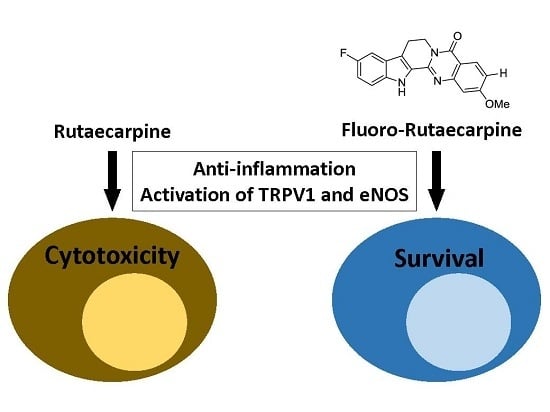
Synthetic Fluororutaecarpine Inhibits Inflammatory Stimuli and Activates Endothelial Transient Receptor Potential Vanilloid-Type 1
Abstract
:1. Introduction
2. Results
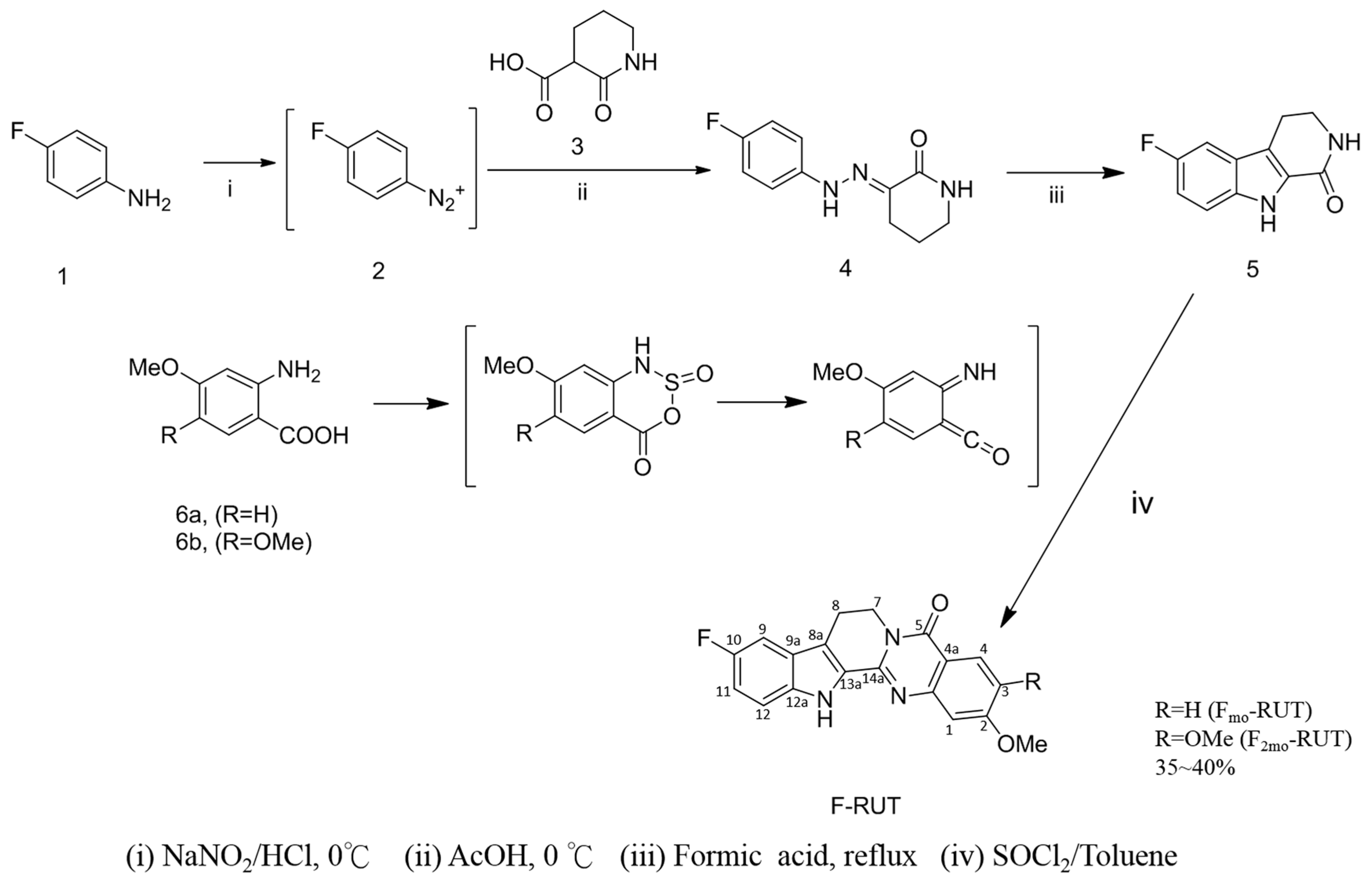
2.1. Design and Synthesis of F-RUT
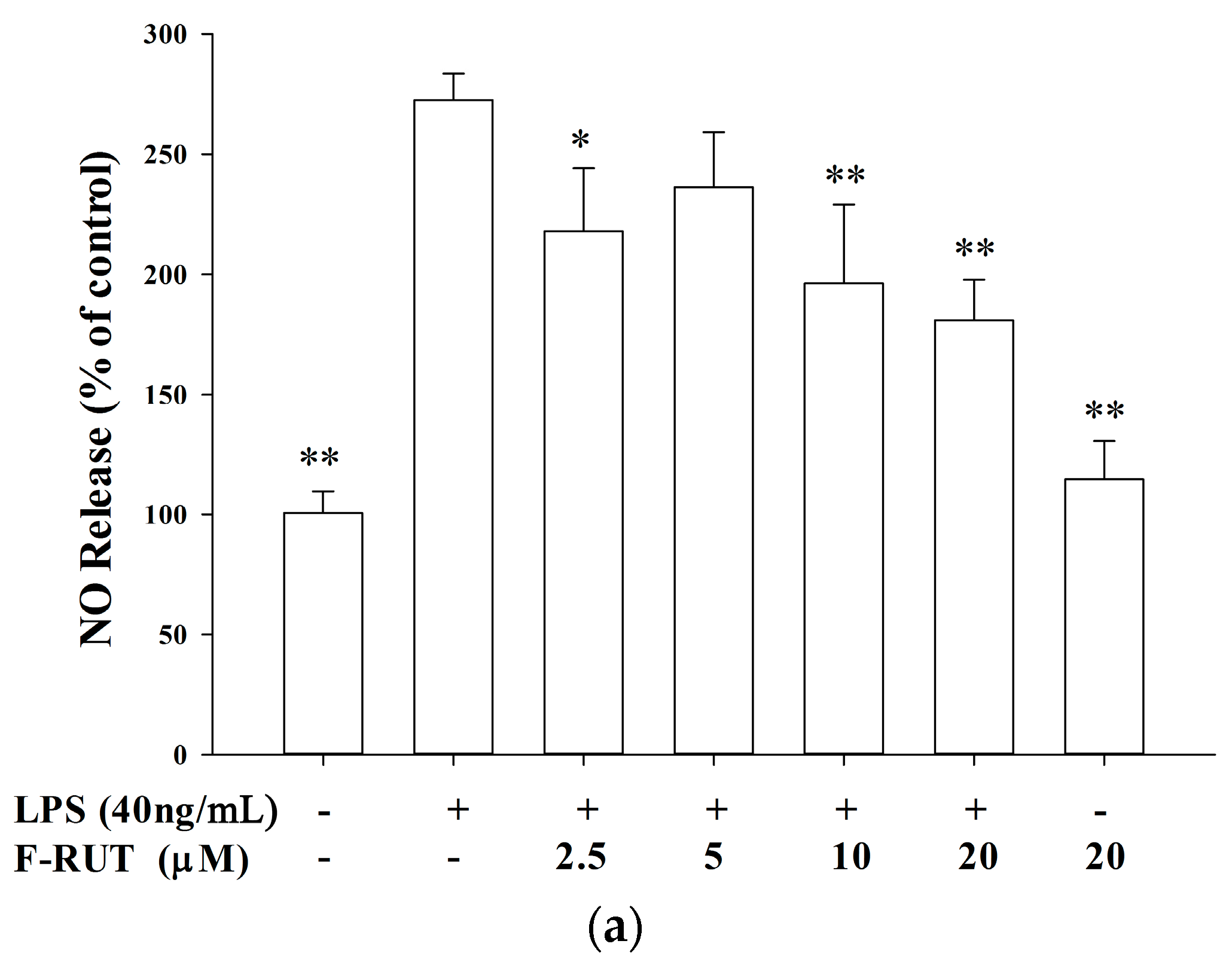
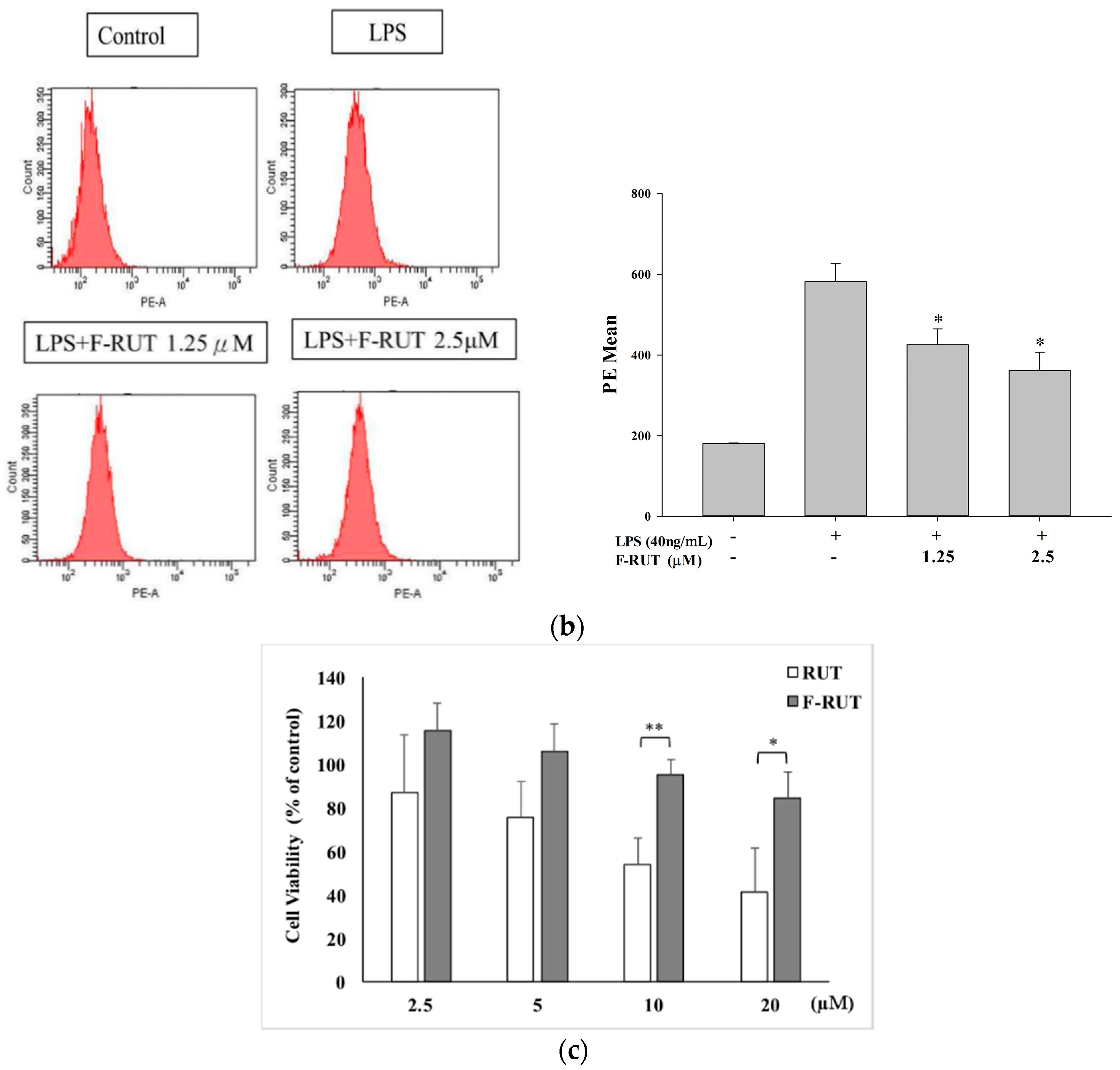
2.2. NO and TNF-Alpha Releases by LPS-Treated Macrophages Were Suppressed by F-RUT
2.3. F-RUT Suppresses Inducible (i)NOS and COX-2 Expressions which Was Correlated with Inhibition of Nuclear Factor (NF)-κB Activity in LPS-Activated Macrophages
2.4. F-RUT Inhibited Cell Migration/Invasion
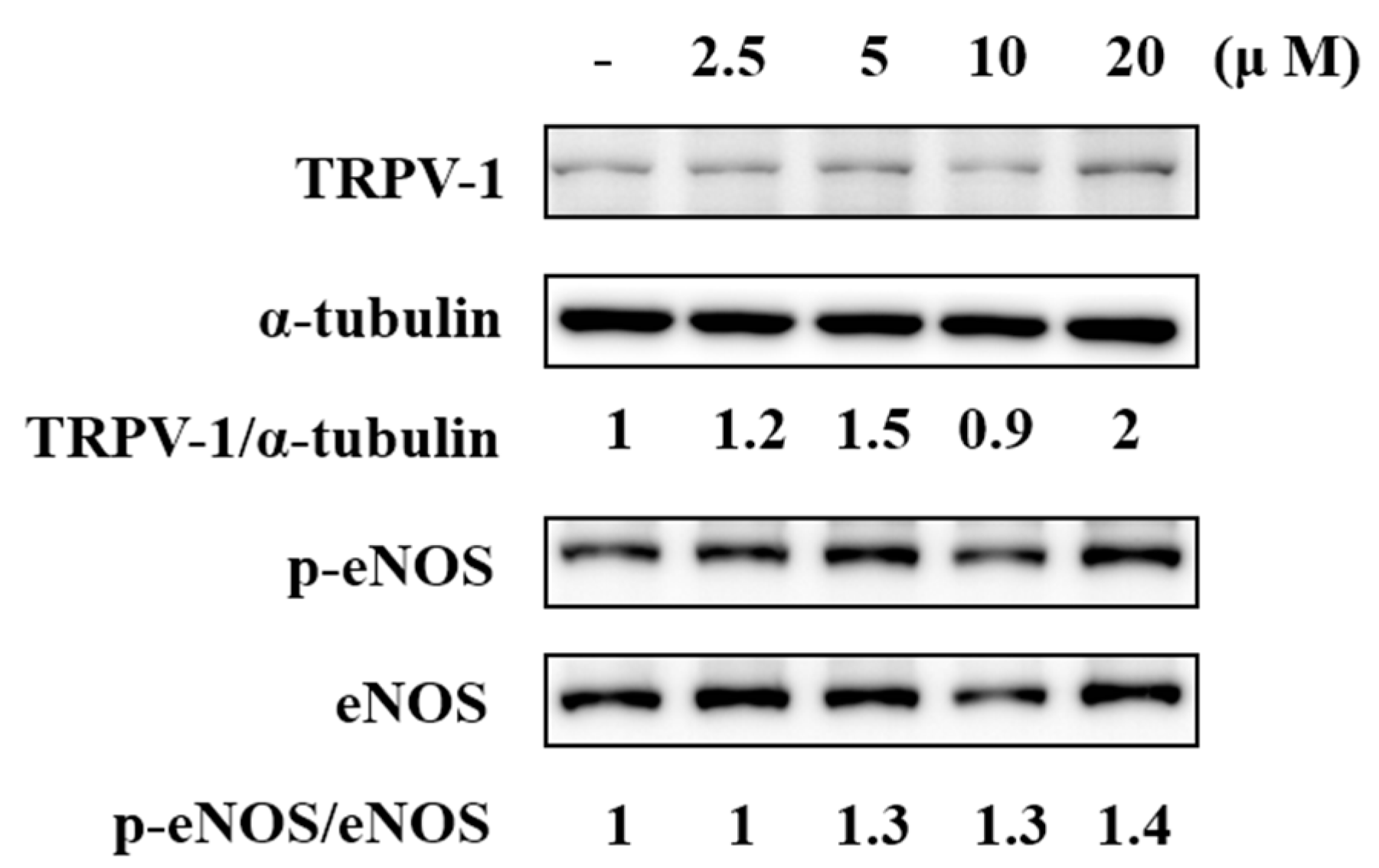
2.5. F-RUT Activated TRPV1 and eNOS
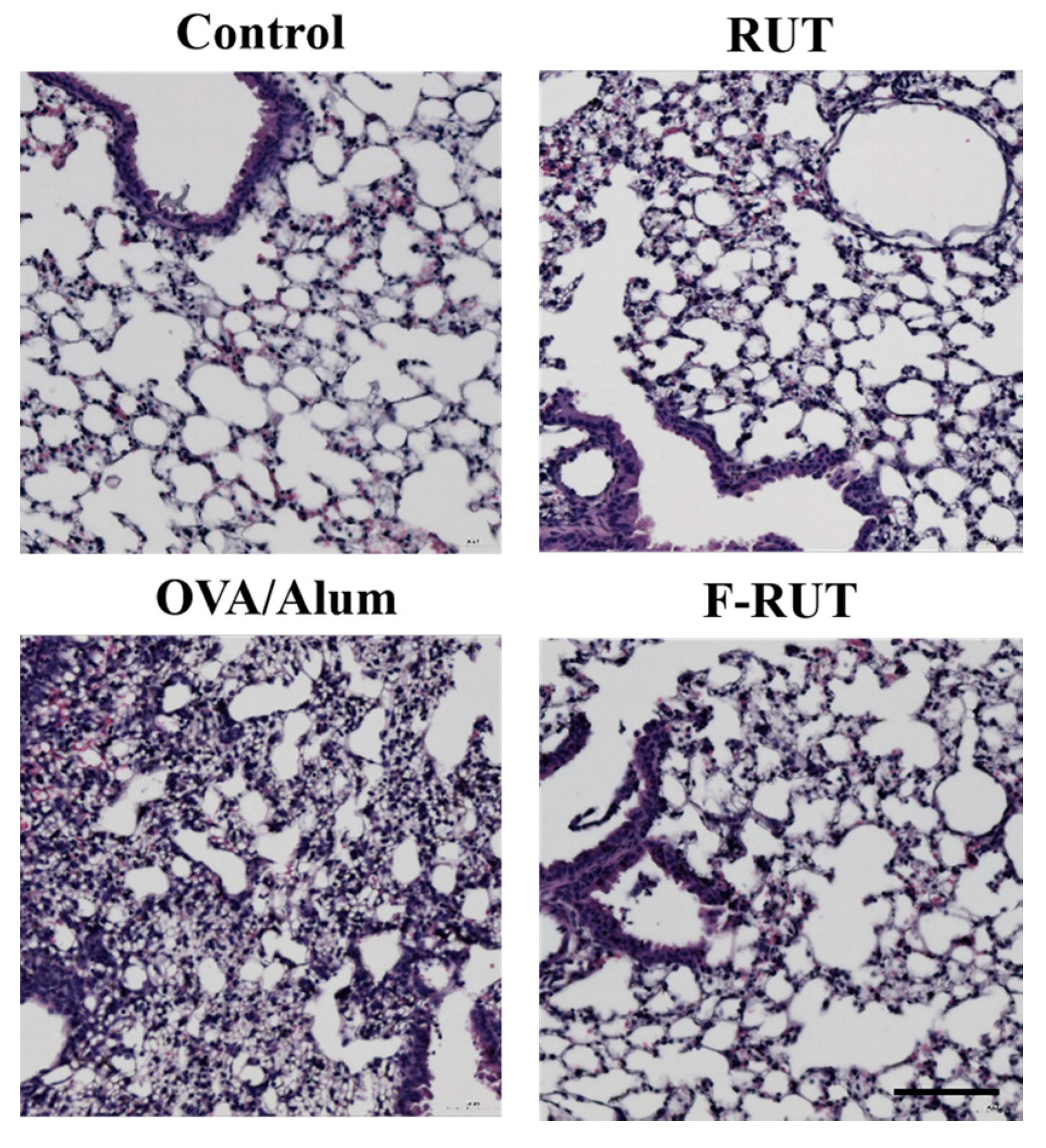
2.6. F-RUT Ameliorates Inflammation in OVA/Alum-Challenged Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals and General Methods
4.2. Synthesis of 10-Fluoro-2-methoxyrutaecarpine (FMO-RUT)
4.3. Cell Culture
4.4. Nitrite Assay
4.5. TNF-α Assay
4.6. Cell Viability Assay
4.7. Western Blot Analysis
4.8. Transient Transfection and Luciferase Assay
4.9. Wound-Healing Migration Assay
4.10. In Vitro Invasion Assays
4.11. Animal Experiment
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Perrotta, I. Ultrastructural features of human atherosclerosis. Ultrastruct. Pathol. 2013, 37, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Garre, D.; Munoz-Pacheco, P.; Gonzalez-Rubio, M.L.; Aragoncillo, P.; Granados, R.; Fernandez-Cruz, A. Ezetimibe reduces plaque inflammation in a rabbit model of atherosclerosis and inhibits monocyte migration in addition to its lipid-lowering effect. Br. J. Pharmacol. 2009, 156, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Bao, J.L.; Chen, X.P.; Huang, M.; Wang, Y.T. Alkaloids isolated from natural herbs as the anticancer agents. Evid. Based Complement. Altern. Med. 2012, 2012, 485042. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jin, H.; Gong, W.; Wang, Z.; Liang, H. Pharmacological actions of multi-target-directed evodiamine. Molecules 2013, 18, 1826–1843. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, Y.Q.; Peng, W.J.; Yu, Y.R.; Wu, Y.S.; Yan, H.; Huang, Q.R.; He, M.; Luo, D. Rutaecarpine reverses the altered connexin expression pattern induced by oxidized low-density lipoprotein in monocytes. J. Cardiovasc. Pharmacol. 2016, 67, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.G.; Lee, C.H.; Noh, M.S.; Lee, J.J.; Jung, Y.S.; Baik, E.J.; Moon, C.H.; Lee, S.H. Rutaecarpine, a quinazolinocarboline alkaloid, inhibits prostaglandin production in raw264.7 macrophages. Planta Med. 2001, 67, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.F.; Chou, C.J.; Shum, A.Y.; Chen, C.F. The vasorelaxant effect of evodiamine in rat isolated mesenteric arteries: Mode of action. Eur. J. Pharmacol. 1992, 215, 277–283. [Google Scholar] [CrossRef]
- Chiou, W.F.; Shum, A.Y.; Liao, J.F.; Chen, C.F. Studies of the cellular mechanisms underlying the vasorelaxant effects of rutaecarpine, a bioactive component extracted from an herbal drug. J. Cardiovasc. Pharmacol. 1997, 29, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.J.; Wu, X.C.; Chen, C.F.; Lin, L.C.; Huang, Y.T.; Shan, J.; Pang, P.K. Vasorelaxing action of rutaecarpine: Effects of rutaecarpine on calcium channel activities in vascular endothelial and smooth muscle cells. J. Pharmacol. Exp. Ther. 1999, 289, 1237–1244. [Google Scholar] [PubMed]
- Li, D.; Zhang, X.J.; Chen, L.; Yang, Z.; Deng, H.W.; Peng, J.; Li, Y.J. Calcitonin gene-related peptide mediates the cardioprotective effects of rutaecarpine against ischaemia-reperfusion injury in spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2009, 36, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Peng, J.; Xin, H.Y.; Luo, D.; Zhang, Y.S.; Zhou, Z.; Jiang, D.J.; Deng, H.W.; Li, Y.J. Calcitonin gene-related peptide-mediated antihypertensive and anti-platelet effects by rutaecarpine in spontaneously hypertensive rats. Peptides 2008, 29, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Hu, C.P.; Wang, C.J.; Li, T.T.; Peng, J.; Li, Y.J. Calcitonin gene-related peptide inhibits angiotensin ii-induced endothelial progenitor cells senescence through up-regulation of klotho expression. Atherosclerosis 2010, 213, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Ching, L.C.; Chen, C.Y.; Su, K.H.; Hou, H.H.; Shyue, S.K.; Kou, Y.R.; Lee, T.S. Implication of amp-activated protein kinase in transient receptor potential vanilloid type 1-mediated activation of endothelial nitric oxide synthase. Mol. Med. 2012, 18, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Ching, L.C.; Kou, Y.R.; Shyue, S.K.; Su, K.H.; Wei, J.; Cheng, L.C.; Yu, Y.B.; Pan, C.C.; Lee, T.S. Molecular mechanisms of activation of endothelial nitric oxide synthase mediated by transient receptor potential vanilloid type 1. Cardiovasc. Res. 2011, 91, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.P.; Xiao, L.; Deng, H.W.; Li, Y.J. The depressor and vasodilator effects of rutaecarpine are mediated by calcitonin gene-related peptide. Planta Med. 2003, 69, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.Y.; Ye, F.; Cai, W.J.; Tan, G.S.; Hu, C.P.; Deng, H.W.; Li, Y.J. Stimulation of calcitonin gene-related peptide synthesis and release: Mechanisms for a novel antihypertensive drug, rutaecarpine. J. Hypertens. 2004, 22, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Torres-Narvaez, J.C.; Mondragon Ldel, V.; Varela Lopez, E.; Perez-Torres, I.; Diaz Juarez, J.A.; Suarez, J.; Hernandez, G.P. Role of the transient receptor potential vanilloid type 1 receptor and stretch-activated ion channels in nitric oxide release from endothelial cells of the aorta and heart in rats. Expe. Clin. Cardiol. 2012, 17, 89–94. [Google Scholar]
- Wei, J.; Ching, L.C.; Zhao, J.F.; Shyue, S.K.; Lee, H.F.; Kou, Y.R.; Lee, T.S. Essential role of transient receptor potential vanilloid type 1 in evodiamine-mediated protection against atherosclerosis. Acta Physiol. (Oxf.) 2013, 207, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.R.; Hung, W.C.; Wu, C.H.; Lee, Y.M.; Yen, M.H. Antithrombotic effect of rutaecarpine, an alkaloid isolated from evodia rutaecarpa, on platelet plug formation in in vivo experiments. Br. J. Haematol. 2000, 110, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.R.; Kan, Y.C.; Hung, W.C.; Su, C.H.; Lin, C.H.; Lee, Y.M.; Yen, M.H. The antiplatelet activity of rutaecarpine, an alkaloid isolated from evodia rutaecarpa, is mediated through inhibition of phospholipase c. Thromb. Res. 1998, 92, 53–64. [Google Scholar] [CrossRef]
- Du, J.; Wang, X.F.; Zhou, Q.M.; Zhang, T.L.; Lu, Y.Y.; Zhang, H.; Su, S.B. Evodiamine induces apoptosis and inhibits metastasis in mdamb-231 human breast cancer cells in vitro and in vivo. Oncol. Rep. 2013, 30, 685–694. [Google Scholar] [PubMed]
- Jia, S.; Hu, C. Pharmacological effects of rutaecarpine as a cardiovascular protective agent. Molecules 2010, 15, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Mahringer, A.; Kunert, O.; Fricker, G.; Efferth, T.; Bauer, R. Cytotoxicity and p-glycoprotein modulating effects of quinolones and indoloquinazolines from the chinese herb evodia rutaecarpa. Planta Med. 2007, 73, 1554–1557. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Son, J.K.; Jeong, B.S.; Jeong, T.C.; Chang, H.W.; Lee, E.S.; Jahng, Y. Progress in the studies on rutaecarpine. Molecules 2008, 13, 272–300. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, G.; Li, D.; Chen, J.; Li, Y.; Zhou, H.; Xie, Y. Synthesis and vasodilator effects of rutaecarpine analogs which might be involved transient receptor potential vanilloid subfamily, member 1 (trpv1). Bioorg. Med. Chem. 2009, 17, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Gu, J.A.; Rau, T.G.; Yang, C.H.; Yang, W.C.; Huang, S.H.; Lin, F.Y.; Lin, C.M.; Huang, S.T. Low-cytotoxic synthetic bromorutaecarpine exhibits anti-inflammation and activation of transient receptor potential vanilloid type 1 activities. BioMed Res. Int. 2013, 2013, 795095. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.; Schindler, L.; Cook, J.M. Carboxyl-mediated pictet-spengler reaction. Direct synthesis of 1,2,3,4-tetrahydro-.Beta.-carbolines from tryptamine-2-carboxylic acids. J. Org. Chem. 1991, 56, 359–365. [Google Scholar] [CrossRef]
- Tschesche, R.; Snatzke, G.; Grimmer, G. Calotropagenin aus asclepias curassavica l. Naturwissenschaften 1959, 46, 263–264. [Google Scholar] [CrossRef]
- Chiou, W.F.; Sung, Y.J.; Liao, J.F.; Shum, A.Y.; Chen, C.F. Inhibitory effect of dehydroevodiamine and evodiamine on nitric oxide production in cultured murine macrophages. J. Nat. Prod. 1997, 60, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.H.; Lee, W.J.; Lee, S.H.; Son, J.K.; Kim, H.L.; Nam, J.M.; Kwon, Y.; Jahng, Y. Synthesis and biological properties of benzo-annulated rutaecarpines. Biol. Pharm. Bull. 2010, 33, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Cooney, R.; Hynes, S.O.; Duffy, A.M.; Sharif, F.; O’Brien, T. Adenoviral-mediated gene transfer of nitric oxide synthase isoforms and vascular cell proliferation. J. Vasc. Res. 2006, 43, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.Y.; Shin, I.S.; Shin, H.K.; Jin, S.E.; Lee, M.Y. Aqueous extract of gumiganghwal-tang, a traditional herbal medicine, reduces pulmonary fibrosis by transforming growth factor-beta1/smad signaling pathway in murine model of chronic asthma. PLoS ONE 2016, 11, e0164833. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.S.; Shin, H.K.; Kim, J.C.; Lee, M.Y. Role of klotho, an antiaging protein, in pulmonary fibrosis. Arch. Toxicol. 2015, 89, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Lee, C.C.; Chiang, B.L. Small interfering rna against interleukin-5 decreases airway eosinophilia and hyper-responsiveness. Gene. Ther. 2008, 15, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Paravicini, T.M.; Touyz, R.M. Redox signaling in hypertension. Cardiovasc. Res. 2006, 71, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Touyz, R.M. Molecular mechanisms of hypertension-reactive oxygen species and antioxidants: A basic science update for the clinician. Can. J. Cardiol. 2012, 28, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Glezeva, N.; Baugh, J.A. Role of inflammation in the pathogenesis of heart failure with preserved ejection fraction and its potential as a therapeutic target. Heart Fail. Rev. 2014, 1, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.L.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, Y.; Watabe, T. Roles of tgf-beta signals in endothelial-mesenchymal transition during cardiac fibrosis. Int. J. Inflamm. 2011, 2011, 724080. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Won, J.Y.; Kim, W.J.; Lee, J.; Kim, K.H.; Youn, S.W.; Kim, J.Y.; Lee, E.J.; Kim, Y.J.; Kim, K.W.; et al. Snail as a potential target molecule in cardiac fibrosis: Paracrine action of endothelial cells on fibroblasts through snail and ctgf axis. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Lu, H.K.; Lee, H.W.; Chen, Y.C.; Li, C.L.; Wang, L.F. Nitric oxide inhibits androgen receptor-mediated collagen production in human gingival fibroblasts. J. Periodontal Res. 2012, 47, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Suk, F.M.; Jou, W.J.; Lin, R.J.; Lin, S.Y.; Tzeng, F.Y.; Liang, Y.C. 15,16-dihydrotanshinone i-induced apoptosis in human colorectal cancer cells: Involvement of atf3. Anticancer Res. 2013, 33, 3225–3231. [Google Scholar] [PubMed]
- Yu, S.H.; Kao, Y.T.; Wu, J.Y.; Huang, S.H.; Huang, S.T.; Lee, C.M.; Cheng, K.T.; Lin, C.M. Inhibition of ampk-associated autophagy enhances caffeic acid phenethyl ester-induced cell death in c6 glioma cells. Planta Med. 2011, 77, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Shen, S.C.; Chien, C.C.; Yang, L.Y.; Shia, L.T.; Chen, Y.C. 12-o-Tetradecanoylphorbol-13-acetate-induced invasion/migration of glioblastoma cells through activating pkcalpha/erk/nf-kappab-dependent mmp-9 expression. J. Cell. Physiol. 2010, 225, 472–481. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the F-RUT is available from the authors. |


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-M.; Gu, J.-A.; Rau, T.-G.; Wang, C.; Yen, C.-H.; Huang, S.-H.; Lin, F.-Y.; Lin, C.-M.; Huang, S.-T. Synthetic Fluororutaecarpine Inhibits Inflammatory Stimuli and Activates Endothelial Transient Receptor Potential Vanilloid-Type 1. Molecules 2017, 22, 656. https://doi.org/10.3390/molecules22040656
Lee C-M, Gu J-A, Rau T-G, Wang C, Yen C-H, Huang S-H, Lin F-Y, Lin C-M, Huang S-T. Synthetic Fluororutaecarpine Inhibits Inflammatory Stimuli and Activates Endothelial Transient Receptor Potential Vanilloid-Type 1. Molecules. 2017; 22(4):656. https://doi.org/10.3390/molecules22040656
Chicago/Turabian StyleLee, Chi-Ming, Jiun-An Gu, Tin-Gan Rau, Chi Wang, Chiao-Han Yen, Shih-Hao Huang, Feng-Yen Lin, Chun-Mao Lin, and Sheng-Tung Huang. 2017. "Synthetic Fluororutaecarpine Inhibits Inflammatory Stimuli and Activates Endothelial Transient Receptor Potential Vanilloid-Type 1" Molecules 22, no. 4: 656. https://doi.org/10.3390/molecules22040656