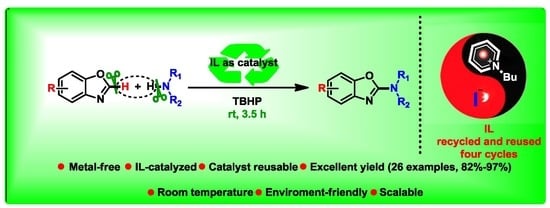
The Synthesis of 2-Aminobenzoxazoles Using Reusable Ionic Liquid as a Green Catalyst under Mild Conditions
Abstract
:1. Introduction
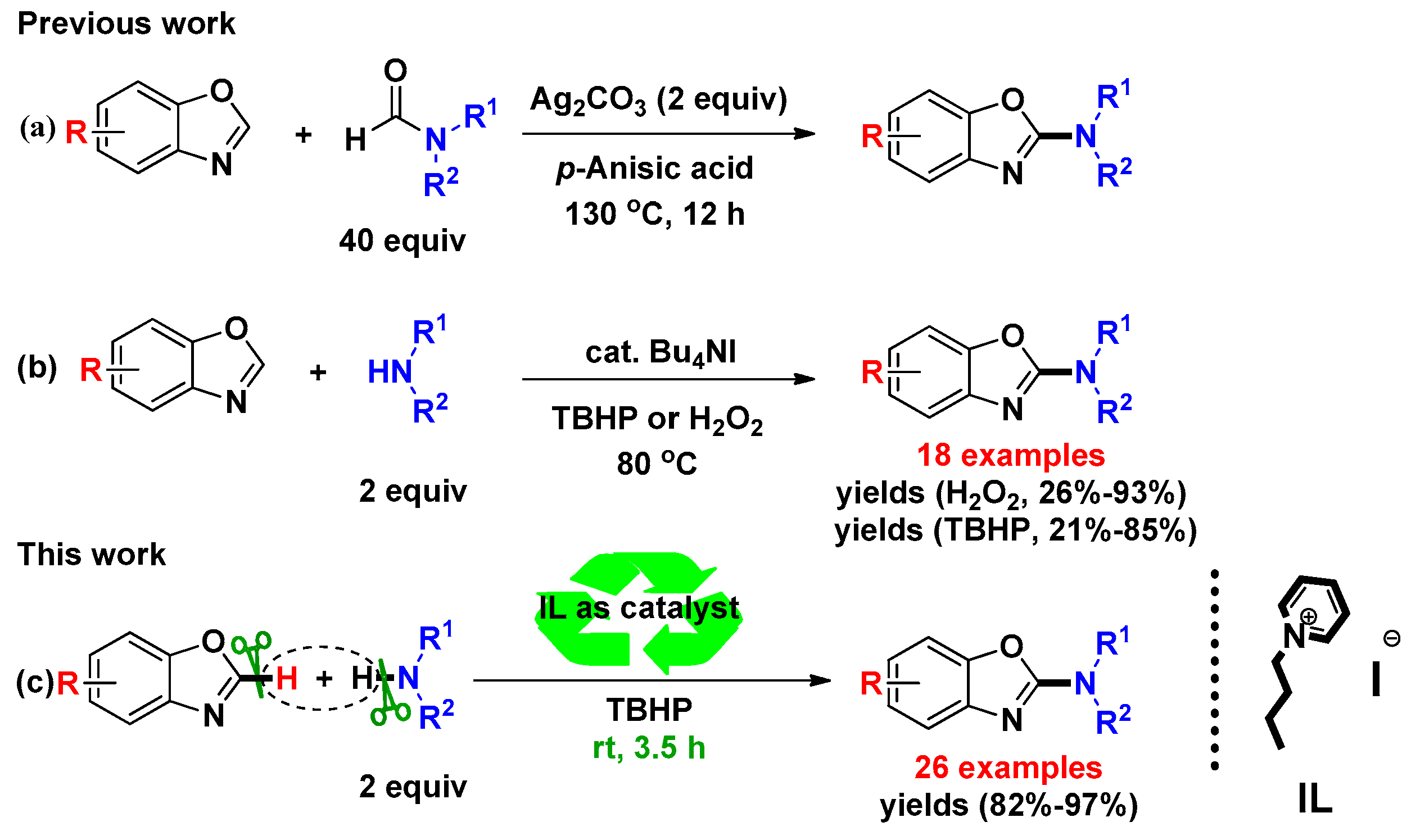
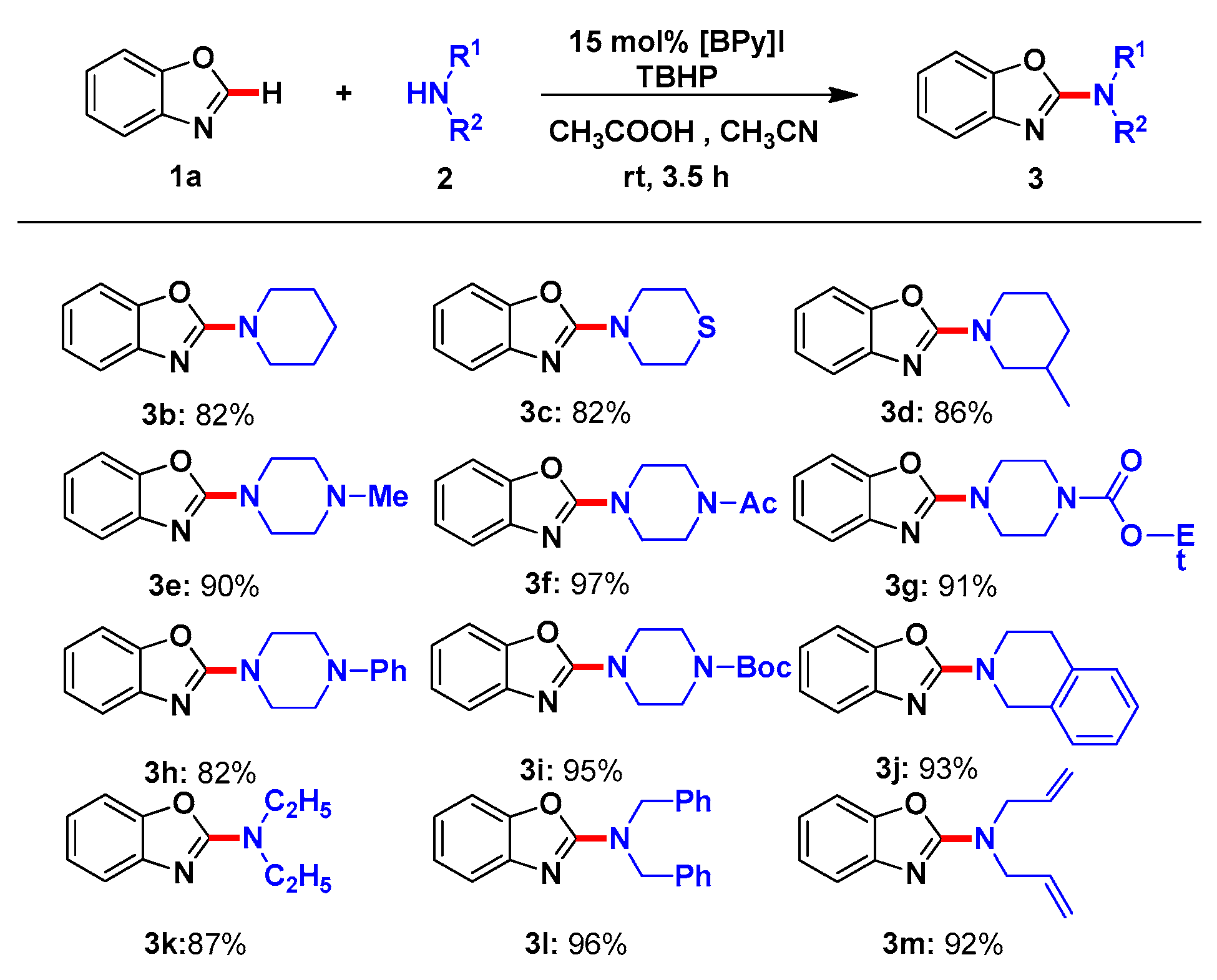
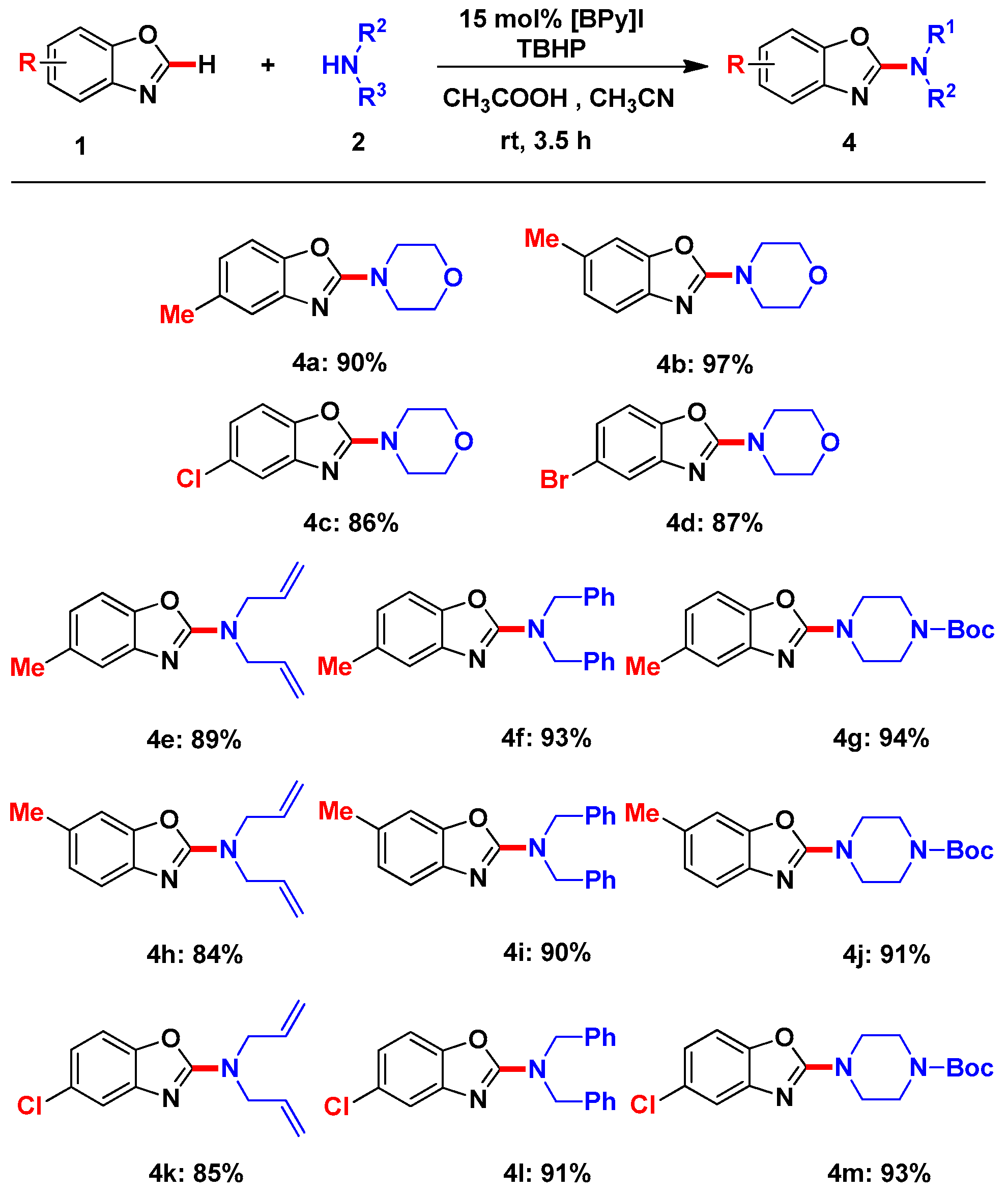
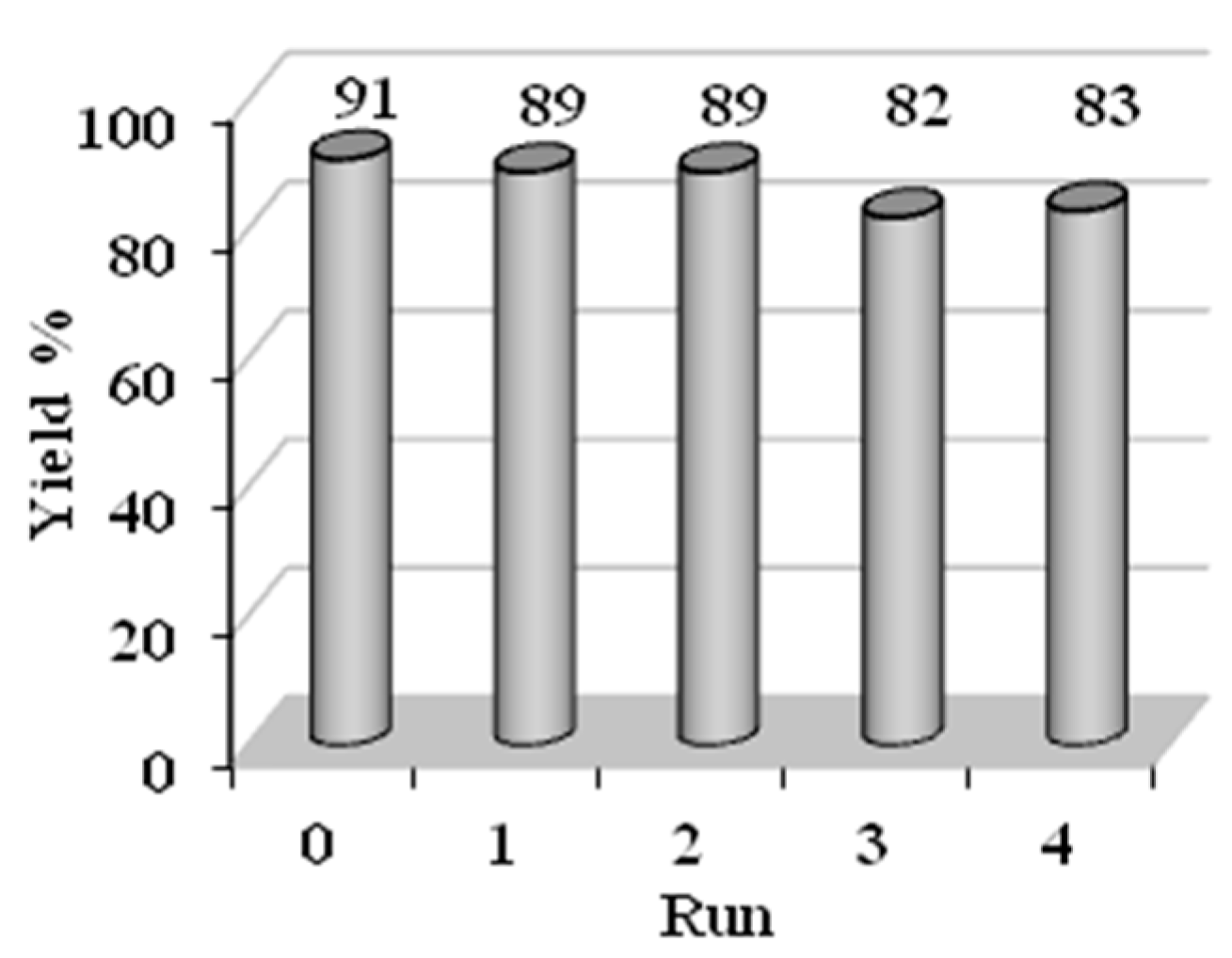
2. Results and Discussion
3. Experimental Section
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kablaoui, N.; Patel, S.; Shao, J.; Demian, D.; Hoffmaster, K.; Berlioz, F.; Vazquez, M.L.; Moore, W.M.; Nugent, R.A. Novel benzoxazole inhibitors of mPGES-1. Bioorg. Med. Chem. Lett. 2013, 23, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.G.; Lo, J.R.; Comery, T.A.; Zhang, G.M.; Zhang, J.Y.; Kowal, D.M.; Smith, D.L.; Di, L.; Kerns, E.H.; Schechter, L.E.; et al. Identification of a series of benzoxazoles as potent 5-HT6 ligands. Bioorg. Med. Chem. Lett. 2009, 19, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Ningaiah, S.; Bhadraiah, U.K.; Keshavamurthy, S.; Javarasetty, C. Novel pyrazoline amidoxime and their 1,2,4-oxadiazole analogues: Synthesis and pharmacological screening. Bioorg. Med. Chem. Lett. 2013, 23, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.Z.; Wang, M.; Hutchins, G.D.; Zheng, Q.H. Synthesis of new carbon-11 labeled benzoxazole derivatives for PET imaging of 5-HT3 receptor. Eur. J. Med. Chem. 2008, 43, 1570–1574. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Shiokawa, S.; Kawano, K.I.; Ito, T.; Murakami, H.; Suzuki, H.; Sato, Y. Orally Active Benzoxazole Derivative as 5-HT3 Receptor Partial Agonist for Treatment of Diarrhea-Predominant Irritable Bowel Syndrome. J. Med. Chem. 2005, 48, 7075–7079. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C.J.; Rogers, B.N.; Bronk, B.S.; Bryce, D.K.; Coe, J.W.; Cook, K.K.; Duplantier, A.J.; Evrard, E.; Hajós, M.; Hoffmann, W.E.; et al. Discovery of 4-(5-Methyloxazolo[4,5-b]pyridin-2-yl)-1,4-diazabicyclo [3.2.2]nonane (CP-810,123), a Novel α7 Nicotinic Acetylcholine Receptor Agonist for the Treatment of Cognitive Disorders in Schizophrenia: Synthesis, SAR Development, and in Vivo Efficacy in Cognition Models. J. Med. Chem. 2010, 53, 1222–1237. [Google Scholar] [PubMed]
- Cox, C.D.; Breslin, M.J.; Whitman, D.B.; Schreier, J.D.; McGaughey, G.B.; Bogusky, M.J.; Roecker, A.J.; Mercer, S.P.; Bednar, R.A.; Lemaire, W.; et al. Discovery of the Dual Orexin Receptor Antagonist [(7R)-4-(5-Chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl] [5-methyl-2-(2H -1,2,3-triazol-2-yl)phenyl] methanone (MK-4305) for the Treatment of Insomnia. J. Med. Chem. 2010, 53, 5320–5332. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Kim, J.Y.; Lee, S.Y.; Chang, S. Silver-Mediated Direct Amination of Benzoxazoles: Tuning the Amino Group Source from Formamides to Parent Amines. Angew. Chem. Int. Ed. 2009, 48, 9127–9130. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Cho, S.H.; Joseph, J.; Chang, S. Cobalt- and Manganese-Catalyzed Direct Amination of Azoles under Mild Reaction Conditions and the Mechanistic Details. Angew. Chem. Int. Ed. 2010, 122, 10095–10099. [Google Scholar] [CrossRef]
- Monguchi, D.; Fujiwara, T.; Furukawa, H.; Mori, A. Direct Amination of Azoles via Catalytic C–H, N–H Coupling. Org. Lett. 2009, 11, 1607–1610. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Schreiber, S.L. Copper-Mediated Amidation of Heterocyclic and Aromatic C–H Bonds. Org. Lett. 2009, 11, 5178–5180. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, M.; Hirano, K.; Satoh, T.; Kowalczyk, R.; Bolm, C.; Miura, M. Copper-Catalyzed Direct Sulfoximination of Azoles and Polyfluoroarenes under Ambient Conditions. Org. Lett. 2011, 13, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Hirano, K.; Satoh, T.; Miura, M. A New Entry of Amination Reagents for Heteroaromatic C-H Bonds: Copper-Catalyzed Direct Amination of Azoles with Chloroamines at Room Temperature. J. Am. Chem. Soc. 2010, 132, 6900–6901. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, N.; Hirano, K.; Satoh, T.; Miura, M. Copper-Catalyzed Direct Amination of Electron-Deficient Arenes with Hydroxylamines. Org. Lett. 2011, 13, 2860–2863. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.J.; Qian, B.; Xie, P.; Huang, H.M. Cooperative Catalysis with Aldehydes and Copper: Development and Application in Aerobic Oxidative CH Amination at Room Temperature. Adv. Synth. Catal. 2013, 355, 1315–1322. [Google Scholar] [CrossRef]
- Wertz, S.; Kodama, S.; Studer, A. Cooperative Catalysis with Aldehydes and Copper: Development and Application in Aerobic Oxidative CH Amination at Room Temperature. Angew. Chem. Int. Ed. 2011, 50, 11511–11515. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hou, J.T.; Wen, J.; Yu, X.Q. Iron-catalyzed direct amination of azoles using formamides or amines as nitrogen sources in air. Chem. Commun. 2011, 47, 3652–3654. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, J.R.; Wei, Z.; Zhang, Q.; Shi, D.X. Direct amination of azoles using CuCl2 complexes of amines under mild conditions. RSC. Adv. 2013, 3, 9622–9624. [Google Scholar] [CrossRef]
- Li, Y.M.; Liu, J.; Xie, Y.S.; Zhang, R.; Jin, K.; Wang, X.N.; Duan, C.Y. Nickel-catalyzed C–H direct amination of benzoxazoles with secondary Amines. Org. Biomol. Chem. 2012, 10, 3715–3720. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, C.L.; Lansing, J.J.; Yüksel, H. Synthesis of 2-Aminobenzoxazoles Using Tetramethyl Orthocarbonate or 1,1-Dichlorodiphenoxymethane. J. Org. Chem. 2010, 75, 7942–7945. [Google Scholar] [CrossRef] [PubMed]
- Parumala, S.K.R.; Peddinti, R.K. Iodine catalyzed cross-dehydrogenative C–S coupling by C(sp2)–H bond activation: Direct access to aryl sulfides from aryl thiols. Green Chem. 2015, 17, 4068–4072. [Google Scholar] [CrossRef]
- Yang, K.; Ke, M.L.; Lin, Y.G.; Song, Q.L. Sulfonamide formation from sodium sulfinates and amines or ammonia under metal-free conditions at ambient temperature. Green Chem. 2015, 17, 1395–1399. [Google Scholar] [CrossRef]
- Zhang, X.B.; Wang, L. TBHP/I2-promoted oxidative coupling of acetophenones with amines at room temperature under metal-free and solvent-free conditions for the synthesis of α-ketoamides. Green Chem. 2012, 14, 2141–2145. [Google Scholar] [CrossRef]
- Huang, H.; Chen, W.H.; Xu, Y.; Li, J. I−/TBHP catalyzed Csp3–N/Csp2–N bond formation via oxidative coupling with benzophenone imine in water. Green Chem. 2015, 17, 4715–4719. [Google Scholar] [CrossRef]
- Zeng, J.W.; Liu, Y.C.; Hsieh, P.A.; Huang, Y.T.; Yi, C.L.; Badsara, S.S.; Lee, C.F. Metal-free cross-coupling reaction of aldehydes with disulfides by using DTBP as an oxidant under solvent-free conditions. Green Chem. 2014, 16, 2644–2652. [Google Scholar] [CrossRef]
- Wen, J.W.; Wei, W.; Xue, S.N.; Yang, D.S.; Lou, Y.; Gao, C.Y.; Wang, H. Metal-Free Oxidative Spirocyclization of Alkynes with Sulfonylhydrazides Leading to 3-Sulfonated Azaspiro[4,5]trienones. J. Org. Chem. 2015, 80, 4966–4972. [Google Scholar] [CrossRef] [PubMed]
- Panda, N.; Mattan, I.; Nayak, D.K. Synthesis of Dibenzofurans via C−H Activation of o-Iodo Diaryl Ethers. J. Org. Chem. 2015, 80, 6590–6597. [Google Scholar] [CrossRef] [PubMed]
- Tinnis, F.; Stridfeldt, E.; Lundberg, H.; Adolfsson, H.; Olofsson, B. Metal-Free N-Arylation of Secondary Amides at Room Temperature. Org. Lett. 2015, 17, 2688–2691. [Google Scholar] [CrossRef] [PubMed]
- Danneman, M.W.; Hong, K.B.; Johnston, J.N. Oxidative Inter-/Intermolecular Alkene Diamination of Hydroxy Styrenes with Electron-Rich Amines. Org. Lett. 2015, 17, 2558–2561. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Kim, J.Y.; Chang, S. A Metal-Free Route to 2-Aminooxazoles by Taking Advantage of the Unique Ring Opening of Benzoxazoles and Oxadiazoles with Secondary Amines. Chem. Eur. J. 2011, 17, 8294–8298. [Google Scholar] [CrossRef] [PubMed]
- Wagh, Y.S.; Tiwari, N.J.; Bhanage, B.M. Metal-free synthesis of 2-aminobenzoxazoles using hypervalent iodine reagent. Tetrahedron Lett. 2013, 54, 1290–1293. [Google Scholar] [CrossRef]
- Lamani, M.; Prabhu, K.R. Iodine-Catalyzed Amination of Benzoxazoles: A Metal-Free Route to 2-Aminobenzoxazoles under Mild Conditions. J. Org. Chem. 2011, 76, 7938–7944. [Google Scholar] [CrossRef] [PubMed]
- Froehr, T.; Sindlinger, C.P.; Kloeckner, U.; Finkbeiner, P.; Nachtsheim, B.J. A Metal-Free Amination of Benzoxazoles The First Example of an Iodide-Catalyzed Oxidative Amination of Heteroarenes. Org. Lett. 2011, 13, 3754–3757. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.J.; Li, W.C.; Zeng, C.C.; Tian, H.Y.; Hu, L.M.; Little, R.D. Electrochemically Initiated Oxidative Amination of Benzoxazoles Using Tetraalkylammonium Halides as Redox Catalysts. J. Org. Chem. 2014, 79, 9613–9618. [Google Scholar] [CrossRef] [PubMed]
- Wagh, Y.S.; Sawant, D.N.; Bhanage, B.M. Metal-free N-iodosuccinimide-catalyzed mild oxidative C–H bond amination of benzoxazoles. Tetrahedron Lett. 2012, 53, 3482–3485. [Google Scholar] [CrossRef]
- Wang, R.; Liu, H.; Yue, L.; Zhang, X.K.; Tan, Q.Y.; Pan, R.L. Transition metal-free direct amination of benzoxazoles using formamides as nitrogen sources. Tetrahedron Lett. 2014, 55, 2233–2237. [Google Scholar] [CrossRef]
- Wang, X.; Xu, D.Q.; Miao, C.X.; Zhang, Q.H.; Sun, W. N-Bromosuccinimide as an oxidant for the transition-metal-free synthesis of 2-aminobenzoxazoles from benzoxazoles and secondary amines. Org. Biomol. Chem. 2014, 12, 3108–3113. [Google Scholar] [CrossRef] [PubMed]
- Yotphan, S.; Beukeaw, D.; Reutrakul, V. A Convenient One-Pot Synthesis of N-Substituted 2-Aminoazole Derivatives. Synthesis 2013, 45, 936–942. [Google Scholar] [CrossRef]
- Martins, M.P.; Frizzo, C.; Moreira, D.; Zanatta, N.; Bonacorso, H. Ionic Liquids in Heterocyclic Synthesis. Chem. Rev. 2008, 108, 2015–2050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shreeve, J.M. Energetic Ionic Liquids as Explosives and Propellant Fuels: A New Journey of Ionic Liquid Chemistry. Chem. Rev. 2014, 114, 10527–10574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, S.; Deng, Y. Recent advances in ionic liquid catalysis. Green Chem. 2011, 13, 2619–2637. [Google Scholar] [CrossRef]
- Giernoth, R. Task-Specific Ionic Liquids. Angew. Chem. Int. Ed. 2010, 49, 2834–2839. [Google Scholar] [CrossRef] [PubMed]
- Taheri, A.; Liu, C.H.; Lai, B.B.; Cheng, C.; Pan, X.J.; Gu, Y.L. Brønsted acid ionic liquid catalyzed facile synthesis of 3-vinylindoles through direct C3 alkenylation of indoles with simple ketones. Green Chem. 2014, 16, 3715–3719. [Google Scholar] [CrossRef]
- Glasnov, T.N.; Holbrey, J.D.; Kappe, C.O.; Seddon, K.R.; Yan, T. Methylation using dimethylcarbonate catalysed by ionic liquids under continuous flow conditions. Green Chem. 2012, 14, 3071–3076. [Google Scholar] [CrossRef]
- Li, J.X.; Yang, W.F.; Yang, S.R.; Huang, L.B.; Wu, W.Q.; Sun, Y.D.; Jiang, H.F. Palladium-Catalyzed Cascade Annulation to Construct Functionalized β- and γ-Lactones in Ionic Liquids. Angew. Chem. Int. Ed. 2014, 53, 7219–7222. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, P.; Petrosyan, A.; Baumgard, J.; Jopp, S.; Steinfeld, N.; Ghochikyan, T.V.; Saghyan, A.S.; Fischer, C.; Langer, P. Synthesis of 2,5-Diarylpyrroles by Ligand-Free Palladium-Catalyzed CH Activation of Pyrroles in Ionic Liquids. ChemCatChem 2013, 5, 2504–2511. [Google Scholar] [CrossRef]
- Baslé, O.; Borduas, N.; Dubois, P.; Chapuzet, J.M.; Chan, T.H.; Lessard, J.; Li, C.J. Aerobic and Electrochemical Oxidative Cross-Dehydrogenative-Coupling (CDC) Reaction in an Imidazolium-Based Ionic Liquid. Chem. Eur. J. 2010, 16, 8162–8166. [Google Scholar] [CrossRef] [PubMed]
- Biffis, A.; Gazzola, L.; Tubaro, C.; Basato, M. Alkyne Hydroarylation in Ionic Liquids Catalyzed by Palladium(II) Complexes. ChemSusChem 2010, 3, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Yang, S.R.; Jiang, H.F.; Wu, W.Q.; Zhao, J.W. Palladium-Catalyzed Coupling of Alkynes with Unactivated Alkenes in Ionic Liquids: A Regio-and Stereoselective Synthesis of Functionalized 1,6-Dienes and Their Analogues. J. Org. Chem. 2013, 78, 12477–12486. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, C.J.; Zhang, Y.H.; Sun, Y.D.; Wang, B.; Liu, W.B. Green Method for the Synthesis of Chromeno[2,3-c]pyrazol-4(1H)-ones through Ionic Liquid Promoted Directed Annulation of 5-(Aryloxy)-1H-pyrazole-4-carbaldehydes in Aqueous Media. Org. Lett. 2015, 17, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.B.; Liu, C.J.; Zhang, Y.H.; Sun, Y.D.; Abdukadera, A.; Wang, B.; Li, H.; Ma, X.C.; Zhang, Z.P. Reusable ionic liquid-catalyzed oxidative coupling of azoles and benzylic compounds via sp3 C–N bond formation under metal-free conditions. Org. Biomol. Chem. 2015, 13, 7154–7158. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: All samples are available from the authors. |
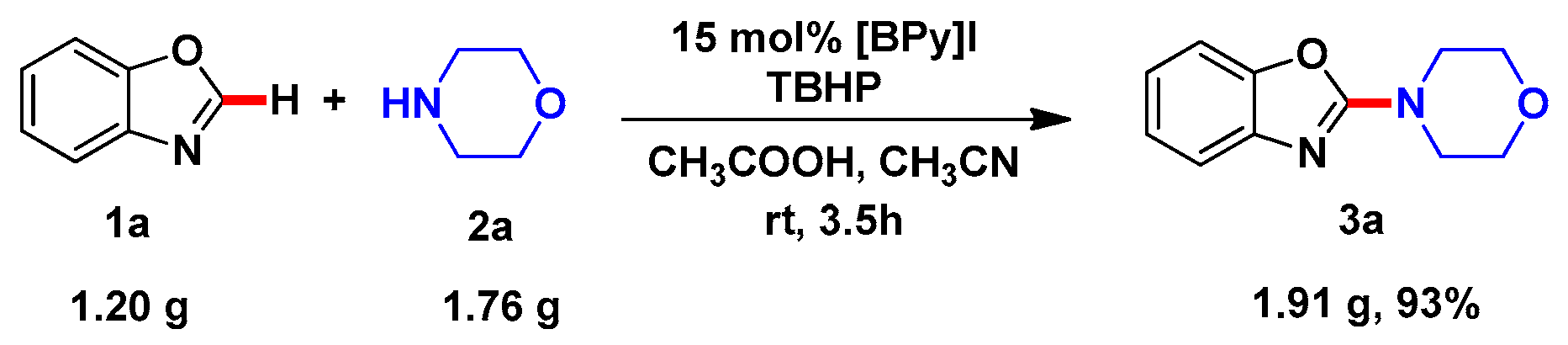


| Entry | Catalyst (mol%) | Oxidant b | Solvent | Time (h) | Yield (%) c |
|---|---|---|---|---|---|
| 1 | [BPy]I (5) | TBHP | CH3CN | 7 | 94 |
| 2 | [BPy]Cl (5) | TBHP | CH3CN | 7 | Trace |
| 3 | [BPy]Br (5) | TBHP | CH3CN | 7 | N.R. d |
| 4 | - | TBHP | CH3CN | 7 | N.R. |
| 5 | [BPy]I (10) | TBHP | CH3CN | 5 | 91 |
| 6 | [BPy]I (15) | TBHP | CH3CN | 3.5 | 94 |
| 7 | [BPy]I (20) | TBHP | CH3CN | 3.5 | 94 |
| 8 c | [BPy]I (15) | TBHP | CH2Cl2 | 3.5 | 88 |
| 9 | [BPy]I (15) | TBHP | THF | 3.5 | 85 |
| 10 | [BPy]I (15) | TBHP | toluene | 3.5 | 86 |
| 11 | [BPy]I (15) | TBHP | H2O | 3.5 | 57 |
| 12 | [BPy]I (15) | TBHP | Neat | 3.5 | 51 |
| 13 | [BPy]I (15) | BPO | CH3CN | 3.5 | 65 |
| 14 | [BPy]I (15) | m-CPBA | CH3CN | 3.5 | N.R. |
| 15 | [BPy]I (15) | DTBP | CH3CN | 3.5 | Trace |
| 16 | [BPy]I (15) | H2O2 | CH3CN | 3.5 | 79 |

| Entry | 1a (Equiv.) | C.HI (Equiv.) | 2a (Equiv.) | TBHP (Equiv.) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 0 | 0 | 25 |
| 2 | 1 | 2 | 0 | 0 | 53 |
| 3 | 1 | 0.15 | 2 | 1.5 | 95 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Liu, Z.; Yuan, T.; Huang, J.; Liu, C. The Synthesis of 2-Aminobenzoxazoles Using Reusable Ionic Liquid as a Green Catalyst under Mild Conditions. Molecules 2017, 22, 576. https://doi.org/10.3390/molecules22040576
Zhou Y, Liu Z, Yuan T, Huang J, Liu C. The Synthesis of 2-Aminobenzoxazoles Using Reusable Ionic Liquid as a Green Catalyst under Mild Conditions. Molecules. 2017; 22(4):576. https://doi.org/10.3390/molecules22040576
Chicago/Turabian StyleZhou, Ya, Zhiqing Liu, Tingting Yuan, Jianbin Huang, and Chenjiang Liu. 2017. "The Synthesis of 2-Aminobenzoxazoles Using Reusable Ionic Liquid as a Green Catalyst under Mild Conditions" Molecules 22, no. 4: 576. https://doi.org/10.3390/molecules22040576