ferrocenyl by michael reaction under solvent-free condition. J. Organomet. Chem. 2001, 637–639, 719–722. [Google Scholar] [CrossRef]
- Smith, N.M.; Raston, C.L.; Smith, C.B.; Sobolev, A.N. PEG mediated synthesis of amino-functionalised 2,4,6-triarylpyridines. Green Chem. 2007, 9, 1185–1190. [Google Scholar] [CrossRef]
- Shankar, R.; Jha, A.K.; Singh, U.S.; Hajela, K. An efficient and improved synthesis of 1,5-diketones: Versatile conjugate addition of nucleophiles to α,β-unsaturated enones and alkynones. Tetrahedron Lett. 2006, 47, 3077–3079. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.; Pang, Y.; Ge, Z.; Li, R. Unexpected synthesis of 1,3,5-triarly-1,5-diketones from aryl ketones via di-enamine mechanism. Tetrahedron 2014, 70, 9240–9244. [Google Scholar] [CrossRef]
- Takahashi, H.; Arai, T.; Yanagisawa, A. 1,5-diketone synthesis promoted by barium hydride or barium alkoxides. Synlett 2006, 2006, 2833–2835. [Google Scholar] [CrossRef]
- Muzart, J. Self-condensation of ketones catalyzed by basic aluminum oxide. Synthesis 2002, 1982, 60–61. [Google Scholar] [CrossRef]
- An excellent suggestion made by a reviewer of this manuscript was to include the amount of self-condensation products formed in each reaction. However, given the various self-aldol products generated, 1,5 diketone (product 7) in addition to several additional 1,2 addition products (products 8 and 9) and the inability to isolate and full characterize these products, we cannot determine the actual yields of self-condensation.
- Clarke, S.L.; McSweeney, C.M.; McGlacken, G.P. Investigation of a novel diamine based chiral auxiliary in the asymmetric alkylation of ketones. Tetrahedron Asymmetry 2014, 25, 356–361. [Google Scholar] [CrossRef]
- Hanawa, H.; Abe, N.; Maruoka, K. Double coordination and activation ability of methylalumoxane (MAO) for hetero functionality: Pivotal role as polymerization cocatalyst. Tetrahedron Lett. 1999, 40, 5365–5368. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
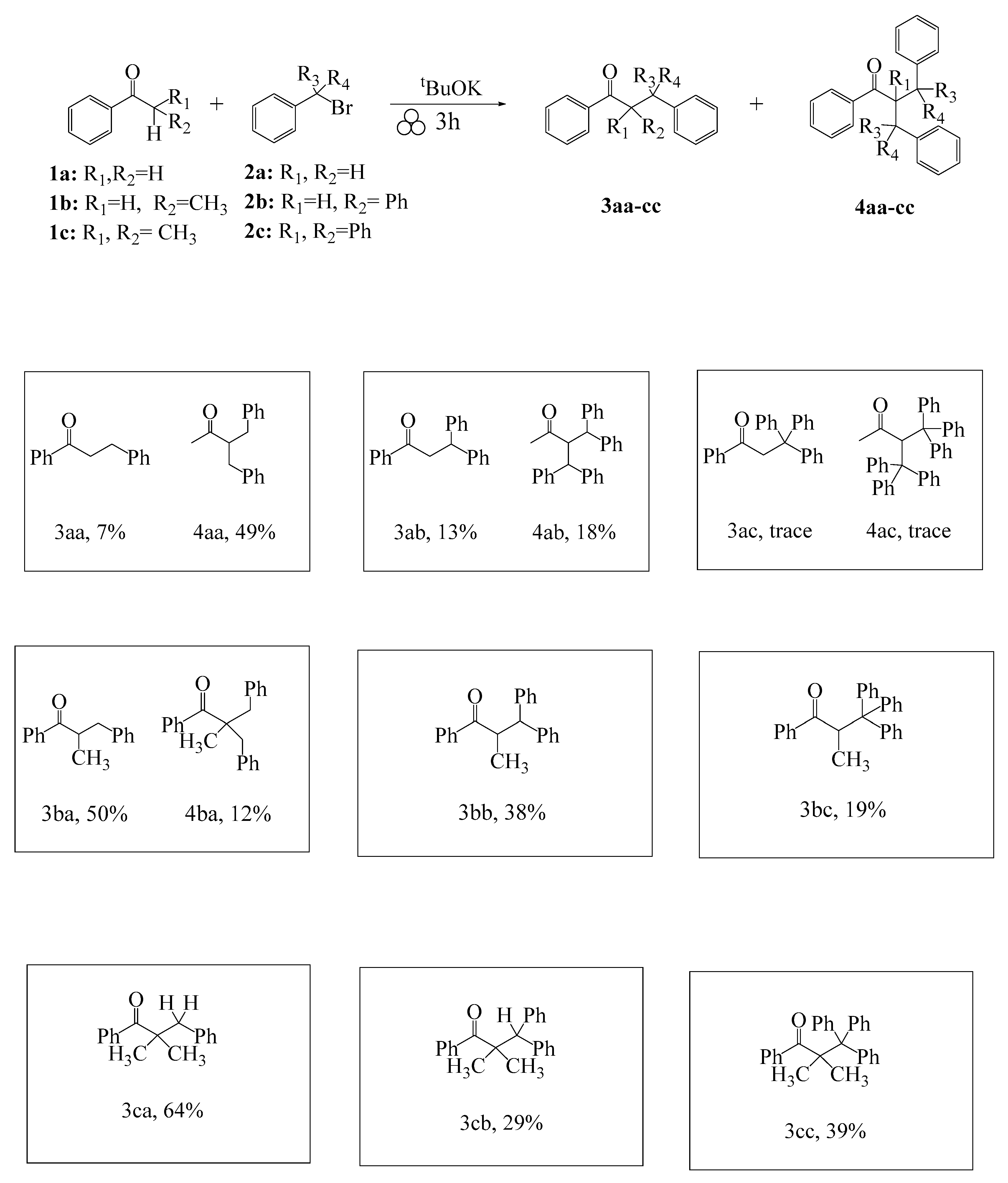

| Entry | Base | Yield | Product Ratio 3aa/4aa |
|---|---|---|---|
| 1 | tBuOK | 94% | 12/88 |
| 2 | NaNH2 | 91% | 12/88 |
| 3 | KOH | 78% | 10/90 |
| 4 | LiHMDS | 52% | 42/58 |
| 5 | NaOH | 11% | 36/64 |
| 6 | CS2CO3 | 3% | 100/0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hopgood, H.; Mack, J. An Increased Understanding of Enolate Additions under Mechanochemical Conditions. Molecules 2017, 22, 696. https://doi.org/10.3390/molecules22050696
Hopgood H, Mack J. An Increased Understanding of Enolate Additions under Mechanochemical Conditions. Molecules. 2017; 22(5):696. https://doi.org/10.3390/molecules22050696
Chicago/Turabian StyleHopgood, Heather, and James Mack. 2017. "An Increased Understanding of Enolate Additions under Mechanochemical Conditions" Molecules 22, no. 5: 696. https://doi.org/10.3390/molecules22050696