Structure-Activity and Lipophilicity Relationships of Selected Antibacterial Natural Flavones and Flavanones of Chilean Flora
Abstract
:1. Introduction
2. Results
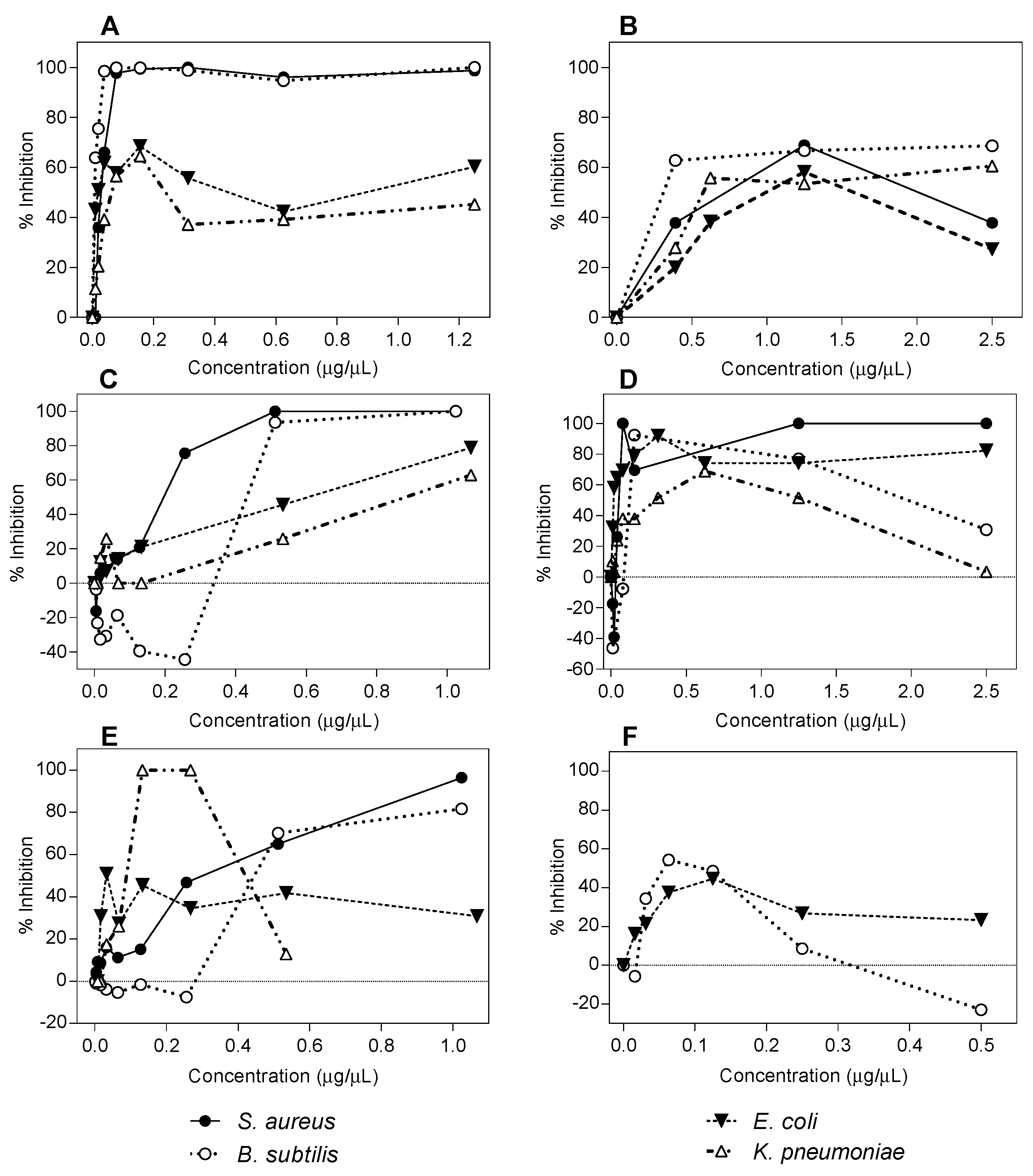
2.1. Antibacterial Activity Determination
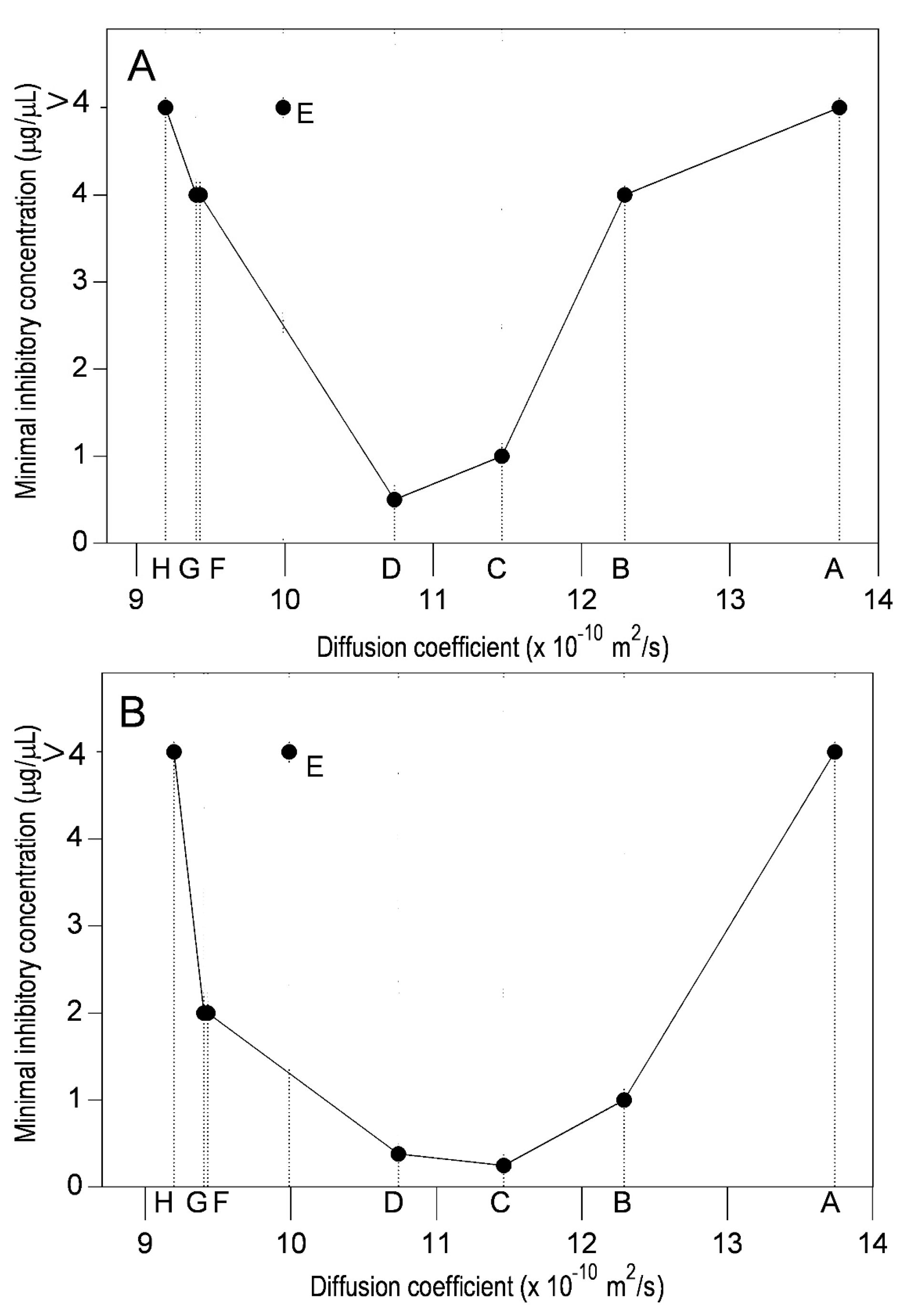
2.2. Diffusion Coefficient Measurements
2.3. Lipophilicity and Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Chemical Reagents
4.3. Antibacterial Activity
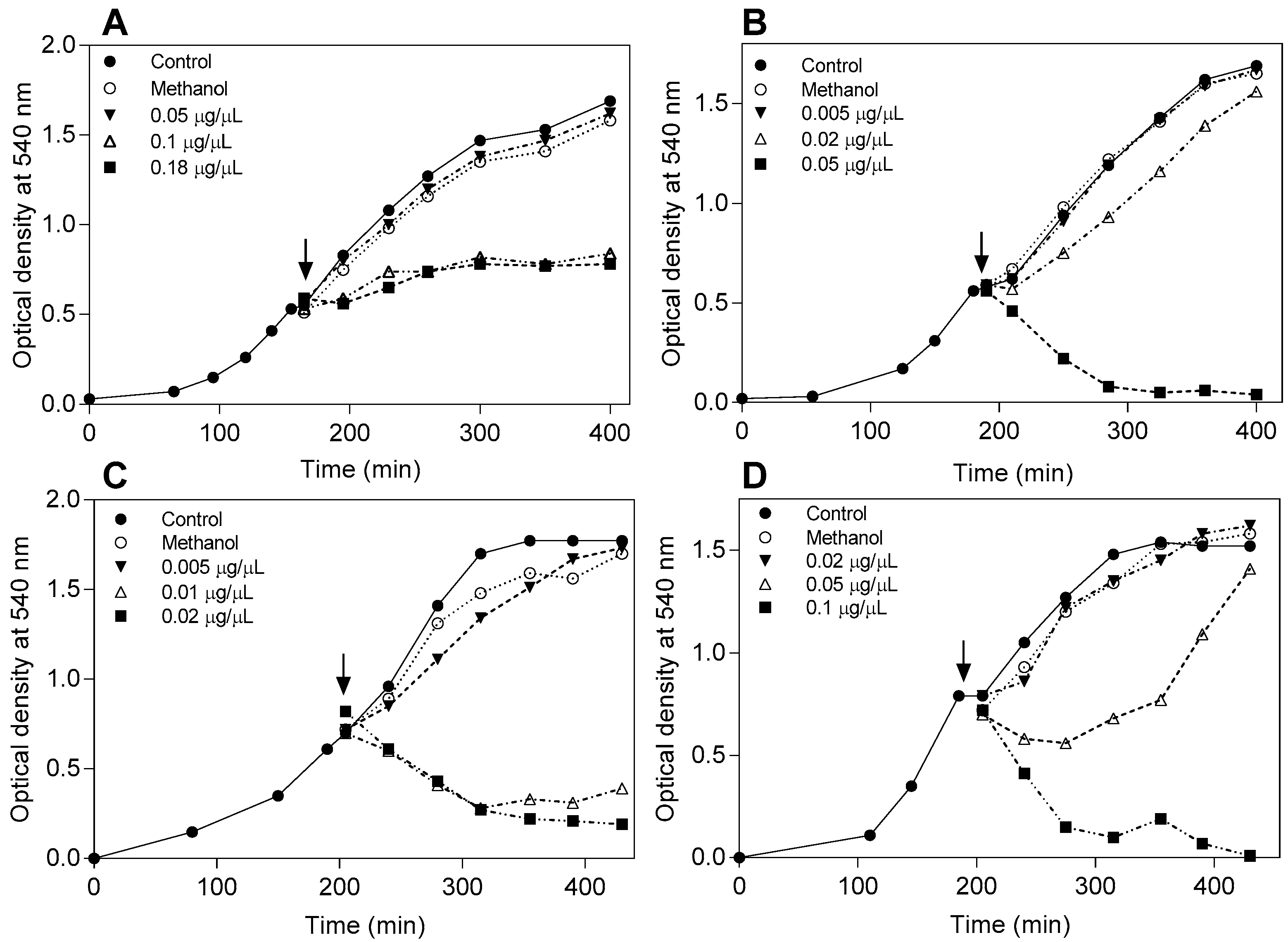
4.4. Effect of the Flavonoids on the Bacterial Growth Curve
4.5. Diffusion Coefficient Determination
4.6. Theoretical Estimation of Lipophilicity
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Romano, B.; Pagano, E.; Montanaro, V.; Fortunato, A.L.; Milic, N.; Borrelli, F. Novel insights into the pharmacology of flavonoids. Phytother. Res. 2013, 27, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Haenen, G.R.M.M.; Arts, M.J.T.J.; Bast, A.; Coleman, M.D. Structure and activity in assessing antioxidant activity in vitro and in vivo. A critical appraisal illustrated with the flavonoids. Environ. Toxicol. Pharmacol. 2006, 21, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wang, X.; Chen, J.; Jiao, R.; Wang, L.; Li, Y.M.; Zuo, Y.; Liu, Y.; Lei, L.; Ma, K.Y.; et al. Biology of ageing and role of dietary antioxidants. Biomed. Res. Int. 2014, 831841. [Google Scholar] [CrossRef] [PubMed]
- Forbes, A.M.; Lin, H.; Meadows, G.G.; Meier, P.G. Synthesis and anticancer activity of new flavonoid analogs and inconsistencies in assays related to proliferation and viability measurements. Int. J. Oncol. 2014, 45, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Chahar, M.K.; Sharma, N.; Dobhal, M.P.; Joshi, Y.C. Flavonoids: A versatile source of anticancer drugs. Pharmacogn. Rev. 2011, 5, 1–12. [Google Scholar] [PubMed]
- Mendoza, L.; Wilkens, M.; Urzúa, A. Antimicrobial study of the resinous exudates and of diterpenoids and flavonoids isolated from some Chilean Pseudognaphalium (Asteraceae). J. Ethnopharmacol. 1997, 58, 85–88. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Mukne, A.P.; Viswanathan, V.; Phadatare, A.G. Structure pre-requisites for isoflavones as effective antibacterial agents. Pharmacogn. Rev. 2011, 5, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol. J. 2007, 2, 1214–1234. [Google Scholar] [CrossRef] [PubMed]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part II: Reconstruction of multienzyme pathways in plants and microbes. Biotechnol. J. 2007, 2, 1235–1249. [Google Scholar] [CrossRef] [PubMed]
- O´Shea, R.; Moser, H.E. Physicochemical properties of antibacterial compounds: Implications for drug discovery. J. Med. Chem. 2008, 51, 2871–2878. [Google Scholar] [CrossRef] [PubMed]
- Laks, P.; Pruner, M. Flavonoid biocides: Structure/activity relations of flavonoid phytoalexin analogues. Phytochemistry 1989, 28, 27–91. [Google Scholar] [CrossRef]
- Wu, T.; He, M.; Zang, X.; Zhou, Y.; Qiu, T.; Pan, S.; Xu, X. A structure-activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim. Biophys. Acta 2013, 1828, 2751–2756. [Google Scholar] [CrossRef] [PubMed]
- Orbach, E.; Finkelstein, A. The nonelectrolyte permeability of planar lipid bilayer membranes. J. Gen. Phys. 1980, 75, 427–436. [Google Scholar] [CrossRef]
- Urzúa, A.; Echeverría, J.; Rezende, M.C.; Wilkens, M. Antibacterial properties of 3H-spiro[1-benzofuran-2, 1′-cyclohexane] derivatives from Heliotropium filifolium. Molecules 2008, 13, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, H.; Tanimoto, K.; Tamura, Y.; Mizutani, K.; Kinoshita, T. Mode of antibacterial action of retrochalcones from Glycyrrhiza inflate. Phytochemistry 1998, 48, 125–129. [Google Scholar] [CrossRef]
- Foti, M.; Piattelli, M.; Baratta, M.T.; Ruberto, G. Flavonoids, coumarins, and cinnamic acids as antioxidants in a micellar system. Structure-activity relationship. J. Agric. Food Chem. 1996, 44, 497–501. [Google Scholar]
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Rezende, M.C.; Urzúa, A.; Cárdenas, L.; Beezer, A.; Mitchell, J.C. Solute-solvent interactions from diffusion of flavonoids in methanol. J. Solut. Chem. 1999, 28, 1107–1112. [Google Scholar] [CrossRef]
- Sandoval, C.; Rezende, M.C.; González-Nilo, F. Solute-solvent interactions of flavonoids in organic solvents. J. Solut. Chem. 2003, 32, 781–790. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Hamilton, V.E.S.; Lamb, A.J. Assessment of the antibacterial activity of selected flavonoids and consideration of discrepancies between previous reports. Microbiol. Res. 2003, 158, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Fallarero, A.; Hanski, L.; Vuorela, P. How to translate a bioassay into a screening assay for natural products: General considerations and implementation of antimicrobial screens. Planta Med. 2014, 80, 1182–1199. [Google Scholar] [CrossRef] [PubMed]
- Pohjala, L.; Tammela, P. Aggregating behavior of phenolic compounds—A source of false bioassay results? Molecules 2012, 17, 10774–10790. [Google Scholar] [CrossRef] [PubMed]
- Masaoka, Y.; Ueno, Y.; Morita, Y.; Kuroda, T.; Mizushima, T.; Tsuchiya, T. A two-component multidrug efflux pump, EbrAB, in Bacillus subtilis. J. Bacteriol. 2000, 182, 2307–2310. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, M.; Alarcón, C.; Urzúa, A.; Mendoza, M. Characterization of the bactericidal activity of the natural diterpene kaurenoic acid. Planta Med. 2002, 68, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Hamilton, V.E.S.; Chapman, D.G.; Taylor, P.W.; Lamb, A.J. Aggregation of Staphylococcus aureus following treatment with the antibacterial flavonol galangin. J. Appl. Microbiol. 2007, 103, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Sufian, A.S.; Ramasamy, K.; Ahmat, N.; Zakaria, Z.A.; Yusof, M.I.M. Isolation and identification of antibacterial and cytotoxic compounds from the leaves of Muntingia calabura L. J. Ethnopharmacol. 2013, 146, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Microdermatology: Cell surface in the interaction of microbes with the external world. J. Bacteriol. 1999, 181, 4–8. [Google Scholar] [PubMed]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Villarroel, L.; Urzúa, A. Secondary metabolites from the resinous exudate of Heliotropium stenophyllum. Bol. Soc. Chil. Quím. 1990, 35, 309–311. [Google Scholar]
- Urzúa, A.; Villarroel, L.; Torres, R.; Teillier, S. Flavonoids in the resinous exudate of Chilean Heliotropium species from Cochranea section. Biochem. Sys. Ecol. 1993, 21, 744. [Google Scholar] [CrossRef]
- Torres, R.; Modak, B.; Villarroel, L.; Urzúa, A.; delle Monache, F.; Sánchez-Ferrando, F. Flavonoids from resinous exudate of Heliotropium sinuatum. Bol. Soc. Chil. Quim. 1996, 41, 195–197. [Google Scholar]
- Villarroel, L.; Torres, R.; Urzúa, A.; Reina, M.; Cabrera, R.; González-Coloma, A. Heliotropium huascoense resin exudate: Chemical constituents and defensive properties. J. Nat. Prod. 2001, 64, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Urzúa, A.; Cuadra, P. Flavonoids from the resinous exudate of Gnaphalium robustum. Bol. Soc. Chil. Quím. 1989, 34, 247–251. [Google Scholar]
- Urzúa, A.; Torres, R.; Bueno, C.; Mendoza, L. Flavonoids and diterpenoids in the trichome resinous exudates from Pseudognaphalium cheiranthifolium, P. heterotrichium and P. vira vira. Biochem. Syst. Ecol. 1995, 23, 459. [Google Scholar] [CrossRef]
- Mendoza, L; Urzúa, A. Minor flavonoids and diterpenoids in the resinous trichome exudates from Pseudognaphalium cheiranthifolium, P. heterotrichium, P. vira vira and P. robustum. Biochem. Syst. Ecol. 1998, 26, 469–471. [Google Scholar] [CrossRef]
- Mayr-Härting, A.; Hedges, A.J.; Berkeley, C.W. Methods for studying bacteriocins. In Methods in Microbiology; Norris, J.R., Rebbon, D.W., Eds.; Academic Press: London, UK; New York, NY, USA, 1972; Volume 7, Part A; pp. 315–422. [Google Scholar]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.; Domínguez, H.C.; Costas, M. Determination of mutual diffusion coefficients in water-rich 2-butoxyethanol/water mixtures using dispersion technique. J. Phys. Chem. 1990, 94, 8731–8734. [Google Scholar] [CrossRef]
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; et al. Virtual computational chemistry laboratory—Design and description. J. Comput. Aid. Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef] [PubMed]
- VCCLAB, Virtual Computational Chemistry Laboratory. Available online: http://www.vcclab.org (accessed on 28 September 2016).
- Tetko, I.V.; Tanchuk, V.Y.; Kasheva, T.N.; Villa, A.E.P. Estimation of aqueous solubility of chemical compounds using E-state indices. J. Chem. Inf. Comp. Sci. 2001, 41, 1488–1493. [Google Scholar] [CrossRef]
- Cheng, T.; Zhao, Y.; Li, X.; Lin, F.; Zhang, X.; Li, Y.; Wang, R. Computation of octanol-water partition coefficients by guiding an additive model with knowledge. J. Chem. Inf. Model. 2007, 47, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the all compounds are available from the authors. |
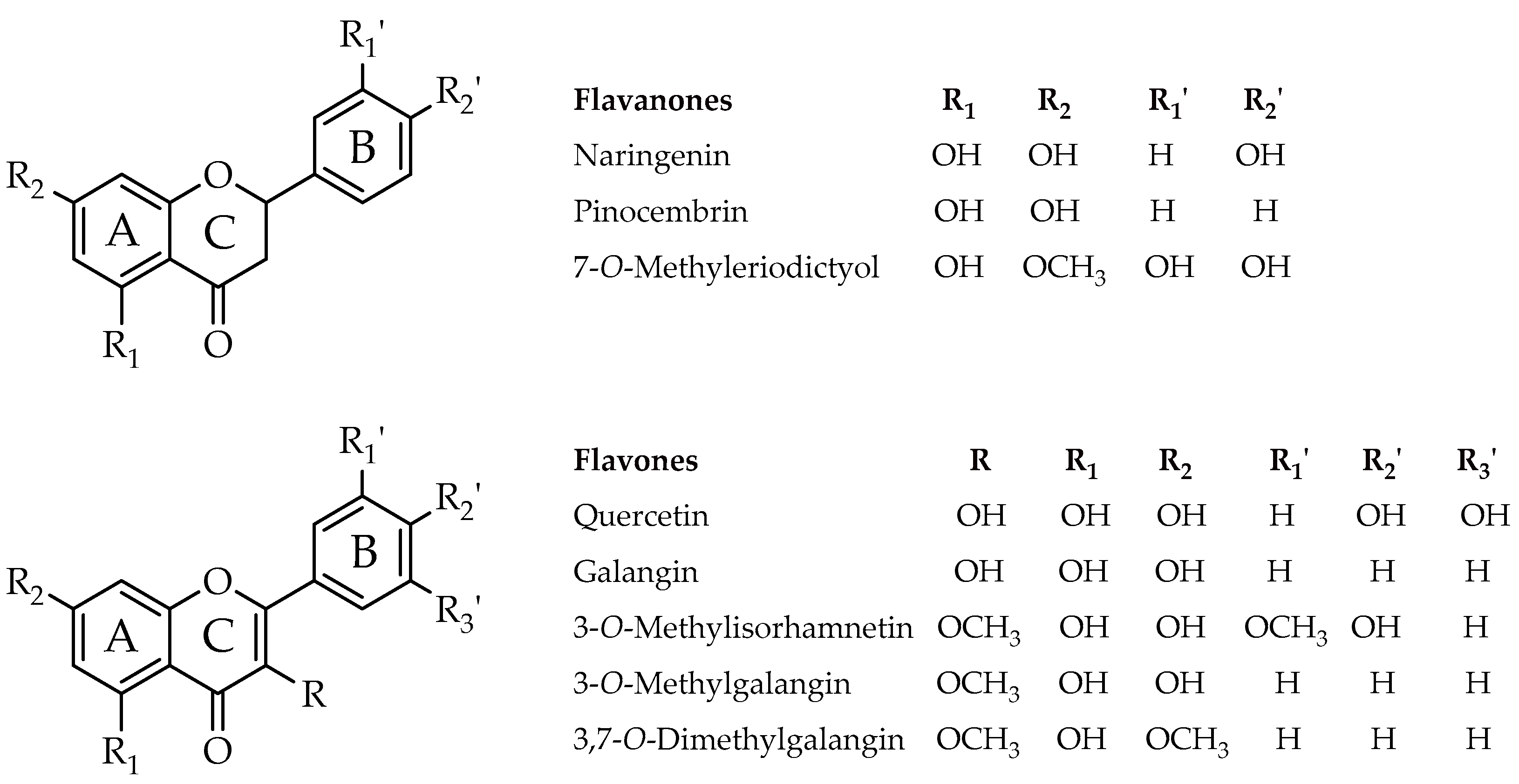

| Compound | Minimal Inhibitory Concentration in Solid Media (µg/µL) * | |||||||
|---|---|---|---|---|---|---|---|---|
| Gram-Negative Bacteria | Gram-Positive Bacteria | |||||||
| E. cloacae | E. coli | K. pneumoniae | P. mirabilis | B. cereus | B. coagulans | B. subtilis | S. aureus | |
| Naringenin (4,5,7-Trihydroxyflavanone) | 2.00 | 4.00 | >4.00 | 2.00 | 2.00 | 2.00 | 2.00 | >4.00 |
| Pinocembrin (5,7-Dihydroxyflavanone) | 1.00 | 4.00 | 1.00 | 4.00 | 2.00 | 1.00 | 1.00 | >4.00 |
| 7-O-Methyleriodictyol (3′,4′,5-Trihydroxy-7-methoxyflavanone) | 2.00 | 4.00 | >4.00 | 0.50 | 2.00 | 1.00 | 2.00 | 4.00 |
| Quercetin (3,3′,4′,5-7-Pentahydroxyflavone) | >4.00 | >4.00 | >4.00 | 0.50 | 2.00 | 2.00 | >4.00 | 2.00 |
| Galangin (3,5,7-Trihydroxyflavone) | 1.00 | 1.00 | 0.50 | 0.25 | 0.25 | 0.25 | 0.25 | 0.50 |
| 3-O-Methylisorhamnetin (5,7,4′-Trihydroxy-3,3′-dimethoxyflavone) | >4.00 | >4.00 | >4.00 | >4.00 | 2.00 | 1.00 | >4.00 | >4.00 |
| 3-O-Methylgalangin (5,7-Dihydroxy-3-methoxyflavone) | 1.00 | 0.50 | 0.50 | 0.25 | 0.25 | 0.38 | 0.38 | 0.50 |
| 3,7-O-Dimethylgalangin (5-Hydroxy-3,7-dimethoxyflavone) | >4.00 | >4.00 | >4.00 | >4.00 | >4.00 | >4.00 | >4.00 | >4.00 |
| Compound a | trb (min) | w1/2 c (cm) | Diffusion Coefficient d (×10−9) | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | 2 | Mean | |
| Naringenin (4,5,7-Trihydroxyflavanone) | 48.7 | 48.8 | 1.1 | 1.1 | 0.90 | 0.99 | 0.95 |
| Pinocembrin (5,7-Dihydroxyflavanone) | 49.5 | 49.2 | 1.0 | 0.9 | 1.10 | 1.35 | 1.23 |
| 7-O-Methyleriodictyol (3′,4′,5-Trihydroxy-7-methoxyflavanone) | 50.8 | 51.2 | 1.1 | 1.1 | 0.94 | 0.94 | 0.94 |
| Quercetin (3,3′,4′,5-7-Pentahydroxyflavone) | 49.4 | 50.3 | 1.1 | 1.1 | 0.91 | 0.93 | 0.92 |
| Galangin (3,5,7-Trihydroxyflavone) | 50.7 | 52.1 | 1.0 | 1.0 | 1.13 | 1.16 | 1.15 |
| 3-O-Methylisorhamnetin (5,7,4′-Trihydroxy-3,3′-dimethoxyflavone) | 49.2 | 49.5 | 1.1 | 1.1 | 0.96 | 1.00 | 0.98 |
| 3-O-Methylgalangin (5,7-Dihydroxy-3-methoxyflavone) | 48.2 | 48.1 | 1.0 | 1.0 | 1.08 | 1.07 | 1.08 |
| 3,7-O-Dimethylgalangin (5-Hydroxy-3,7-dimethoxyflavone) | 49.6 | 50.2 | 0.9 | 0.9 | 1.37 | 1.38 | 1.38 |
| Flavonoids | ALOGPs | ACLogP | AB/LogP | miLogP | ALOGP | MLOGP | KOWWIN | XLOGP2 | XLOGP3 | Average Lipophilicity |
|---|---|---|---|---|---|---|---|---|---|---|
| Naringenin (4,5,7-Trihydroxyflavanone) | 2.47 | 2.50 | 2.33 | 2.12 | 2.30 | 1.45 | 2.61 | 1.57 | 2.39 | 2.19 ± 0.41 |
| Pinocembrin (5,7-Dihydroxyflavanone) | 2.85 | 2.80 | 2.95 | 2.60 | 2.57 | 2.24 | 3.09 | 2.40 | 2.75 | 2.69 ± 0.27 |
| 7-O-Methyleriodictyol (3′,4′,5-Trihydroxy-7-methoxyflavanone) | 2.54 | 2.40 | 2.31 | 2.16 | 2.28 | 0.93 | 2.69 | 1.49 | 2.36 | 2.13 ± 0.56 |
| Quercetin (3,3′,4′,5-7-Pentahydroxyflavone) | 1.81 | 1.80 | 2.34 | 1.68 | 1.50 | 0.23 | 1.48 | 1.52 | 1.54 | 1.55 ± 0.56 |
| Galangin (3,5,7-Trihydroxyflavone) | 2.39 | 2.40 | 3.42 | 2.65 | 2.04 | 1.76 | 2.44 | 3.17 | 2.25 | 2.50 ± 0.52 |
| 3-O-Methylisorhamnetin (5,7,4′-Trihydroxy-3,3′-dimethoxyflavone) | 3.00 | 2.45 | 2.55 | 2.27 | 1.82 | 0.73 | 1.96 | 1.72 | 2.82 | 2.15 ± 0.69 |
| 3-O-Methylgalangin (5,7-Dihydroxy-3-methoxyflavone) | 3.49 | 2.86 | 3.33 | 2.93 | 2.10 | 2.01 | 2.62 | 3.06 | 2.58 | 2.78 ± 0.50 |
| 3,7-O-Dimethylgalangin (5-Hydroxy-3,7-dimethoxyflavone) | 3.74 | 3.05 | 3.77 | 3.46 | 2.35 | 2.26 | 3.18 | 3.38 | 3.53 | 3.19 ± 0.55 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echeverría, J.; Opazo, J.; Mendoza, L.; Urzúa, A.; Wilkens, M. Structure-Activity and Lipophilicity Relationships of Selected Antibacterial Natural Flavones and Flavanones of Chilean Flora. Molecules 2017, 22, 608. https://doi.org/10.3390/molecules22040608
Echeverría J, Opazo J, Mendoza L, Urzúa A, Wilkens M. Structure-Activity and Lipophilicity Relationships of Selected Antibacterial Natural Flavones and Flavanones of Chilean Flora. Molecules. 2017; 22(4):608. https://doi.org/10.3390/molecules22040608
Chicago/Turabian StyleEcheverría, Javier, Julia Opazo, Leonora Mendoza, Alejandro Urzúa, and Marcela Wilkens. 2017. "Structure-Activity and Lipophilicity Relationships of Selected Antibacterial Natural Flavones and Flavanones of Chilean Flora" Molecules 22, no. 4: 608. https://doi.org/10.3390/molecules22040608








