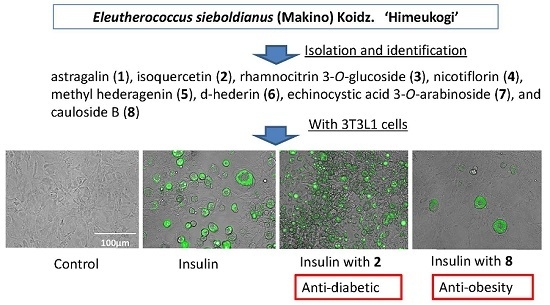
Effects of Flavonoids and Triterpene Analogues from Leaves of Eleutherococcus sieboldianus (Makino) Koidz. ‘Himeukogi’ in 3T3-L1 Preadipocytes
Abstract
:1. Introduction
2. Results
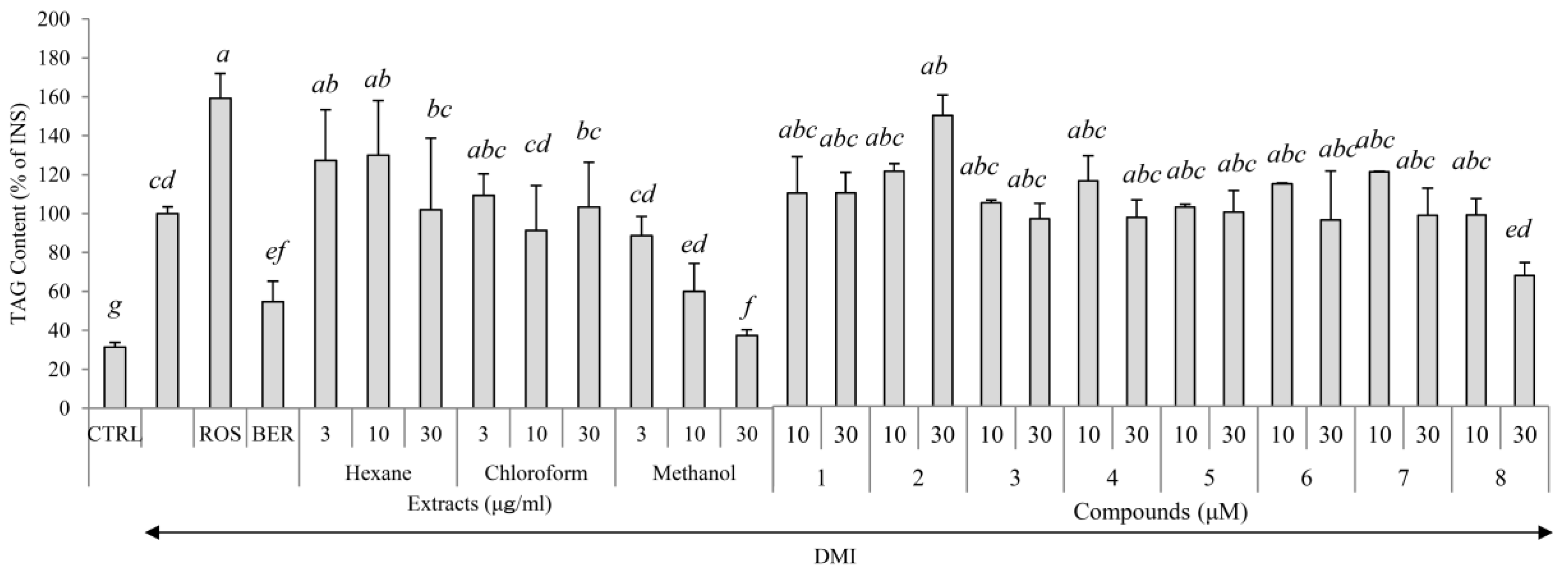
2.1. Yields, Cytotoxicity, and Regulatory Effects on Adipogenesis of the Extracts from E. sieboldianus
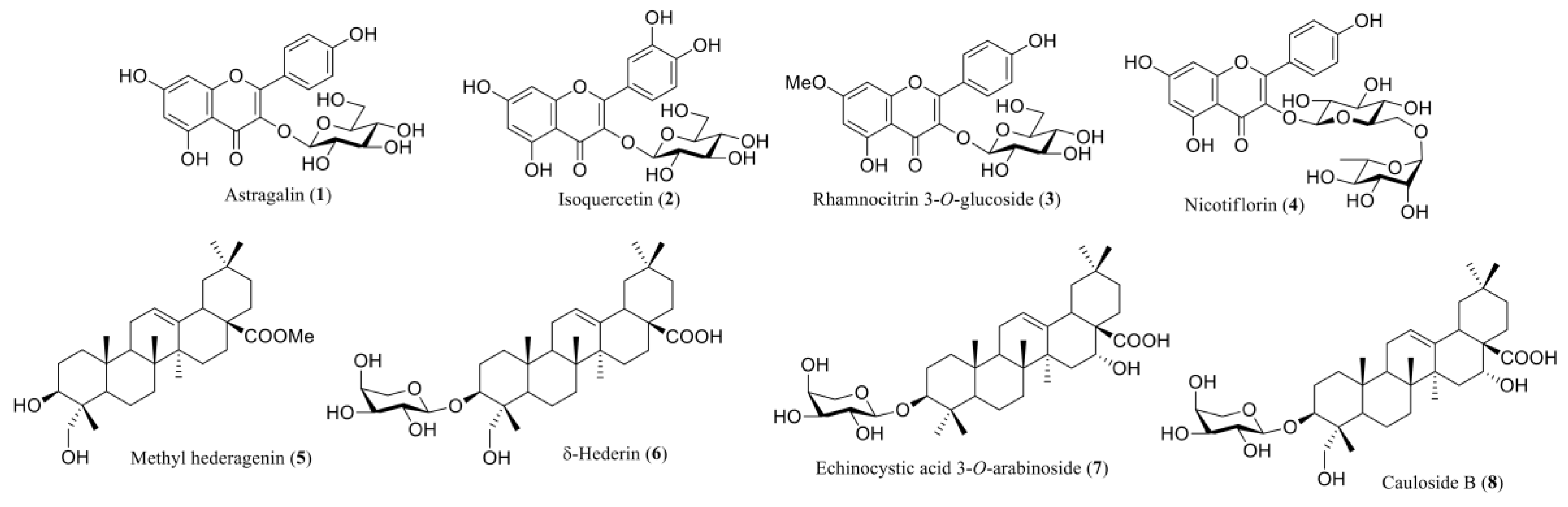
2.2. Characterization of the Compounds Isolated
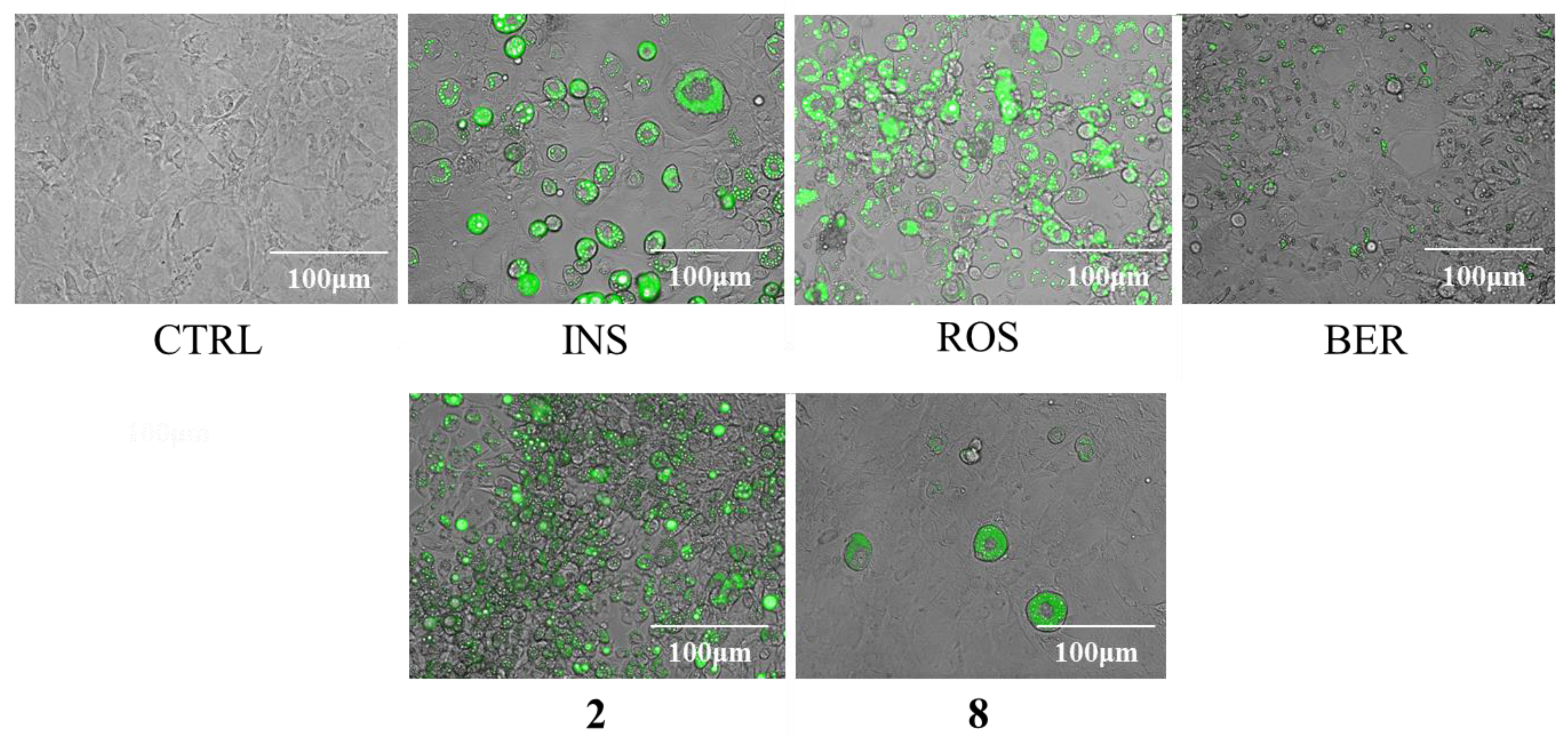
2.3. Cytotoxicity and Regulatory Effects on Adipogenesis of the Compounds Isolated from E. sieboldianus
2.4. The Effects of 2 and 8 on Adipogenic Proteins
2.5. The Effects of 2 and 8 on the Expression of Lipogenic Proteins
2.6. The Effects of 2 and 8 on the Expression of a Lipolytic Protein
2.7. The Effects of 2 and 8 on the Expression of Adiponectin
2.8. The Effects of 2 and 8 on the Expression of GLUT4
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Solvent Fractionation
4.3. Isolation of Active Constituents
4.4. Analytical Instrument of Active Components
4.5. Spectral Data of Isolated Components
4.6. Cell Culture
4.7. Cell Toxicity Assay
4.8. Measurement of Intracellular Triglycerol Level
4.9. Detection of Proteins
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ueno, T.; Noda, H.; Ogata, T. Bioavailability of dietary antioxidants in Acanthopanax sieboldianus Makino leaves. Stud. Sci. Technol. 2013, 2, 35–42. [Google Scholar]
- Komatsu, K.; Nanba, T.; Park, J.H.; Chang, K.W. Studies on the Crude Drug from Korea. (VIII). On the Folk Medicine “Min Gal Pi” Derived from Genus Acanthopanax. Syouyakugaku Zasshi 1992, 46, 195–201. [Google Scholar]
- Sawada, H.; Miyakoshi, M.; Isoda, S.; Ida, Y.; Shoji, J. Saponins from leaves of Acanthopanax sieboldianus. Phytochemistry 1993, 34, 1117–1121. [Google Scholar] [CrossRef]
- Moto, M.; Uchida, N.; Kurakane, S.; Yamakada, N. Antidiabetic effect of himeukogi (Acanthopanax sieboldianus) leaf tea on type 2 diabetic mice. Kasei Gakkaishi 2010, 61, 647–653. [Google Scholar]
- Kato, M.; Kurakane, S.; Nishina, A.; Park, J.; Chang, H. The blood lactate increase in high intensity exercise is depressed by Acanthopanax sieboldianus. Nutrients 2013, 5, 4134–4144. [Google Scholar] [CrossRef] [PubMed]
- Moto, M.; Tamura, A.; Yamada, N. Inhibitory effects of himeukogi leaf tea on postprandial blood glucose elevation in rats and humans. Nihon Eiyou Syokuryou Gakkaishi 2008, 61, 111–117. [Google Scholar] [CrossRef]
- Kang, M.C.; Kang, N.; Ko, S.C.; Kim, Y.B.; Jeon, Y.J. Anti-obesity effects of seaweeds of Jeju Island on the differentiation of 3T3-L1 preadipocytes and obese mice fed a high-fat diet. Food Chem. Toxicol. 2016, 90, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, T.; Inaguma, T.; Han, J.; Villareal, M.O.; Isoda, H. Cyanidin-3-glucoside derived from black soybeans ameliorate type 2 diabetes through the induction of differentiation of preadipocytes into smaller and insulin-sensitive adipocytes. J. Nutr. Biochem. 2015, 26, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Lafontan, M. Adipose tissue and adipocyte dysregulation. Diabetes Metab. 2014, 40, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Shao, J. Role of adipocytokines in obesity-associated insulin resistance. J. Nutr. Biochem. 2008, 19, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Capurso, C.; Capurso, A. From excess adiposity to insulin resistance: The role of free fatty acids. Vasc. Pharmacol. 2012, 57, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Salans, L.B.; Knittle, J.L.; Hirsch, J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J. Clin. Investig. 1968, 47, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A. Central role of the adipocyte in the insulin-sensitising and cardiovascular risk modifying actions of the thiazolidinediones. Biochimie 2003, 85, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.G.; Javorschi, S.; Hevener, A.L.; Kruszynska, Y.T.; Norman, R.A.; Sinha, M.; Olefsky, J.M. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes 2002, 51, 2968–2974. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Olefsky, J.M. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes 1996, 45, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Rangwala, S.M.; Lazar, M.A. Peroxisome proliferator-activated receptor gamma in diabetes and metabolism. Trends Pharmacol. Sci. 2004, 25, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Arner, P. The adipocyte in insulin resistance: Key molecules and the impact of the thiazolidinediones. Trends Endocrinol. Metab. 2003, 14, 137–145. [Google Scholar] [CrossRef]
- Gonzalez-Castejon, M.; Rodriguez-Casado, A. Dietary phytochemicals and their potential effects on obesity: A review. Pharmacol. Res. 2011, 64, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Leiherer, A.; Mundlein, A.; Drexel, H. Phytochemicals and their impact on adipose tissue inflammation and diabetes. Vasc. Pharmacol. 2013, 58, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Abe, D.; Sekiya, K. Nobiletin enhances differentiation and lipolysis of 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2007, 357, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Abe, D.; Sekiya, K. Sakuranetin induces adipogenesis of 3T3-L1 cells through enhanced expression of PPARγ2. Biochem. Biophys. Res. Commun. 2008, 372, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Cha, B.Y.; Lee, Y.S.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.T. Magnolol enhances adipocyte differentiation and glucose uptake in 3T3-L1 cells. Life Sci. 2009, 84, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Chen, Y.; Jin, M.N.; Zhang, C.; Qiao, W.; Yue, X.L.; Duan, H.Q.; Niu, W.Y. Anti-obesity and anti-diabetic effects of flavonoid derivative (Fla-CN) via microRNA in high fat diet induced obesity mice. Eur. J. Pharm. Sci. 2016, 82, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, C.; Jin, M.N.; Qin, N.; Qiao, W.; Yue, X.L.; Duan, H.Q.; Niu, W.Y. Flavonoid derivative exerts an antidiabetic effect via AMPK activation in diet-induced obesity mice. Nat. Prod. Res. 2015, 30, 1988–1992. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Zhang, L.; Dong, J.; Zhang, X.; Chen, Y.G.; Bao, B.; Liu, J. Low-dose diet supplement of a natural flavonoid, luteolin, ameliorates diet-induced obesity and insulin resistance in mice. Mol. Nutr. Food Res. 2014, 58, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Chang, W.L.; Huang, S.F.; Lin, C.Y.; Lin, H.C.; Chang, T.C. Pachymic acid stimulates glucose uptake through enhanced GLUT4 expression and translocation. Eur. J. Pharmacol. 2010, 648, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Ha, Y.J.; Shim, E.K.; Choi, S.Y.; Jin, J.L.; Yun-Choi, H.S.; Lee, J.R. Insulin-mimetic and insulin-sensitizing activities of a pentacyclic triterpenoid insulin receptor activator. Biochem. J. 2007, 403, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Slavin, M.; Lu, Y.; Kaplan, N.; Yu, L.L. Effects of baking on cyanidin-3-glucoside content and antioxidant properties of black and yellow soybean crackers. Food Chem. 2013, 141, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, J.S.; Yoo, H.; Choung, M.G.; Sung, M.K. Effects of black soybean [Glycine max (L.) Merr.] seed coats and its anthocyanidins on colonic inflammation and cell proliferation in vitro and in vivo. J. Agric. Food Chem. 2008, 56, 8427–8433. [Google Scholar] [CrossRef] [PubMed]
- Ionkova, I. Astragalus species (milk vetch): In vitro culture and the production of saponins, astragaline, and other biologically active compounds. Biotechnol. Agric. For. 1995, 33, 97–138. [Google Scholar]
- Park, S.; Kim, J.; Lee, S.; Bae, K.; Kang, S. Chemical constituents of Lathyrus davidii. Nat. Prod. Sci. 2008, 14, 281–288. [Google Scholar]
- Kuang, H.; Zhang, N.; Tian, Z.; Zhang, P.; Okada, Y.; Okuyama, T. Studies of the constituents of Astragalus membranaceus. II. Structures of triterpenoidal glycosides, huangquiyenin D, from the leaves. Nat. Med. 1997, 51, 358–360. [Google Scholar]
- Furuya, T.; Orihara, Y.; Hayashi, C. Triterpenoids from Eucalyptus perriniana cultured cells. Phytochemistry 1987, 26, 715–719. [Google Scholar] [CrossRef]
- Li, X.-C.; Wang, D.-Z.; Wu, S.-G.; Yang, C.-R. Triterpenoid saponins from Pulsatilla campanella. Phytochemistry 1990, 29, 595–599. [Google Scholar] [CrossRef]
- Tava, A.; Pecetti, L.; Romani, M.; Mella, M.; Avato, P. Triterpenoid glycosides from the leaves of two cultivars of Medicago polymorpha L. J. Agric. Food Chem. 2011, 59, 6142–6149. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, W.W.; Nanz, D.; Sticher, O.; Leonticins, D.-H. Five triterpene saponins from Leontice kiangnanensis. Phytochemistry 1997, 44, 497–504. [Google Scholar] [CrossRef]
- Janevski, M.; Ratnayake, S.; Siljanovski, S.; McGlynn, M.A.; Cameron-Smith, D.; Lewandowski, P. Fructose containing sugars modulate mRNA of lipogenic genes ACC and FAS and protein levels of transcription factors ChREBP and SREBP1c with no effect on body weight or liver fat. Food Funct. 2012, 3, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Souza, S.C.; de Vargas, L.M.; Yamamoto, M.T.; Lien, P.; Franciosa, M.D.; Moss, L.G.; Greenberg, A.S. Overexpression of perilipin A and B blocks the ability of tumor necrosis factor alpha to increase lipolysis in 3T3-L1 adipocytes. J. Biol. Chem. 1998, 273, 24665–24669. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.S.; Egan, J.J.; Wek, S.A.; Garty, N.B.; Blanchette-Mackie, E.J.; Londos, C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J. Biol. Chem. 1991, 266, 11341–11346. [Google Scholar] [PubMed]
- Sargeant, R.J.; Paquet, M.R. Effect of insulin on the rates of synthesis and degradation of GLUT1 and GLUT4 glucose transporters in 3T3-L1 adipocytes. Biochem. J. 1993, 290, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.J.; Pang, S.; Harris, D.S.; Heuser, J.; James, D.E. Translocation of the glucose transporter (GLUT4) to the cell surface in permeabilized 3T3-L1 adipocytes: effects of ATP insulin, and GTP gamma S and localization of GLUT4 to clathrin lattices. J. Cell Biol. 1992, 117, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kang, H.J.; Kim, S.Z.; Kwon, T.O.; Jeong, S.I.; Jang, S.I. Antioxidant effect of astragalin isolated from the leaves of Morus alba L. against free radical-induced oxidative hemolysis of human red blood cells. Arch. Pharm. Res. 2013, 36, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Kondeva-Burdina, M.; Krasteva, I.; Mitcheva, M. Effects of rhamnocitrin 4-β-d-galactopyranoside, isolated from Astragalus hamosus on toxicity models in vitro. Pharmacogn. Mag. 2014, 10 (Suppl. 3), S487–S493. [Google Scholar] [PubMed]
- Li, Y.; Wang, C.; Li, H.; Yu, T.; Tan, L. Simultaneous Determination of Formononetin, Calycosin and Rhamnocitrin from Astragalus Complanatus by UHPLC-MS-MS in Rat Plasma: Application to a Pharmacokinetic Study. J. Chromatogr. Sci. 2016, 54, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Avila-Villarreal, G.M.; Aguilar-Guadarrama, B.; Castillo-Espana, P.; Gonzalez-Trujano, M.E.; Villalobos Azucena Ibeth, C.; Estrada-Soto, S. Anxiolytic-like effects of Brickellia cavanillesii and their bioactive compounds nicotiflorin and acacetin in experimental models in mice. Planta Medica 2016, 81, S1–S381. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, H.; Wang, Q.H.; Yang, B.Y.; Kuang, H.X. A new feruloyl tyramine glycoside from the roots of Achyranthes bidentata. Chin. J. Nat. Med. 2012, 10, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Majester-Savornin, B.; Elias, R.; Diaz-Lanza, A.M.; Balansard, G.; Gasquet, M.; Delmas, F. Saponins of the ivy plant, Hedera helix, and their leishmanicidic activity. Planta Medica 1991, 57, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Samadi, A.K.; Rao, K.V.; Cohen, M.S.; Timmermann, B.N. Cytotoxic oleanane-type saponins from Albizia inundata. J. Nat. Prod. 2011, 74, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Aftab, K.; Shaheen, F.; Mohammad, F.V.; Noorwala, M.; Ahmad, V.U. Phyto-pharmacology of saponins from Symphytum officinale L. Adv. Exp. Med. Biol. 1996, 404, 429–442. [Google Scholar] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Jin, Y.C.; Chung, J.I.; Shin, S.C.; Lee, S.J.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. The anti-diabetic effect of anthocyanins in streptozotocin-induced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Mol. Nutr. Food Res. 2009, 53, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Zunino, S. Type 2 diabetes and glycemic response to grapes or grape products. J. Nutr. 2009, 139, 1794S–1800S. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Yang, Y.; Jiang, B.; Jin, H.; Zhou, L.; Liu, S.; Chen, M. Ginsenoside Rb1 promotes adipogenesis in 3T3-L1 cells by enhancing PPARγ2 and C/EBPα gene expression. Life Sci. 2007, 80, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kang, R.; Kim, Y.S.; Chung, S.I.; Yoon, Y. Platycodin D inhibits adipogenesis of 3T3-L1 cells by modulating Kruppel-like factor 2 and peroxisome proliferator-activated receptor gamma. Phytother. Res. PTR 2010, 24 (Suppl. 2), S161–S167. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Y.; Zhao, T.; Wang, Y.; Sun, C. Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through LKB1/AMPK pathway. PLoS ONE 2013, 8, e70135. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.S.; Yeh, C.H.; Yeh, M.F.; Cheng, J.T. Increase of adipogenesis by ginsenoside (Rh2) in 3T3-L1 cell via an activation of glucocorticoid receptor. Horm. Metab. Res. 2009, 41, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Savkur, R.S.; Miller, A.R. Investigational PPAR-gamma agonists for the treatment of Type 2 diabetes. Expert Opin. Investig. Drugs 2006, 15, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Yun, Z.; Maecker, H.L.; Johnson, R.S.; Giaccia, A.J. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: A mechanism for regulation of adipogenesis by hypoxia. Dev. Cell 2002, 2, 331–341. [Google Scholar] [CrossRef]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 1999, 3, 151–158. [Google Scholar] [CrossRef]
- Garin-Shkolnik, T.; Rudich, A.; Hotamisligil, G.S.; Rubinstein, M. FABP4 attenuates PPARγ and adipogenesis and is inversely correlated with PPARγ in adipose tissues. Diabetes 2014, 63, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Tschop, M.H.; Wilding, J.P. Anti-obesity drugs: Past, present and future. Dis. Models Mech. 2012, 5, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.X.; Yang, L.Z.; Li, L.X.; Zhao, X.P. Diabetic ketoacidosis in pregnancy tends to occur at lower blood glucose levels: case-control study and a case report of euglycemic diabetic ketoacidosis in pregnancy. J. Obstet. Gynaecol. Res. 2008, 34, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Hellriegel, C.; Rueck, A.; Sauermoser, R.; Wuethrich, J. Using high-performance quantitative NMR (HP-qNMR) for certifying traceable and highly accurate purity values of organic reference materials with uncertainties <0.1%. Accredit. Qual. Assur. 2013, 18, 91–98. [Google Scholar] [CrossRef]
- Fujimori, K.; Shibano, M. Avicularin, a plant flavonoid, suppresses lipid accumulation through repression of C/EBPα-activated GLUT4-mediated glucose uptake in 3T3-L1 cells. J. Agric. Food Chem. 2013, 61, 5139–5147. [Google Scholar] [CrossRef] [PubMed]
- Nishina, A.; Kimura, H.; Sekiguchi, A.; Fukumoto, R.H.; Nakajima, S.; Furukawa, S. Lysophosphatidylethanolamine in Grifola frondosa as a neurotrophic activator via activation of MAPK. J. Lipid Res. 2006, 47, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available. |


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishina, A.; Itagaki, M.; Suzuki, Y.; Koketsu, M.; Ninomiya, M.; Sato, D.; Suzuki, T.; Hayakawa, S.; Kuroda, M.; Kimura, H. Effects of Flavonoids and Triterpene Analogues from Leaves of Eleutherococcus sieboldianus (Makino) Koidz. ‘Himeukogi’ in 3T3-L1 Preadipocytes. Molecules 2017, 22, 671. https://doi.org/10.3390/molecules22040671
Nishina A, Itagaki M, Suzuki Y, Koketsu M, Ninomiya M, Sato D, Suzuki T, Hayakawa S, Kuroda M, Kimura H. Effects of Flavonoids and Triterpene Analogues from Leaves of Eleutherococcus sieboldianus (Makino) Koidz. ‘Himeukogi’ in 3T3-L1 Preadipocytes. Molecules. 2017; 22(4):671. https://doi.org/10.3390/molecules22040671
Chicago/Turabian StyleNishina, Atsuyoshi, Masaya Itagaki, Yuusuke Suzuki, Mamoru Koketsu, Masayuki Ninomiya, Daisuke Sato, Takashi Suzuki, Satoshi Hayakawa, Makoto Kuroda, and Hirokazu Kimura. 2017. "Effects of Flavonoids and Triterpene Analogues from Leaves of Eleutherococcus sieboldianus (Makino) Koidz. ‘Himeukogi’ in 3T3-L1 Preadipocytes" Molecules 22, no. 4: 671. https://doi.org/10.3390/molecules22040671