Morphology Analysis and Optimization: Crucial Factor Determining the Performance of Perovskite Solar Cells
Abstract
:1. Introduction
2. Surface Morphology and Spectrum Analysis
2.1. Fabrication Processions Related Surface Morphology of the Perovskite Layer
2.1.1. PVSK Materials
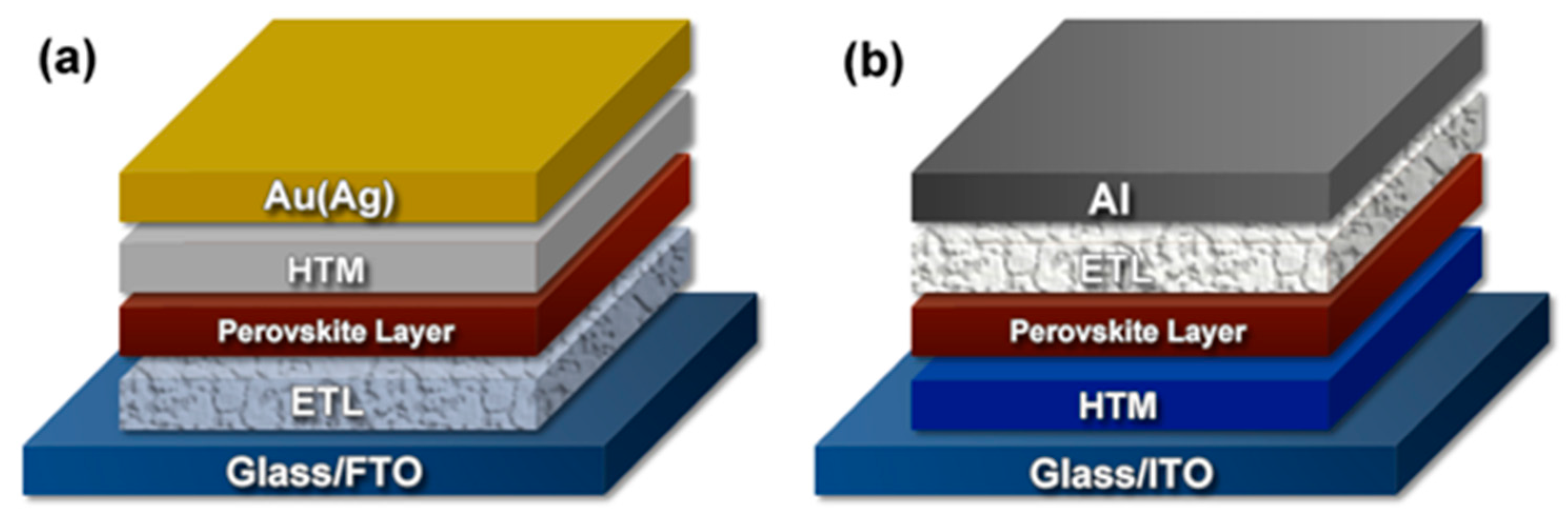
2.1.2. Carrier-Transporting Layers
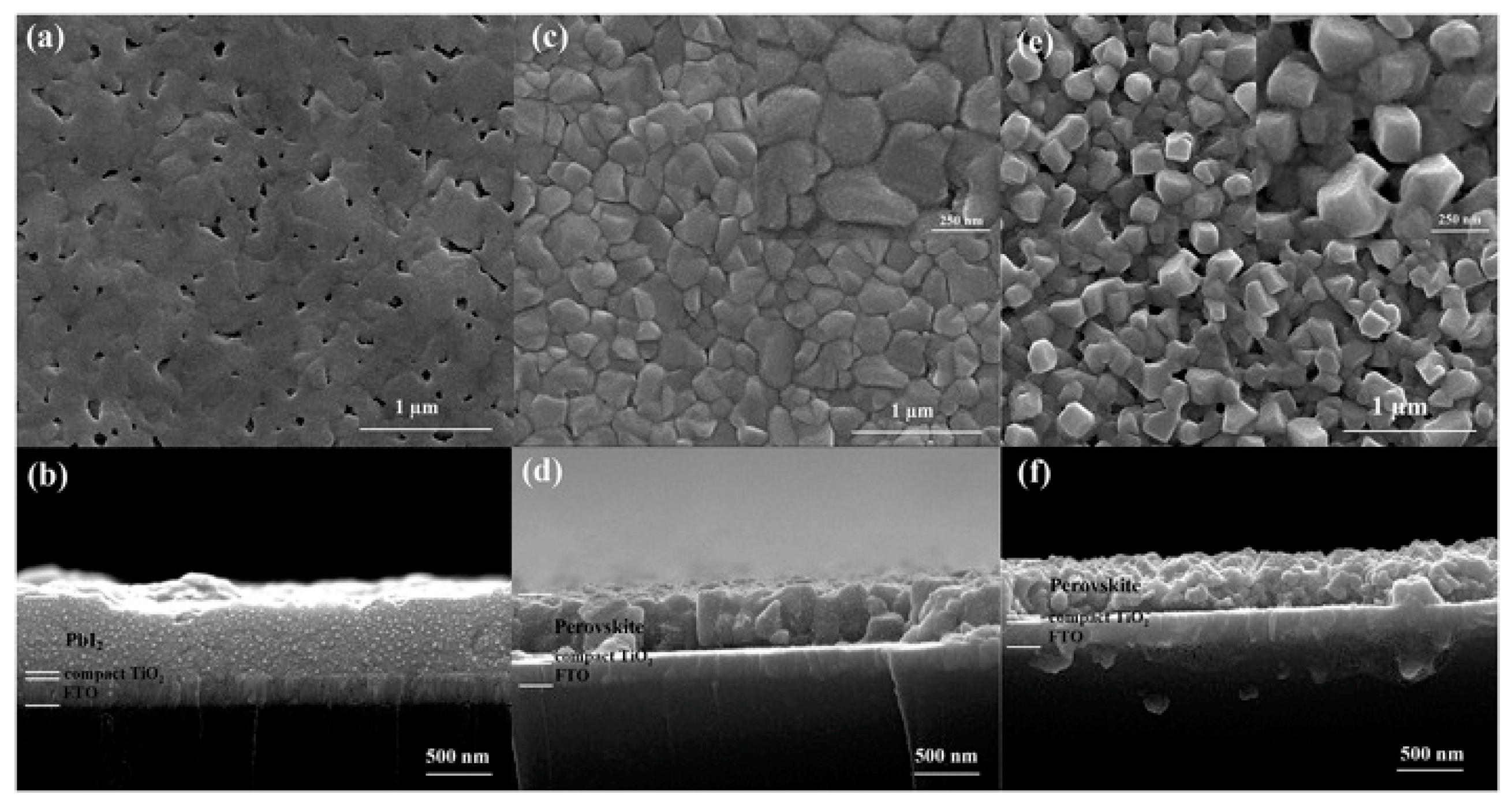
2.1.3. Film-Deposition Approaches
2.2. Impact Factors to the Perovskite Morphology
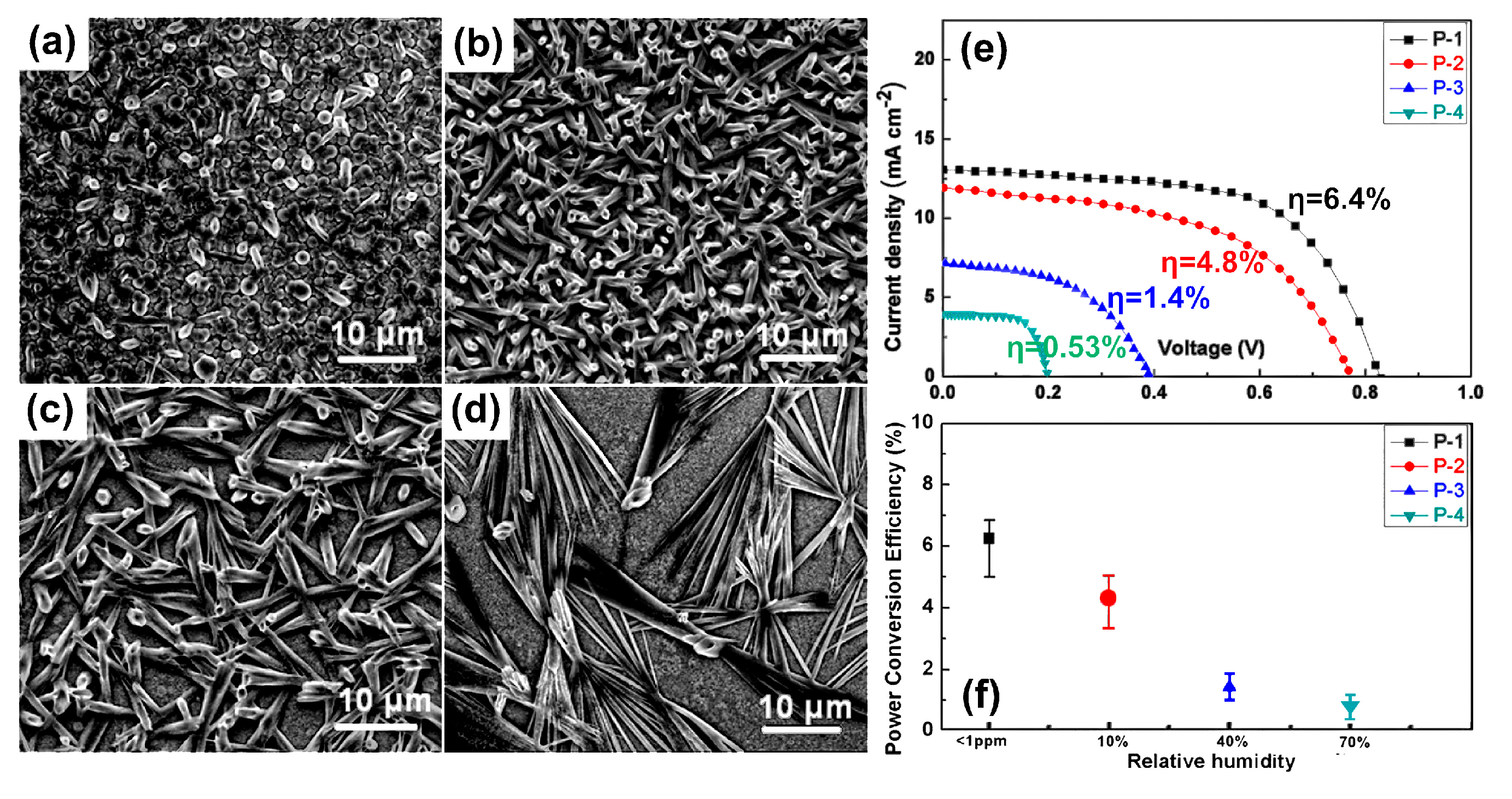
2.2.1. Effect of Humidity
2.2.2. Effect of Solutions
2.2.3. Effect of Pressure
2.3. Spectra Analysis of the Perovskite Layer
2.3.1. Fourier Transform Infrared Spectroscopy
2.3.2. Raman Spectroscopy
3. Interface Energy Analysis and Alignment
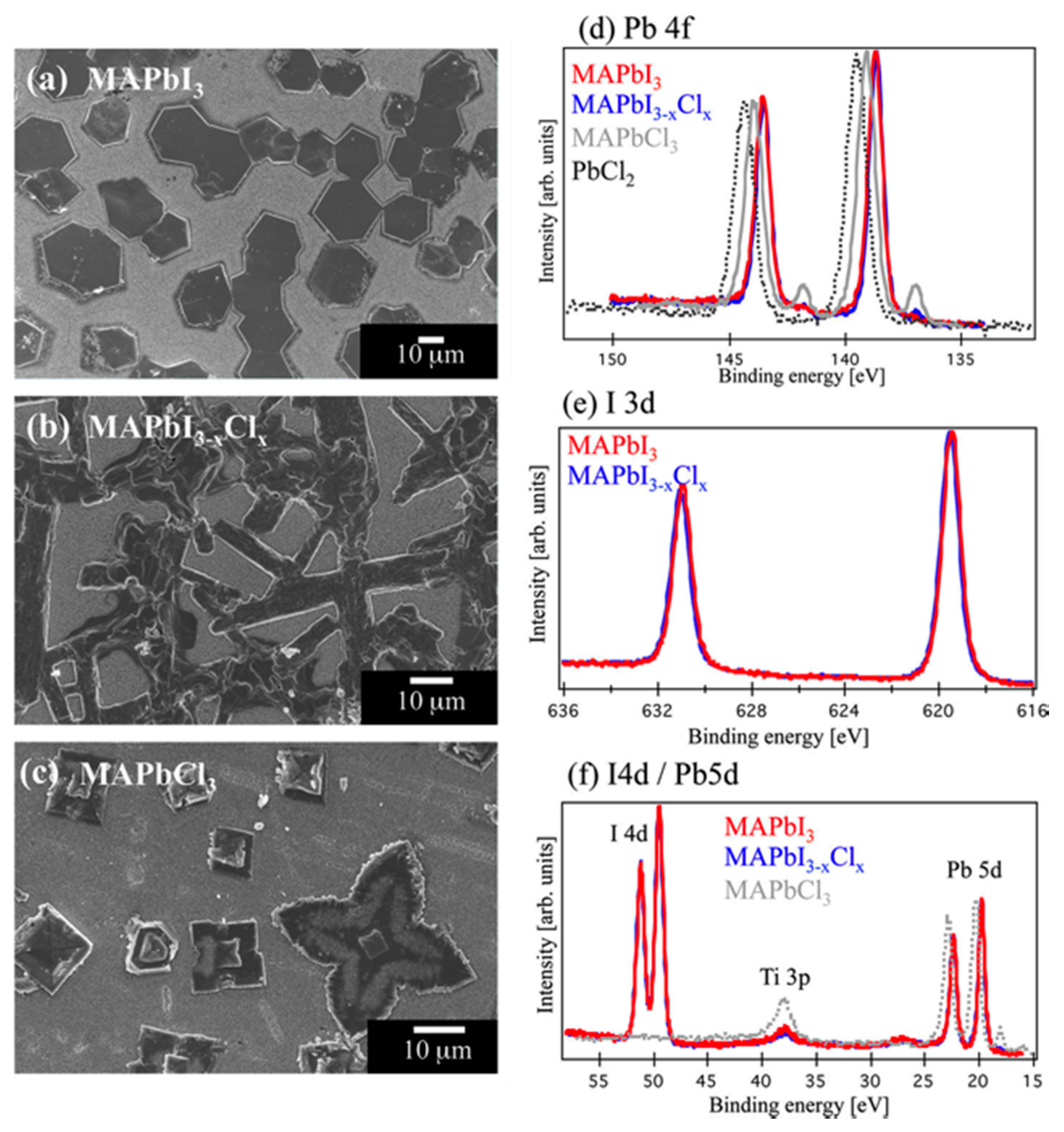
3.1. Characterization by X-ray Photoelectron Spectroscopy
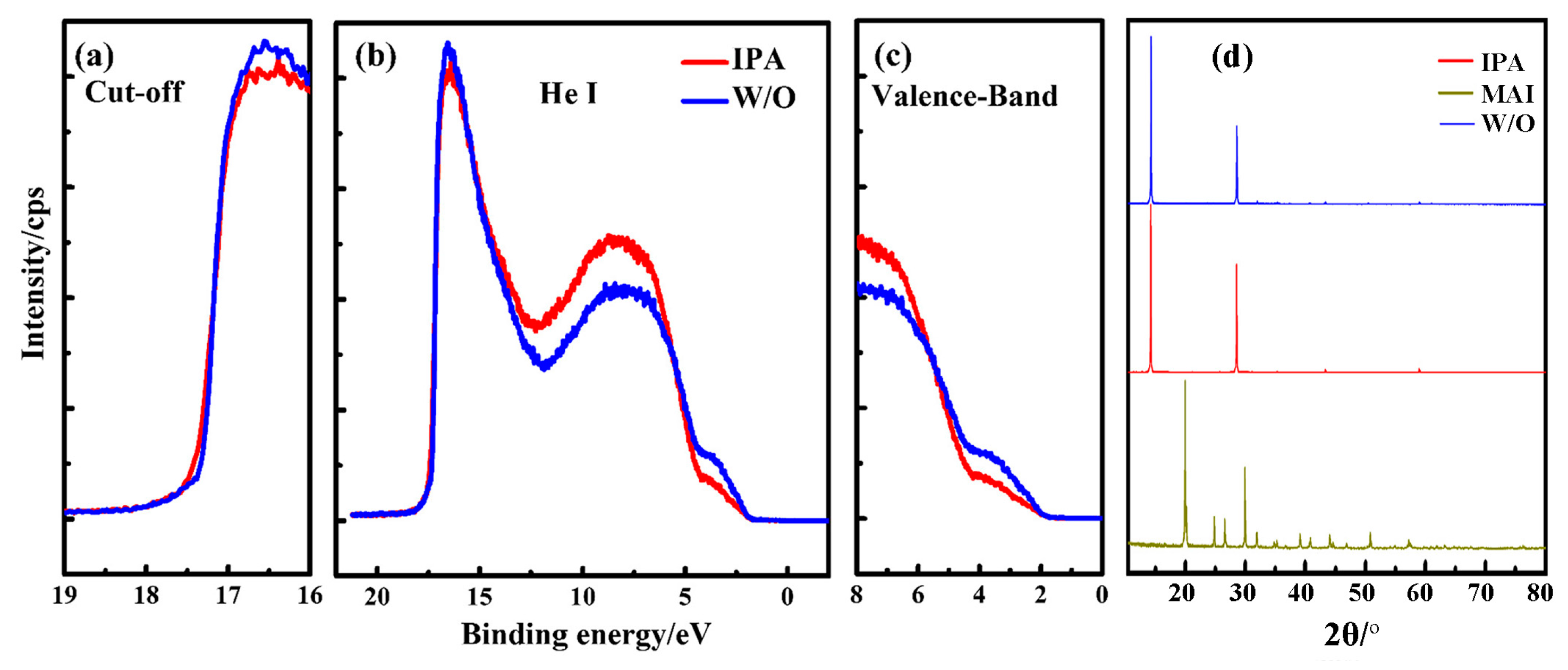
3.2. Characterization by Ultraviolet Photoelectron Spectroscopy
4. Approach for Morphology Control and Optimization
4.1. Effect of Additives
4.2. Solvent Treatment
4.3. Thermal Annealing
4.4. Other Process Optimization
5. Summary and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Burschka, J.; Pellet, N.; Moon, S.; Humphry, R.; Gao, P.; Nazeeruddin, M.K.; Gratzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.; Duan, H.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domanski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.R.; Jain, A.; Voznyy, O.; Lan, X.; de Arquer, F.P.G.; Fan, J.Z.; Quintero-Bermudez, R.; Yuan, M.; Zhang, B.; Zhao, Y.; et al. Efficient and stable solution-processed planar perovskite solar cells via contact passivation. Science 2017, 355, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shrotriya, V.; Huang, J.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 2005, 4, 864–868. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Yang, T.; Zhang, W.; Gong, X. Solution-processed ultrasensitive polymer photodetectors with high external quantum efficiency and detectivity. ACS Appl. Mater. Interface 2012, 4, 3701–3705. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yao, Y.; Yang, H.; Shrotriya, V.; Yang, G.; Yang, Y. “Solvent Annealing” Effect in polymer solar cells based on poly (3-hexylthiophene) and methanofullerenes. Adv. Funct. Mater. 2007, 17, 1636–1644. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, H.; Hong, Z.; Luo, S.; Duan, H.S.; Wang, H.H.; Liu, Y.; Li, G.; Yang, Y. Planar heterojunction perovskite solar cells via vapor-assisted solution process. J. Am. Chem. Soc. 2013, 136, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Park, J.S.; Han, I.K.; Shin, T.J.; Jo, W.H. CH3NH3PbI3 crystal orientation and photovoltaic performance of planar heterojunction perovskite solar cells. Sol. Energy Mat. Sol. Cells 2017, 160, 77–84. [Google Scholar] [CrossRef]
- Xiao, Z.; Bi, C.; Shao, Y.; Dong, Q.; Wang, Q.; Yuan, Y.; Wang, C.; Gao, Y.; Huang, J. Efficient, High yield perovskite photovoltaic devices grown by interdiffusion of solution-processed precursor stacking layers. Energy Environ. Sci. 2014, 7, 2619. [Google Scholar] [CrossRef]
- Qin, P.; Tanaka, S.; Ito, S.; Tetreault, N.; Manabe, K.; Nishino, H.; Nazeeruddin, M.K.; Grätzel, M. Inorganic hole conductor-based lead halide perovskite solar cells with 12.4% conversion efficiency. Nat. Commun. 2014, 5, 3834. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Seo, J.; Park, S.; Shin, S.S.; Kim, Y.C.; Jeon, N.J.; Shin, H.W.; Ahn, T.K.; Noh, J.H.; Yoon, S.C.; et al. Efficient CH3NH3PbI3 perovskite solar cells employing nanostructured p-type NiO electrode formed by a pulsed laser deposition. Adv. Mater. 2015, 27, 4013–4019. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Tiepelt, J.O.; Christians, J.A.; Levine, I.; Edri, E.; Sanehira, E.M.; Hodes, G.; Cahen, D.; Kahn, A. High-work-function molybdenum oxide hole extraction contacts in hybrid organic-inorganic perovskite solar cells. ACS Appl. Mat. Interfaces 2016, 8, 31491–31499. [Google Scholar] [CrossRef] [PubMed]
- Christians, J.A.; Fung, R.C.M.; Kamat, P.V. An inorganic hole conductor for organo-lead halide perovskite solar cells. Improved hole conductivity with copper iodide. J. Am. Chem. Soc. 2014, 136, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.C.; Lin, K.W.; Guo, T.F.; Lee, J. Perovskite-based solar cells with nickel-oxidized nickel oxide hole transfer layer. IEEE Trans. Electron Dev. 2015, 65, 1590–1595. [Google Scholar]
- Zhao, K.; Munir, R.; Yan, B.; Yang, Y.; Kim, T.; Amassian, A. Solution-processed inorganic copper (I) thiocyanate (CuSCN) hole transporting layers for efficient p-i-n perovskite solar cells. J. Mater. Chem. A 2015, 3, 20554–20559. [Google Scholar] [CrossRef]
- Zuo, C.T.; Ding, LM. Solution-processed Cu2O and CuO as hole transport materials for efficient perovskite solar cells. Small 2015, 11, 5528–5532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lyu, M.Q.; Yu, H.; Yun, J.H.; Wang, Q.; Wang, L.Z. Stable and Low-cost mesoscopic CH3NH3PbI2Br perovskite solar cells by using a thin poly(3-hexylthiophene) layer as a hole transporter. Chem. Eur. J. 2015, 21, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.X.; Sun, K.; Ouyang, J.Y.; Lu, X.M. Layered V2O5/PEDOT nanowires and ultrathin nanobelts fabricated with a silk reelinglike process. Chem. Mater. 2015, 27, 5813–5819. [Google Scholar] [CrossRef]
- Chao, D.; Xia, X.; Liu, J.; Fan, Z.; Ng, C.F.; Lin, J.; Zhang, H.; Shen, Z.X.; Fan, H.J. A V2O5/conductive-polymer core/shell nanobelt array on three-dimensional graphite foam: A high-rate, ultrastable, and freestanding cathode for lithium-ion batteries. Adv. Mater. 2014, 26, 5794–5800. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, H.P.; Yusoff, A.R.; Jang, J. Organic photovoltaic with PEDOT:PSS and V2O5 mixture as hole transport layer. Sol. Energy Mat. Sol. Cells 2014, 120, 238–243. [Google Scholar] [CrossRef]
- Guo, C.X.; Yilmaz, G.; Chen, S.; Chen, S.; Lu, X. Hierarchical nanocomposite composed of layered V2O5/PEDOT/MnO2 nanosheets for high-performance asymmetric supercapacitors. Nano Energy 2015, 12, 76–87. [Google Scholar] [CrossRef]
- Song, H.M.; Yoo, D.Y.; Hong, S.K.; Kim, J.S.; Cho, W.I.; Mho, S.I. Electrochemical impedance analysis of V2O5 and PEDOT composite film cathodes. Electroanalsis 2011, 23, 2094–2102. [Google Scholar] [CrossRef]
- Seo, J.; Park, S.; Kim, Y.C.; Jeon, N.J.; Noh, J.H.; Yoon, S.C.; Seok, S.I. Benefits of very thin PCBM and LiF layers for solution processed p-i-n perovskite solar cells. Energy Environ. Sci. 2014, 7, 2642–2646. [Google Scholar] [CrossRef]
- Yang, H.; Song, Q.; Lu, Z.; Guo, C.; Gong, C.; Hu, W.; Li, C.M. Electrochemically polymerized nanostructured poly(3.4-ethylenedioxythiophene)-poly(styrenesulfonate) buffer layer for a high performance polymer solar cell. Energy Environ. Sci. 2010, 3, 1580–1586. [Google Scholar] [CrossRef]
- Chen, W.Y.; Bao, X.C.; Zhu, Q.Q.; Zhu, D.Q.; Qiu, M.; Sun, M.L.; Yang, R.Q. Simple planar perovskite solar cells with a dopant-free benzodithiophene conjugated polymer as hole transporting material. J. Mater. Chem. C 2015, 3, 10070–10073. [Google Scholar] [CrossRef]
- Wang, J.T.W.; Ball, J.M.; Barea, E.M.; Abate, A.; Alexander-Webber, J.A.; Huang, J.; Saliba, M.; Mora-Sero, I.; Bisquert, J.; Snaith, H.J.; et al. Low-temperature processed electron collection layers of graphene/TiO2 nanocomposites in thin film perovskite solar cells. Nano Lett. 2014, 14, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Son, D.Y.; Im, J.Y.; Kim, H.S.; Park, N.G. 11% Efficient perovskite solar cell based on ZnO nanorods: An effective charge collection system. J. Phys. Chem. C 2014, 118, 16567–16573. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Elumalai, N.K.; Upama, M.B.; Wang, D.A.; Chan, K.H.; Wright, M.; Xu, C.; Hague, F.; Uddin, A. Low temperature processed ZnO thin film as electron transport layer for efficient perovskite solar cells. Sol. Energy Mat. Sol. Cells 2017, 159, 251–264. [Google Scholar] [CrossRef]
- Niu, G.; Li, W.; Meng, F.; Wang, L.; Dong, H.; Qiu, Y.J. Study on the stability of CH3NH3PbI3 films and the effect of post-modification by aluminum oxide in all-solid-state hybrid solar cells. Mater. Chem. 2014, 2, 705–710. [Google Scholar] [CrossRef]
- Mali, S.S.; Shim, C.S.; Park, H.K.; Heo, J.; Patil, P.S.; Hong, C.K. Ultrathin atomic layer deposited TiO2 for surface passivation of hydrothermally grown 1D TiO2 nanorod arrays for efficient solid-state perovskite solar cells. Chem. Mater. 2015, 27, 1541–1551. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.H.; Xing, Y.J.; Zhang, Z.L.; Zhang, X.F.; Li, Z.; Shi, Y.T.; Ma, T.L.; Ma, R.Z.; Wang, K.L.; et al. Perovskite solar cell using a two-dimensional titania nanosheet thin film as the compact layer. ACS Appl. Mater. Interfaces 2015, 7, 15117–15122. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Ke, W.J.; Wang, J.; Liu, Q.; Wan, J.W.; Yang, G.; Fang, G.J. Perovskite solar cell based on network nanoporous layer consisted of TiO2 nanowires and its interface optimization. J. Power Source 2015, 290, 144–152. [Google Scholar] [CrossRef]
- Mahmood, K.; Swain, B.S.; Amassian, A. Highly Efficient Hybrid Photovoltaics Based on hyperbranched three-dimensional TiO2 electron transporting materials. Adv. Mater. 2015, 27, 2859–2865. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Paulose, M.; Dar, M.I.; Moehl, T.; Arora, N.; Gao, P.; Varghese, O.K.; Grätzel, M.; Nazeeruddin, M.K. Stable and efficient perovskite solar cells based on titania nanotube arrays. Small 2015, 11, 5533–5539. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Zhong, D.; Yang, Z.; Huang, B.K.; Miao, S.; Zhang, W.H.; Qiu, J.S.; Li, C. An acid-free medium growth of rutile TiO2 nanorods arrays and their application in perovskite solar cells. J. Mater. Chem. C 2015, 3, 729–733. [Google Scholar] [CrossRef]
- Philippe, B.; Park, B.W.; Lindblad, R.; Oscarsson, J.; Ahmadi, S.; Johansson, E.M.J.; Rensmo, H. Chemical and electronic structure characterization of lead halide perovskites and stability behavior under different exposures-a photoelectron spectroscopy investigation. Chem. Mater. 2015, 27, 1720–1731. [Google Scholar] [CrossRef]
- Liu, J.; Lin, J.H.; Xue, Q.F.; Ye, Q.Y.; He, X.L.; Ouyang, L.Q.; Zhuang, D.M.; Liao, C.; Yip, H.L.; Mei, J.M.; et al. Growth and evolution of solution-processed CH3NH3PbI3-xClx layer for highly efficient planar-heterojunction perovskite solar cells. J. Power Source 2016, 301, 242–250. [Google Scholar] [CrossRef]
- Gao, P.; Grätzel, M.; Nazeeruddin, M.K. Organohalide lead perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 2448–2463. [Google Scholar] [CrossRef]
- Kim, H.S.; Im, S.H.; Park, N.G. Organolead halide perovskite: New horizons in solar cell research. J. Phys. Chem. C 2014, 118, 5615–5625. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.N.; Zhou, S.S.; Jin, S.Y.; Li, H.Q.; Zhai, T.Y. Crystal organometal halide perovskites with promising optoelectronic applications. J. Mater. Chem. C 2016, 4, 11–27. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Fakharuddin, A.; Giacomo, F.D.; Ahmed, I.; Wali, Q.; Brown, T.M.; Jose, R. Role of morphology and crystallinity of nanorod and planar electron transport layers on the performance and long term durability of perovskite solar cells. J. Power Source 2015, 283, 61–67. [Google Scholar] [CrossRef]
- Numata, Y.; Sanehira, Y.; Miyasaka, T. Photocurrent Enhancement of Formamidinium Lead trihalide mesoscopic perovskite solar cells with large size TiO2 nanoparticles. Chem. Lett. 2015, 44, 1619–1621. [Google Scholar] [CrossRef]
- You, J.; Meng, L.; Song, T.B.; Guo, T.F.; Yang, Y.M.; Chang, W.H.; Hong, Z.; Chen, H.; Zhou, H.; Chen, Q.; et al. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat. Nanotechnol. 2016, 11, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Hörantner, M.T.; Choi, K.; Snaith, H.J.; Park, T. Inducing swift nucleation morphology control for efficient planar perovskite solar cells by hot-air quenching. J. Mater. Chem. A 2017, 5, 3812–3818. [Google Scholar] [CrossRef]
- Wang, S.; Sina, M.; Parikh, P.; Uekert, T.; Shahbazian, B.; Devaraj, A.; Meng, Y.S. Role of 4-tert-Butylpyridine as a hole transport layer morphological controller in perovskite solar cells. Nano Lett. 2016, 16, 5594–5600. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Wu, J.; Wu, S.; Xu, X.; Ellis, J.E.; Xu, G.; Star, A.; Gao, D. Perovskite solar cells based on bottom-fused TiO2 nanocones. J. Mater. Chem. A 2016, 4, 1520–1530. [Google Scholar] [CrossRef]
- Du, T.; Wang, N.; Chen, H.J.; Lin, H.; He, H.C. Comparative study of vapor- and solution-crystallized perovskite for planar heterojunction solar cells. ACS Appl. Mater. Inter. 2015, 7, 3382–3388. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.J.; Zhou, Y.Y.; Zeng, Y.N.; Jiang, C.S.; Padture, N.P.; Zhu, K. Square-centimeter solution-processed planar CH3NH3PbI3 perovskite solar cells with efficiency exceeding 15%. Adv. Mater. 2015, 27, 6363–6370. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bi, D.; Yi, C.; Décoppet, J.D.; Luo, J.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. A vacuum flash-assisted solution process for high-efficiency large-area perovskite solar cells. Science 2016, 353, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Cao, D.H.; Stoumpos, C.C.; Song, T.B.; Sato, Y.; Aramaki, S.; Kanatzidis, M.G. Overcoming short-circuit in lead-free CH3NH3SnI3 perovskite solar cells via kinetically controlled gas–solid reaction film fabrication process. J. Phys. Chem. Lett. 2016, 7, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, C.; Qiao, W.; Ma, S.; Wang, F.; Zhang, B.; Hu, L.; Dai, S.; Tan, Z.A. The growth of a CH3 NH3PbI3 thin film using simplified close space sublimation for efficient and large dimensional perovskite solar cells. Energy Environ. Sci. 2016, 9, 1486–1494. [Google Scholar] [CrossRef]
- Aranda, C.; Cristobal, C.; Shooshtari, L.; Li, C.; Huettner, S.; Guerrero, A. Formation criteria of high efficiency perovskite solar cells under ambient conditions. Sustain. Energy Fuels 2017. [Google Scholar] [CrossRef]
- Gao, H.; Bao, C.X.; Li, F.M.; Yu, T.; Yang, J.; Zhu, W.D.; Zhou, X.X.; Fu, G.; Zou, Z.G. Nucleation and crystal growth of organic-inorganic lead halide perovskites under different relative humidity. ACS Appl. Mater. Interfaces 2015, 7, 9110–9117. [Google Scholar] [CrossRef] [PubMed]
- Eperson, G.E.; Burlakov, V.M.; Docampo, P.; Goriely, A.; Snaith, H.J. Morphological control for high performance, solution-processed planar heterojunction perovskite solar cells. Adv. Funct. Mater. 2014, 24, 151–157. [Google Scholar] [CrossRef]
- Jeng, J.Y.; Chiang, Y.F.; Lee, M.H.; Peng, S.R.; Guo, T.F.; Chen, P.; Wen, T.C. CH3NH3PbI3 perovskite/fullerene planar-heterojunction hybrid solar cells. Adv. Mater. 2013, 25, 3727–3732. [Google Scholar] [CrossRef] [PubMed]
- Docampo, P.; Ball, J.M.; Darwich, M.; Eperon, G.E.; Snaith, H.J. Efficient organometal trihalide perovskite planar-heterojunction solar cells on flexible polymer substrates. Nat. Commun. 2013, 4, 2761. [Google Scholar] [CrossRef] [PubMed]
- Eperon, G.E.; Habisreutinger, S.N.; Leijtens, T.; Bruijnaers, B.J.; Franeker, J.J.; deQuilettes, D.W.; Pathak, S.; Sutton, R.J.; Grancini, G.; Ginger, D.S.; et al. The importance of moisture in hybrid lead halide perovskite thin film fabrication. ACS Nano 2015, 9, 9380–9393. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Johansson, E.M.J.; Philippe, B.; Gustafsson, T.; Sveinbjörnsson, K.; Hagfeldt, A.; Boschloo, G. Enhanced crystallinity in organic–inorganic lead halide perovskites on mesoporous TiO2 via disorder–order phase transition. Chem. Mater. 2014, 26, 4466–4471. [Google Scholar] [CrossRef]
- Tosun, B.S.; Hillhouse, H.W. Enhanced carrier lifetimes of pure iodide hybrid perovskite via vapor-equilibrated re-growth (VERG). J. Phys. Chem. Lett. 2015, 6, 2503–2508. [Google Scholar] [CrossRef] [PubMed]
- NREL Efficiency Chart. Available online: https://www.nrel.gov/pv/assets/images/efficiency_chart.jpg (accessed on 22 March 2017).
- Sum, T.C.; Mathews, N. Advancements in perovskite solar cells: Photophysics behind the photovoltaics. Energy Environ. Sci. 2014, 7, 2518–2534. [Google Scholar] [CrossRef]
- Wang, K.; Liu, C.; Du, P.C.; Zhang, H.L.; Gong, X. Efficient perovskite hybrid solar cells through a homogeneous high-quality organolead iodide layer. Small 2015, 11, 3369–3376. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Luo, J.S.; Franckevičius, M.; Pellet, N.; Gao, P.; Moehl, T.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Grätzel, M.; Park, N.G. Nanowire perovskite solar cell. Nano Lett. 2015, 15, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Di, D.W.; Musselman, K.P.; Li, G.R.; Sadhanala, A.; Ievskaya, Y.; Song, Q.L.; Tan, Z.K.; Lai, M.L.; MacManus-Driscoll, J.L.; Greenham, N.C.; et al. Size-dependent photon emission from organometal halide, perovskite nanocrystals embedded in an organic matrix. J. Phys. Chem. Lett. 2015, 6, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.P.; Meng, F.; Rowley, M.B.; Thompson, B.J.; Shearer, M.J.; Ma, D.W.; Hamers, R.J.; Wright, J.C.; Jin, S. Solution growth of single crystal methylammonium lead halide perovskite nanostructures for optoelectronic and photovoltaic applications. J. Am. Chem. Soc. 2015, 137, 5810–5818. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, K. Solution chemistry engineering toward high-efficiency perovskite solar cells. J. Phys. Chem. Lett. 2014, 5, 4175–4186. [Google Scholar] [CrossRef] [PubMed]
- Razza, S.; Giacomo, F.D.; Matteocci, F.; Cinà, L.; Palma, A.L.; Casaluci, S.; Cameron, P.; D’Epifanio, A.; Licoccia, S.; Reale, A.; et al. Perovskite solar cells and large area modules (100 cm2) based on an air flow-assisted PbI2 blade coating deposition process. J. Power Source 2014, 277, 286–291. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, K. Three-step sequential solution deposition of PbI2-free CH3NH3PbI3 perovskite. J. Mater. Chem. A 2015, 3, 9086–9091. [Google Scholar] [CrossRef]
- Sun, C.Y.; Guo, Y.P.; Duan, H.N.; Chen, Y.J.; Guo, Y.L.; Li, H.; Liu, H.Z. Solvent-assisted growth of organic–inorganic hybrid perovskites with enhanced photovoltaic performances. Sol. Energy Mater. Sol. Cells 2015, 143, 360–368. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Kovalenko, A.; Forés, S.M.; Aranda, C.; Guerrero, A. Coordination chemistry dictates the structural defects in lead halide perovskites. ChemPhysChem 2016, 17, 2795–2798. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.; Nie, W.; Lin, Y.H.; Blancon, J.C.; Tretiak, S.; Even, J.; Gupta, G.; Ajayan, P.M.; Mohite, A.D. Effect of precursor solution aging on the crystallinity and photovoltaic performance of perovskite solar cells. Adv. Energy Mater. 2017, 7, 1062159. [Google Scholar] [CrossRef]
- Lu, J.J.; Wan, M.X.; Wen, P.; Luo, F.; Liu, X.; Wen, J.; Hu, C.Y.; Guo, J. Investigation on the high pressure annealing induced re crystallization mechanism of CH3NH3PbI3 film. J. Alloys Compd. 2017, 694, 1365–1370. [Google Scholar] [CrossRef]
- Salado, M.; Idigoras, J.; Calio, L.; Kazim, S.; Nazeeruddin, M.K.; Anta, J.A.; Ahmad, S. Interface play between perovskite and hole selective layer on the performance and stability of perovskite solar cells. ACS Appl. Mat. Interface 2016, 8, 34414–34421. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Lü, X.J.; Yang, W.G.; Wen, T.; Yang, L.X.; Ren, X.T.; Wang, L.; Lin, Z.S.; Zhao, Y.S. Pressure-induced phase transformation, reversible amorphization, and anomalous visible light response in organolead bromide perovskite. J. Am. Chem. Soc. 2015, 137, 11144–11149. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.D.; Bera, A.; Haque, M.A.; Rakhi, R.B.; Gobbo, S.D.; Alshareef, H.N.; Wua, T. Atmospheric effects on the photovoltaic performance of hybrid perovskite solar cells. Sol. Energy Sol. Mat. Cells 2015, 137, 6–14. [Google Scholar] [CrossRef]
- Abate, A.; Leijtens, T.; Pathak, S.; Teuscher, J.; Avolio, R.; Errico, M.E.; Kirkpatrik, J.; Ball, J.M.; Docampo, P.; McPherson, I.; et al. Lithium Salts as “redoxactive” p-type dopants for organic semiconductors and their impact in solid-state dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2013, 15, 2572–2579. [Google Scholar] [CrossRef] [PubMed]
- Park, B.W.; Jain, S.M.; Zhang, X.L.; Hagfeldt, A.; Boschloo, G.; Edvinsson, T. Resonance raman and excitation energy dependent charge transfer mechanism in halide-substituted hybrid perovskite solar cells. ACS Nano 2015, 9, 2088–2101. [Google Scholar] [CrossRef] [PubMed]
- Adli, H.K.; Harada, T.; Septina, W.; Hozan, S.; Ito, S.; Ikeda, S. Effects of porosity and amount of surface hydroxyl groups of a porous TiO2 layer on the performance of a CH3NH3PbI3 perovskite photovoltaic cell. J. Phys. Chem. C 2015, 119, 22304–22309. [Google Scholar] [CrossRef]
- Ledinský, M.; Löper, P.; Niesen, B.; Holovský, J.; Moon, S.J.; Yum, J.H.; Wolf, S.D.; Fejfar, A.; Ballif, C. Raman spectroscopy of organic-inorganic halide perovskites. J. Phys. Chem. Lett. 2015, 6, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Whittaker-Brooks, L.L.; MacLeod, B.A.; Olson, D.C.; Loo, Y.L.; Kahn, A. Electronic level alignment in inverted organometal perovskite solar cells. Adv. Mat. Interfaces 2015, 2, 1400532. [Google Scholar] [CrossRef]
- Ng, T.W.; Chandran, H.T.; Chan, C.Y.; Lo, M.F.; Lee, C.S. Ionic Charge Transfer complex induced visible light harvesting and photocharge generation in perovskite. ACS Appl. Mater. Interfaces 2015, 7, 20280–20284. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Lee, S.; Kim, K.H.; Kim, S.Y.; Lee, J.H.; Kim, J.J. Exciplex-forming co-host for organic light-emitting diodes with ultimate efficiency. Adv. Funct. Mater. 2013, 23, 4914–4920. [Google Scholar] [CrossRef]
- Veldman, D.; Meskers, S.C.J.; Janssen, R.A.J. The energy of charge-transfer states in electron donor-acceptor blends: Insight into the energy losses in organic solar cells. Adv. Funct. Mater. 2009, 19, 1939–1948. [Google Scholar] [CrossRef]
- Qiu, W.M.; Buffière, M.; Brammertz, G.; Paetzold, U.W.; Froyen, L.; Heremans, P.; Cheyns, D. High efficiency perovskite solar cells using a PCBM/ZnO double electron transport layer and a short air-aging step. Org. Electron. 2015, 26, 30–35. [Google Scholar] [CrossRef]
- Kera, S.; Yabuuchi, Y.; Yamane, H.; Setoyama, H.; Okudaira, K.K.; Kahn, A.; Ueno, N. Impact of an interface dipole layer on molecular level alignment at an organic-conductor interface studied by ultraviolet photoemission spectroscopy. Phys. Rev. B 2004, 70, 085304. [Google Scholar] [CrossRef]
- Fukagawa, H.; Yamane, H.; Kera, S.; Okudaira, K.K.; Ueno, N. Experimental estimation of the electric dipole moment and polarizability of titanyl phthalocyanine using ultraviolet photoelectron spectroscopy. Phys. Rev. B 2006, 73, 041302. [Google Scholar] [CrossRef]
- Wang, Q.K.; Wang, R.B.; Shen, P.F.; Li, C.; Li, Y.Q.; Liu, L.J.; Duhm, S.; Tang, J.X. Energy level offsets at lead halide perovskite/organic hybrid interfaces and their impacts on charge separation. Adv. Mater. Interfaces 2015, 2, 1400528. [Google Scholar] [CrossRef]
- Calloni, A.; Abate, A.; Bussetti, G.; Berti, G.; Yivlialin, R.; Ciccacci, F.; Duò, L. Stability of organic cations in solution-processed CH3NH3PbI3 perovskites: Formation of modified surface layers. J. Phys. Chem. C 2015, 119, 21329–21335. [Google Scholar] [CrossRef]
- Tsai, H.; Nie, W.Y.; Cheruku, P.; Mack, N.H.; Xu, P.; Gupta, G.; Mohite, A.D.; Wang, H.L. Optimizing composition and morphology for large-grain perovskite solar cells via chemical control. Chem. Mater. 2015, 27, 5570–5576. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chu, C.Y.; Huang, Y.C.; Huang, C.W.; Chang, S.Y.; Chen, C.A.; Chao, C.Y.; Su, W.F. Tuning perovskite morphology by polymer additive for high efficiency solar cell. ACS Appl. Mater. Interface 2015, 7, 4955–4961. [Google Scholar] [CrossRef] [PubMed]
- Ahn, N.; Son, D.Y.; Jang, I.H.; Kang, S.M.; Choi, M.; Park, N.G. Highly reproducible perovskite solar cells with average efficiency of 18.3% and best efficiency of 19.7% fabricated via lewis base adduct of lead (II) iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Z.; Fan, J.D.; Li, J.W.; Mai, Y.H.; Wang, L.D. Controllable grain morphology of perovskite absorber film by molecular self-assembly toward efficient solar cell exceeding 17%. J. Am. Chem. Soc. 2015, 137, 10399–10405. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xue, Q.F.; Hu, Z.C.; Chen, Z.M.; Huang, F.; Yip, H.L.; Cao, Y. Phosphonium halides as both processing additives and interfacial modifiers for high performance planar-heterojunction perovskite solar cells. Small 2015, 11, 3344–3350. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; Zhou, Y.Y.; Pang, S.P.; Xiao, Z.W.; Zhang, J.L.; Chai, W.Q.; Xu, H.X.; Liu, Z.H.; Padture, N.P.; Cui, G.L. Additive-modulated evolution of HC(NH2)2PbI3 black polymorph for mesoscopic perovskite solar cells. Chem. Mater. 2015, 27, 7149–7155. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Williams, S.T.; Jen, A.K.J. Low-temperature processed high-performance flexible perovskite solar cells via rationally optimized solvent washing treatments. RSC Adv. 2014, 4, 62971–62977. [Google Scholar] [CrossRef]
- Xiao, M.; Huang, F.; Huang, W.; Dkhissi, Y.; Zhu, Y.; Etheridge, J.; Gray-Weale, A.; Bach, U.; Cheng, Y.B.; Spiccia, L. A fast deposition-crystallization procedure for highly efficient lead iodide perovskite thin-film solar cells. Angew. Chem. Int. Ed. 2014, 53, 9898–9903. [Google Scholar] [CrossRef] [PubMed]
- Kara, K.; Kara, D.A.; Kırbıyık, C.; Ersoz, M.; Usluer, O.; Briseno, A.L.; Kus, M. Solvent washing with toluene enhances efficiency and increases reproducibility in perovskite solar cells. RSC Adv. 2016, 6, 26606–26611. [Google Scholar] [CrossRef]
- El-Henawey, M.I.; Gebhardt, R.S.; El-Tonsy, M.M.; Chaudhary, S. Organic solvent vapor treatment of lead iodide layers in the two-step sequential deposition of CH3NH3PbI3-based perovskite solar cells. J. Mater. Chem. A 2016, 4, 1947–1952. [Google Scholar] [CrossRef]
- Yu, J.C.; Kim, D.B.; Baek, G.; Lee, B.R.; Jung, E.D.; Lee, S.; Chu, J.H.; Lee, D.K.; Choi, K.J.; Cho, S.; et al. High-performance planar perovskite optoelectronic devices: A morphological and interfacial control by polar solvent treatment. Adv. Mater. 2015, 27, 3492–3500. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.F.; Chang, S.H.; Wang, K.H.; Cheng, H.M.; Chiu, K.Y.; Lee, K.M.; Chen, S.H.; Wu, C.G. Unraveling the high performance of tri-iodide perovskite absorber based photovoltaics with a non-polar solvent washing treatment. Sol. Energy Mater. Sol. Cells 2014, 141, 309–314. [Google Scholar] [CrossRef]
- Chern, Y.C.; Wu, H.R.; Chen, Y.C.; Zan, H.W.; Meng, H.F.; Horng, S.F. Reliable solution processed planar perovskite hybrid solar cells with large-area uniformity by chloroform soaking and spin rinsing induced surface precipitation. AIP Adv. 2015, 5, 087125. [Google Scholar] [CrossRef]
- Liu, Z.H.; Lee, E.C. Solvent engineering of the electron transport layer using 1,8-diiodooctane for improving the performance of perovskite solar cells. Org. Electron. 2015, 24, 101–105. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, B.C.; Wu, R.S.; Cao, C.H.; Huang, Y.L.; Liu, C.B.; Hu, Z.K.; Huang, H.; Gao, Y.L.; Yang, J.L. Efficient and non-hysteresis CH3NH3PbI3/PCBM planar heterojunction solar cells. Org. Electron. 2015, 24, 106–112. [Google Scholar] [CrossRef]
- Cao, J.; Yin, J.; Yuan, S.F.; Zhao, Y.; Li, J.; Zheng, N.F. Thiols as interfacial modifiers to enhance the performance and stability of perovskite solar cells. Nanoscale 2015, 7, 9443–9447. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Li, X.D.; Tang, G.; Zhao, L.X.; Zhang, W.J.; Jiu, T.G.; Fang, J.F. Improving efficiency of planar hybrid CH3NH3PbI3−xClx perovskite solar cells by isopropanol solvent treatment. Org. Electron. 2015, 24, 205–211. [Google Scholar] [CrossRef]
- Solis-Ibarra, D.; Smith, I.C.; Karunadasa, H.I. Post-synthetic halide conversion and selective halogen capture in hybrid perovskites. Chem. Sci. 2015, 6, 4054–4059. [Google Scholar] [CrossRef]
- Liu, J.; Gao, C.; He, X.L.; Ye, Q.Y.; Ouyang, L.Q.; Zhuang, D.M.; Liao, C.; Mei, J.; Lau, W. Improved crystallization of perovskite films by optimized solvent annealing for high efficiency solar cell. ACS Appl. Mater. Interface 2015, 7, 24008–24015. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Tanaka, S.; Nishino, H. Substrate-preheating effects on PbI2 spin coating for perovskite solar cells via sequential deposition. Chem. Lett. 2015, 44, 849–851. [Google Scholar] [CrossRef]
- Song, D.D.; Cui, P.; Wang, T.Y.; Wei, D.; Li, M.C.; Cao, F.H.; Yue, X.P.; Fu, P.F.; Li, Y.Y.; He, Y.; et al. Managing carrier lifetime and doping property of lead halide perovskite by post annealing processes for highly efficient perovskite solar cells. J. Phys. Chem. C 2015, 119, 22812–22819. [Google Scholar] [CrossRef]
- Li, Y.B.; Cooper, J.K.; Buonsanti, R.; Giannini, C.; Liu, Y.; Toma, F.M.; Sharp, I.D. Fabrication of planar heterojunction perovskite solar cells by controlled low-pressure vapor annealing. J. Phys. Chem. Lett. 2015, 6, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hun, Z.Y.; Xu, J.; Zhang, K.; Zhang, J.; Zhu, Y.J. Multi-step slow annealing perovskite films for high performance planar perovskite solar cells. Sol. Energy Mater. Sol. Cells 2015, 141, 377–382. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Chen, W.; Yue, Y.F.; Liu, J.; Bi, E.B.; Yang, X.D.; Islam, A.; Han, L.Y. Consecutive morphology controlling operations for highly reproducible mesostructured perovskite solar cells. ACS Appl. Mater. Interfaces 2015, 7, 20707–20713. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.Y.; Lim, S.L.; Goh, W.P.; Wei, F.X.; Zhang, J. Improvement of CH3NH3PbI3 formation for efficient and better reproducible mesoscopic perovskite solar cells. ACS Appl. Mater. Interfaces 2015, 7, 24726–24732. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Jiang, K.J.; Cui, X.P.; Zhang, Q.Q.; Gao, M.; Su, M.J.; Yang, L.M.; Song, Y.L. Direct conversion of CH3NH3PbI3 from electrodeposited PbO for highly efficient planar perovskite solar cells. Sci. Rep. 2015, 5, 15889. [Google Scholar] [CrossRef] [PubMed]
- Su, T.S.; Hsieh, T.Y.; Hong, C.Y.; Wei, T.C. Electrodeposited ultrathin TiO2 blocking layers for efficient perovskite solar cells. Sci. Rep. 2015, 5, 16098. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, M.; Boopathi, K.M.; Huang, T.Y.; Huang, Y.C.; Tsao, C.S.; Chu, C.W. Using an airbrush pen for layer-by-layer growth of continuous perovskite thin films for hybrid solar cells. ACS Appl. Mater. Interfaces 2015, 7, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, B.R.; Hoogland, S.; Adachi, M.M.; Kanjanaboos, P.; Wong, C.T.; McDowell, J.J.; Xu, J.X.; Voznyy, O.; Ning, Z.J.; Houtepen, A.J.; et al. Perovskite thin films via atomic layer deposition. Adv. Mater. 2015, 27, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.Y.; Fan, G.L.; Jeng, J.Y.; Chen, K.C.; Chen, P.; Wen, T.C.; Guo, T.F.; Wong, K.T. Functional p-type, polymerized organic electrode interlayer in CH3NH3PbI3 perovskite/fullerene planar heterojunction hybrid solar cells. ACS Appl. Mater. Interfaces 2015, 7, 24973–24981. [Google Scholar] [CrossRef] [PubMed]
- Casaluci, S.; Cin, L.; Pockett, A.; Kubiak, P.S.; Niemann, R.G.; Reale, A.; Carlo, A.D.; Cameron, P.J. A simple approach for the fabrication of perovskite solar cells in air. J. Power Sour. 2015, 297, 504–510. [Google Scholar] [CrossRef]
- Yue, Y.F.; Umeyama, T.; Kohara, Y.; Kashio, H.; Itoh, M.; Ito, S.; Sivaniah, E.; Imahori, H. Polymer-assisted construction of mesoporous TiO2 layers for improving perovskite solar cell performance. J. Phys. Chem. C 2015, 119, 22847–22854. [Google Scholar] [CrossRef]
- Bywater, S.; Black, P.E. Thermal depolymerization of polymethyl methacrylate and poly-α-methylstyrene in solution in various solvents. J. Phys. Chem. 1965, 69, 2967–2970. [Google Scholar] [CrossRef]
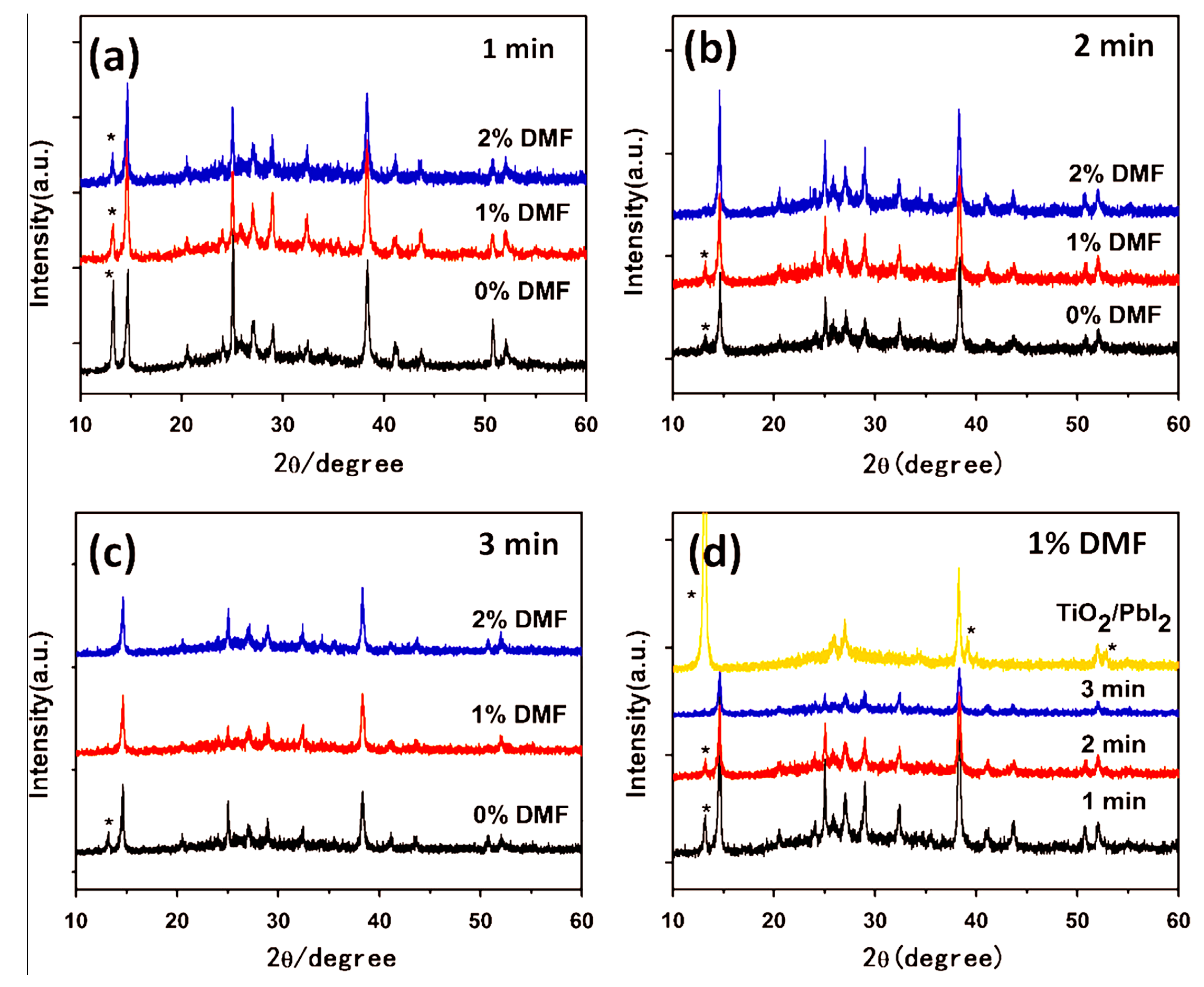

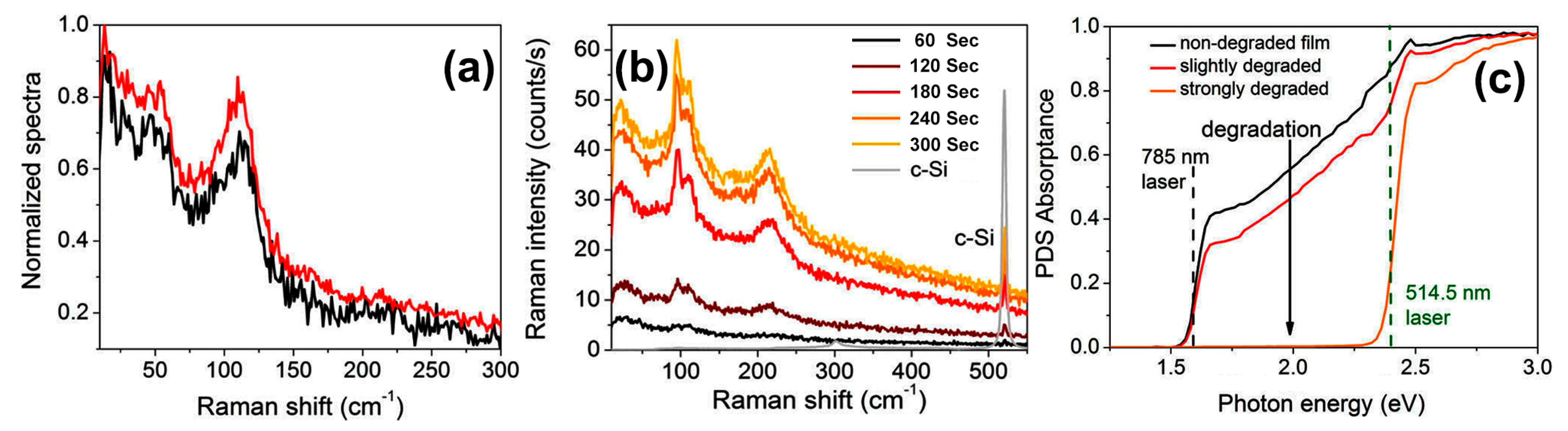
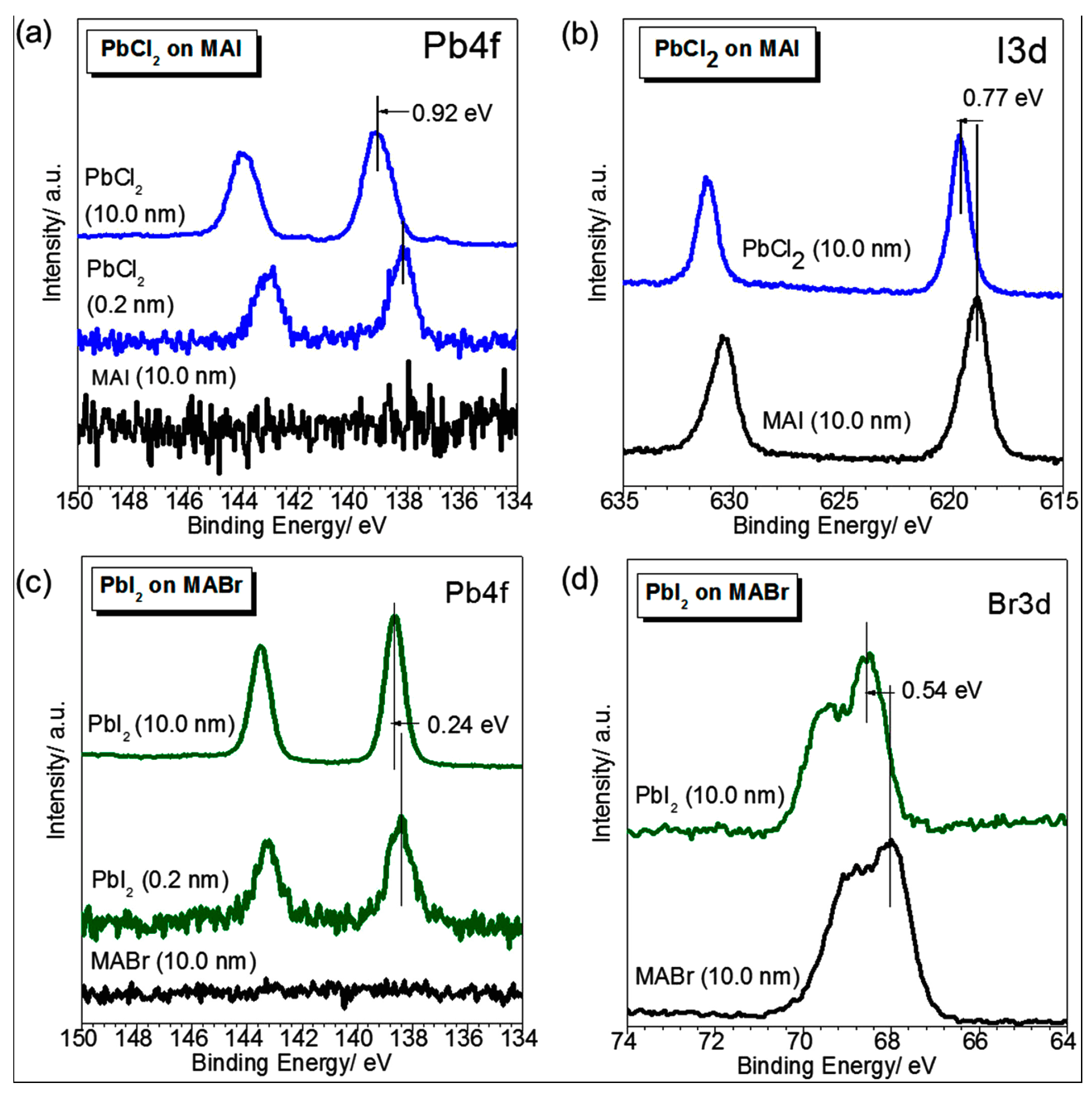
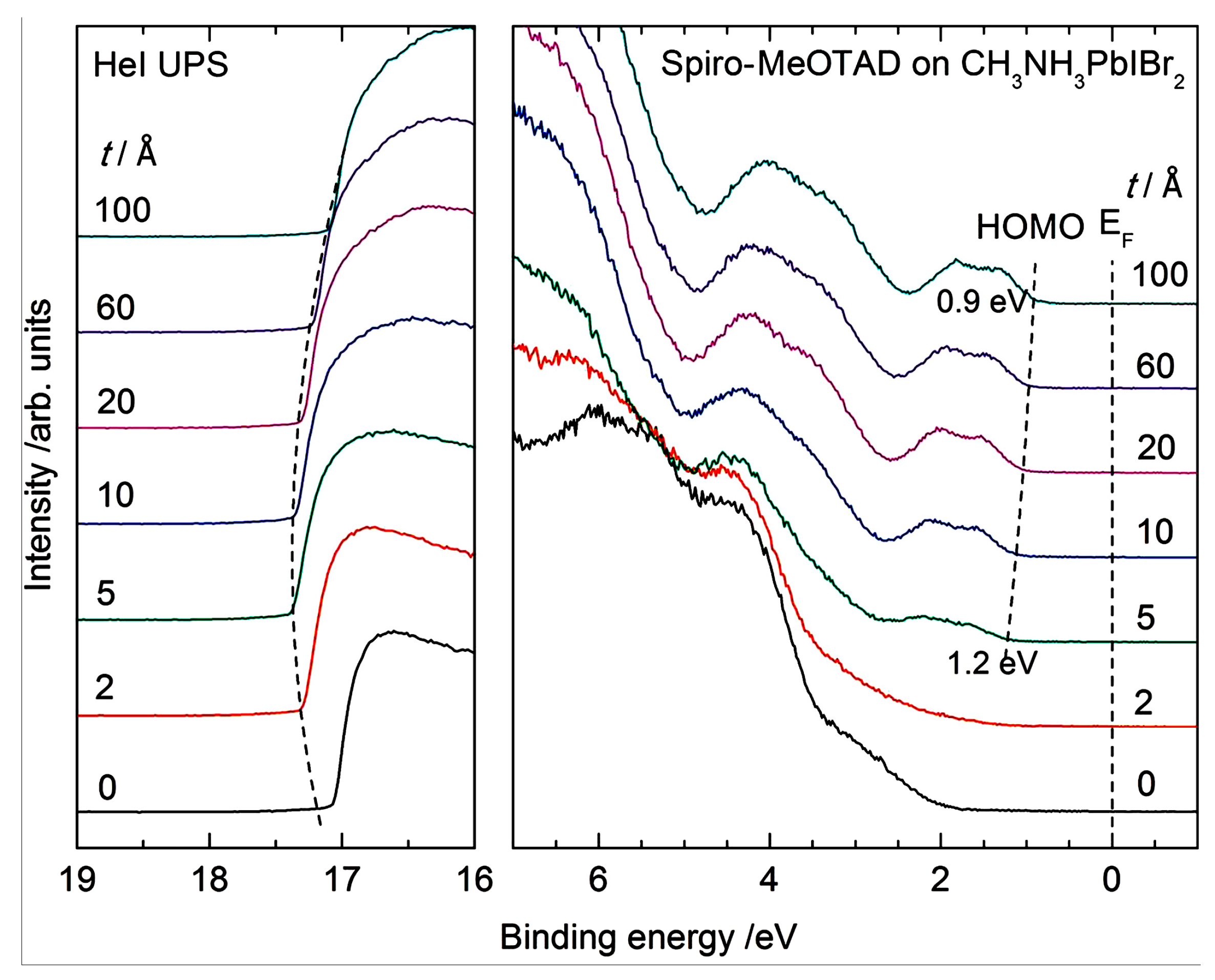




| Precursor Solutions (MA:Pb:I:Cl) | Performance Type | JSC (mA/cm2) | VOC (mV) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| 1 M PbI2 + 1 M MAI (1:1:3:0) | Average | 3.32 ± 1.87 | 649 ± 311 | 46.3 ± 12.6 | 1.40 ± 0.97 |
| Best | 4.74 | 895 | 56.3 | 2.36 | |
| 0.3 M PbCl2 + 0.7 M PbI2 + 1.6 M MAI (1.6:1:3:0.6) | Average | 15.18 ± 2.55 | 843 ± 40 | 40.4 ± 5.4 | 5.19 ± 1.20 |
| Best | 17.15 | 898 | 45.8 | 7.06 | |
| 0.5 M PbCl2 + 0.5 M PbI2 + 2.0 M MAI (2:1:3:1) | Average | 19.08 ± 1.65 | 979 ± 40 | 55.5 ± 4.1 | 10.41 ± 1.52 |
| Best | 19.9 | 996 | 62.1 | 12.32 | |
| 0.8 M PbCl2 + 0.2 M PbI2 + 2.6 M MAI (2.6:1:3:1.6) | Average | 19.15 ± 0.88 | 965 ± 35 | 57.6 ± 4.0 | 10.68 ± 1.26 |
| Best | 20.1 | 961 | 63.0 | 12.16 | |
| 1 M PbCl2 + 3 M MAI (3:1:3:2) | Average | 18.28 ± 1.16 | 857 ± 87 | 50.4 ± 5.4 | 7.95 ± 1.66 |
| Best | 18.74 | 996 | 57.7 | 10.77 |
| Solvent | Scan Direction | VOC (V) | JSC (mA/cm2) | FF (%) | PCEmax (%) | PCEave (%) 1 |
|---|---|---|---|---|---|---|
| N2 | Forward | 0.93 | 16.9 | 0.53 | 8.34 | 7.39 ± 0.62 |
| Reverse | 0.91 | 17.1 | 0.55 | 8.55 | 7.59 ± 0.60 | |
| H2O | Forward | 0.95 | 18.7 | 0.51 | 8.99 | 7.50 ± 1.40 |
| Reverse | 0.94 | 18.6 | 0.51 | 8.85 | 7.62 ± 1.14 | |
| γ-butyrolactone (GBL) | Forward | 0.92 | 20.9 | 0.64 | 12.29 | 11.50 ± 0.74 |
| Reverse | 0.92 | 20.8 | 0.65 | 12.47 | 11.32 ± 0.86 | |
| DMF | Forward | 10.91 | 20.2 | 0.62 | 11.29 | 10.47 ± 0.82 |
| Reverse | 0.91 | 20.3 | 0.64 | 11.89 | 10.65 ± 0.70 | |
| DMSO | Forward | 0.93 | 20.9 | 0.68 | 13.21 | 11.89 ± 1.43 |
| Reverse | 0.93 | 20.9 | 0.69 | 13.59 | 12.04 ± 1.27 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, W.; Liu, X.; Guo, X.; Niu, Q.; Yi, J.; Xia, R.; Min, Y. Morphology Analysis and Optimization: Crucial Factor Determining the Performance of Perovskite Solar Cells. Molecules 2017, 22, 520. https://doi.org/10.3390/molecules22040520
Zeng W, Liu X, Guo X, Niu Q, Yi J, Xia R, Min Y. Morphology Analysis and Optimization: Crucial Factor Determining the Performance of Perovskite Solar Cells. Molecules. 2017; 22(4):520. https://doi.org/10.3390/molecules22040520
Chicago/Turabian StyleZeng, Wenjin, Xingming Liu, Xiangru Guo, Qiaoli Niu, Jianpeng Yi, Ruidong Xia, and Yong Min. 2017. "Morphology Analysis and Optimization: Crucial Factor Determining the Performance of Perovskite Solar Cells" Molecules 22, no. 4: 520. https://doi.org/10.3390/molecules22040520






