Synthetic Peptides Derived from Bovine Lactoferricin Exhibit Antimicrobial Activity against E. coli ATCC 11775, S. maltophilia ATCC 13636 and S. enteritidis ATCC 13076
Abstract
:1. Introduction
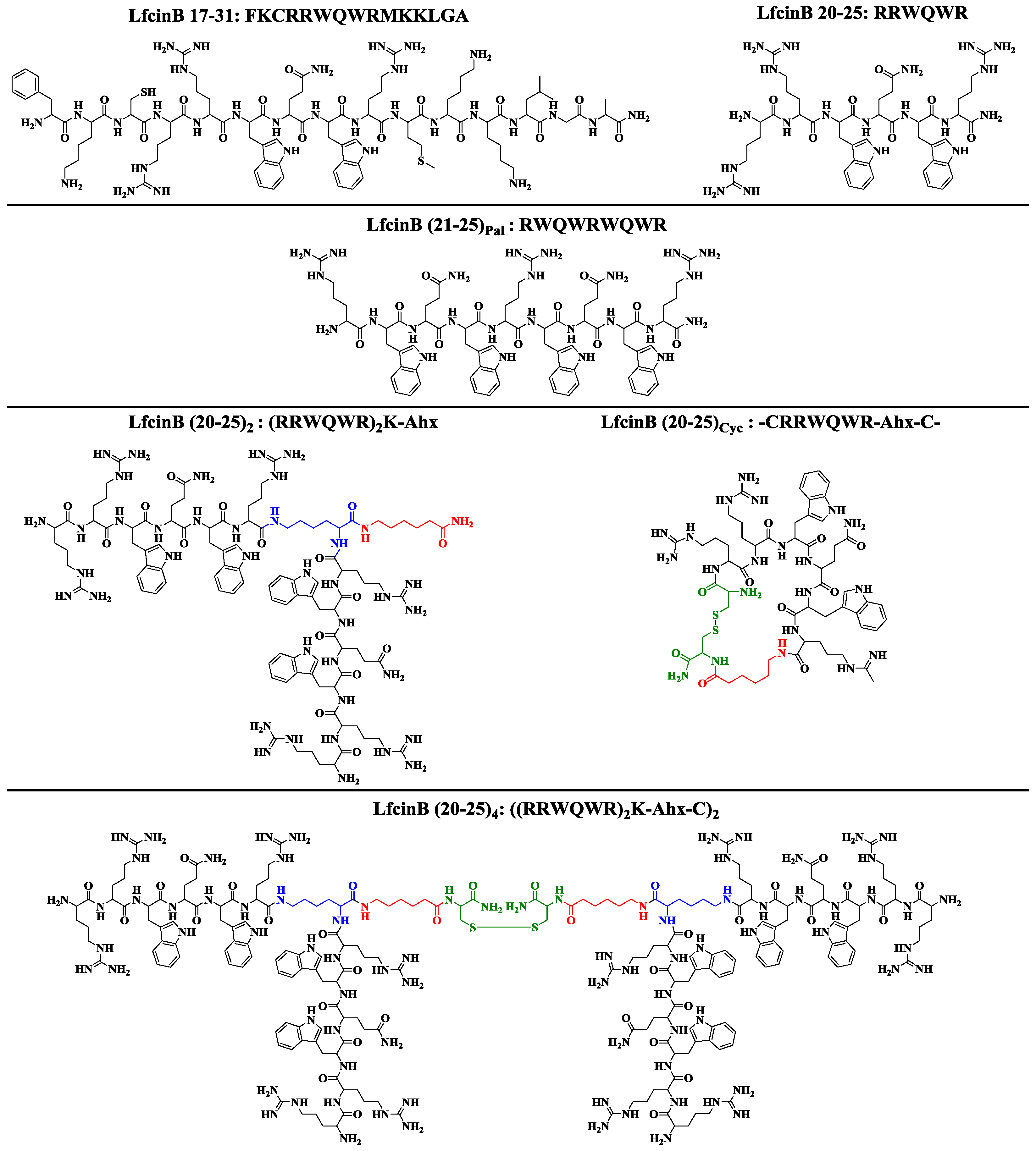
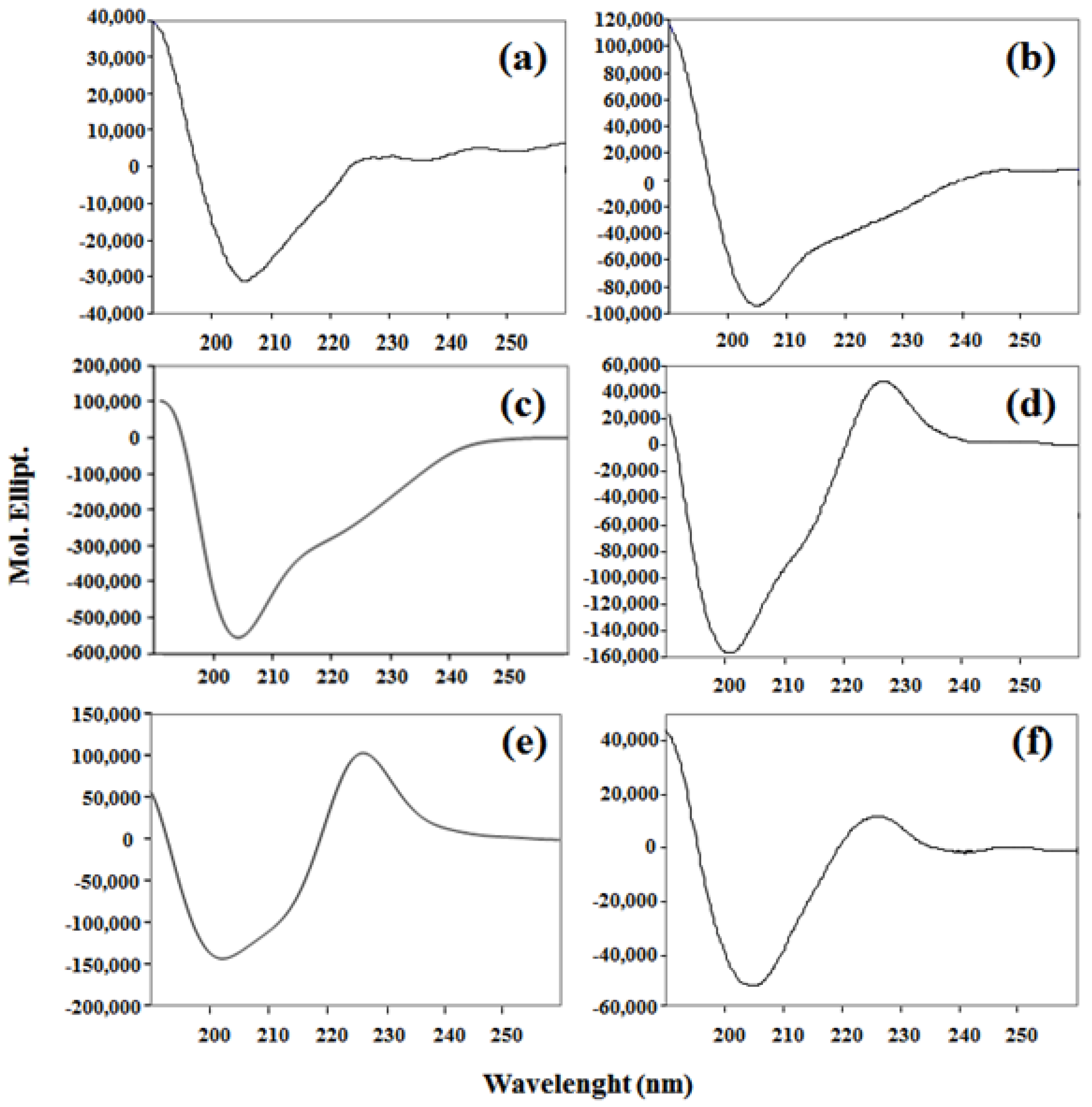
2. Results and Discussion
3. Materials and Methods
3.1. Reagents and Materials
3.2. LfcinB-Derived Peptide Synthesis
3.3. LfcinB-Derived Peptide Characterization
3.3.1. Analytical Methods
3.3.2. Circular Dichroism (CD)
3.4. LfcinB-Derived Peptide Antibacterial Activity
3.4.1. Susceptibility Assays
3.4.2. Antibacterial Activity Assays
3.4.3. Hemolytic Activity Assay
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Antimicrobial Resistance, Global Report on Surveillance. 2014, pp. 1–25. Available online: http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1 (accessed on 5 February 2016).
- Gelband, H.; Miller-Petrie, M.; Pant, S.; Gandra, S.; Levinson, J.; Barter, D.; White, A.; Laxminarayan, R. State of the World’s Antibiotics; Center for Disease Dynamics, Economics & Policy (CDDEP): Washington, DC, USA, 2015; pp. 14–22. [Google Scholar]
- Brooke, J.S. Stenotrophomonas maltophilia: An Emerging Global Opportunistic Pathogen. Clin. Microbiol. Rev. 2012, 25, 2–41. [Google Scholar] [CrossRef] [PubMed]
- Al-Jasser, A.M. Case report. Stenotrophomonas maltophilia resistant to trimethoprim - sulfamethoxazole: An increasing problem. Ann. Clin. Microb. Antimicrob. 2006, 5, 23. [Google Scholar]
- Zhang, L.; Li, X.Z.; Poole, K. Multiple Antibiotic Resistance in Stenotrophomonas maltophilia: Involvement of a Multidrug Efflux System. Antimicrob. Agents. Chemother. 2000, 44, 287–293. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial resistance. Available online: http://www.who.int/mediacentre/factsheets/fs139/en/ (accessed on 5 February 2017).
- Ghazaey, S.; Mirmomeni, M.H. Microbial-resistant Salmonella enteritidis isolated from poultry samples. Rep. Biochem. Mol. Biol. 2012, 1, 9–13. [Google Scholar] [PubMed]
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic therapeutic peptides: Science and market. Drug. Discov. Today 2010, 15, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, T.; Kyprianou, T.; Martinelli, F.; Oppici, C.; Heiligers, D.; Hills, D.; Calvo, X.; Verhaert, P. The emergence of peptides in the pharmaceutical business: From exploration to exploitation. EuPA Open Proteom. 2014, 4, 58–69. [Google Scholar] [CrossRef]
- Reddy, R.; Yedery, R.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.; Ren, D. Antimicrobial Peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed]
- Farnaud, S.; Evans, R. Lactoferrin a multifunctional protein with antimicrobial properties. Mol. Immunol. 2003, 40, 395–405. [Google Scholar] [CrossRef]
- Lonnerdal, B.; Lyer, S. Lactoferrin: Molecular structure and biological function. Annu. Rev. Nutr. 1995, 15, 93–110. [Google Scholar] [CrossRef] [PubMed]
- García-Montoya, I.A.; Cendón, T.S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin a multiple bioactive protein: An overview. Biochim. Biophys. Acta 2012, 1820, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Wakabayashi, H.; Shin, K.; Yamauchi, K.; Yaeshima, T.; Iwatsuki, K. Twenty-five years of research on bovine lactoferrin applications. Biochimie 2009, 91, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K.; Tomita, M. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. Biochim. Biophys. Acta 1992, 73, 472–479. [Google Scholar]
- Arnold, R.; Brewer, M.; Guthier, J. Bactericidal activity of human lactoferrin: Sensitivity of a variety of microorganisms. Infect. Immun. 1980, 28, 893–898. [Google Scholar] [PubMed]
- Brouwer, C.P.; Rahman, M.; Welling, M.M. Discovery and development of a synthetic peptide derived from lactoferrin for clinical use. Peptides 2011, 32, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Strøm, M.B.; Haug, B.E.; Skar, M.L.; Stensen, W.; Stiberg, T.; Svendsen, J.S. The Pharmacophore of Short Cationic Antibacterial Peptides. J. Med. Chem. 2003, 46, 1567–1570. [Google Scholar]
- Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K.; Tomita, M. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1992, 1121, 130–136. [Google Scholar] [CrossRef]
- Sinha, M.; Kaushik, S.; Kaur, P.; Sharma, S.; Singh, T. Antimicrobial Lactoferrin Peptides: The Hidden Players in the Protective Function of a Multifunctional Protein. Int. J. Pep. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ulvatne, H.; Haukland, H.H.; Olsvik, O.; Vorland, L.H. Lactoferricin B causes depolarization of the cytoplasmic membrane of Escherichia coli ATCC 25922 and fusion of negatively charged liposomes. FEBS Lett. 2001, 492, 62–65. [Google Scholar] [CrossRef]
- Andersen, J.H.; Osbakk, S.A.; Vorland, L.H.; Traavik, T.; Gutteberg, T.J. Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts. Antiviral Res. 2001, 51, 141–149. [Google Scholar] [CrossRef]
- Andersen, J.H.; Jenssen, H.; Gutteberg, T.J. Lactoferrin and lactoferricin inhibit Herpes simplex 1 and 2 infection and exhibit synergy when combined with acyclovir. Antiviral Res. 2003, 58, 209–215. [Google Scholar] [CrossRef]
- Aguilera, O.; Ostolaza, H.; Quiros, L.M.; Fierro, L.J. Permeabilizing action of an antimicrobial lactoferricin-derived peptide on bacterial and artificial membranes. FEBS Lett. 1999, 462, 273–277. [Google Scholar] [CrossRef]
- Chan, D.; Prenner, E.; Vogel, H. Tryptophan- and arginine-rich antimicrobial peptides: Structures and mechanisms of action. Biochim. Biophys. Acta 2006, 1758, 1184–1202. [Google Scholar] [CrossRef] [PubMed]
- Haukland, H.H.; Ulvatne, H.; Sandvik, K.; Vorland, L.H. The antimicrobial peptides lactoferricin B and magainin 2 cross over the bacterial cytoplasmic membrane and reside in the cytoplasm. FEBS Lett. 2001, 508, 389–393. [Google Scholar] [CrossRef]
- Liu, Y.; Han, F.; Xie, Y.; Wang, Y. Comparative antimicrobial activity and mechanism of action of bovine lactoferricin-derived synthetic peptides. Biometals 2011, 24, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.H.; Ho, Y.h.; Chuang, Y.C.; Chen, P.C.; Chen, C.S. Identification of Lactoferricin B Intracellular Targets Using an Escherichia coli Proteome Chip. PLoS ONE 2011, 6, e28197. [Google Scholar] [CrossRef] [PubMed]
- Ulvatne, H.; Samuelsen, O.; Haukland, H.H.; Krämer, M.; Vorland, L.H. Lactoferricin B inhibits bacterial macromolecular synthesis in Escherichia coli and Bacillus subtilis. FEMS. Microbiol. Lett. 2004, 15, 377–384. [Google Scholar]
- Vorland, L.H.; Ulvatne, H.; Andersen, J.; Haukland, H.; Rekdal, O.; Svendsen, J.S.; Gutteberg, T.J. Lactoferricin of bovine origin is more active than lactoferricins of human, murine and caprine origin. Scand. J. Infect. Dis. 1998, 30, 513–517. [Google Scholar] [PubMed]
- Schibli, D.; Hwang, P.; Vogel, H. The structure of the antimicrobial active center of lactoferricin B bound to sodium dodecyl sulfate micelles. FEBS Lett. 1999, 446, 213–217. [Google Scholar] [CrossRef]
- Greathouse, D.; Vostrikov, V.; Mcclellan, N.; Chipollini, J.; Lay, J.; Liyanage, R.; Ladd, T. Lipid interactions of acylated tryptophan-methylated lactoferricin peptides by solid-state NMR. J. Pept. Sci. 2008, 14, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Chau, J.K.; Perry, N.A.; de Boer, L.; Zaat, S.A.; Vogel, H.J. Serum stabilities of short tryptophan- and arginine-rich antimicrobial peptide analogs. PLoS ONE 2010, 5, e12684. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Guo, H.Y.; Zhang, M.; Jiang, L.; Ren, F.Z. The six amino acid antimicrobial peptide bLFcin6 penetrates cells and delivers siRNA. FEBS J. 2013, 280, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- León, M.A.; Leal, A.L.; Almanzar, G.; Rosas, J.E.; García, J.E.; Rivera, Z.J. Antibacterial activity of synthetic peptides derived from Lactoferricin against Escherichia coli ATCC 25922 and Enterococcus faecalis ATCC 29212. Biomed. Res. Int. 2015, 2015, 1–8. [Google Scholar]
- Rekdal, O.; Andersen, J.; Vorland, L.H.; Svendsen, J.S. Construction and synthesis of lactoferricin derivatives with enhanced antibacterial activity. J. Pept. Sci. 1999, 5, 32–45. [Google Scholar] [CrossRef]
- Haug, B.E.; Skar, M.L.; Svendsen, J.S. Bulky aromatic amino acids increase the antibacterial activity of 15-residue bovine lactoferricin derivatives. J. Pept. Sci. 2001, 7, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Matsumoto, H.; Hashimoto, K.; Teraguchi, S.; Takase, M.; Hayasawa, H. N-Acylated and D enantiomer derivatives of a nonamer core peptide of lactoferricin B showing improved antimicrobial activity. Antimicrob. Agents Chemother. 1999, 43, 1267–1269. [Google Scholar] [PubMed]
- Hoek, K.S.; Milne, J.M.; Grieve, P.A.; Dionysius, D.A.; Smith, R. Antibacterial activity in bovine lactoferrin-derived peptides. Antimicrob. Agents Chemother. 1997, 41, 54–59. [Google Scholar] [PubMed]
- Svenson, J.; Vergote, V.; Karstad, R.; Burvenich, C.; Svendsen, J.S.; Spiegeleer, B. Metabolic fate of lactoferricin-based antimicrobial peptides: Effect of truncation and incorporation of amino acid analogs on the in vitro metabolic stability. J. Pharmacol. Exp. Ther. 2010, 332, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Strøm, M.B.; Rekdal, O.; Svendsen, J.S. Antibacterial activity of 15-residue lactoferricin derivatives. J. Pept. Res. 2000, 56, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Shyu, Ch.; Mao, F. Antibacterial activity of short hydrophobic and basic rich peptides. Am. J. Vet. Res. 2003, 64, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, W.; Carnelocce, L.; Silva, J.; Oliveira, A.; Goncalves, R. Conformational changes in bovine lactoferrin induced by slow or fast temperature increases. Biol. Chem. 2008, 389, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Vorland, L.H.; Ulvatne, H.; Andersen, J.; Haukland, H.H.; Rekdal, O.; Svendsen, J.S.; Gutteberg, T.J. Antibacterial effects of lactoferricin B. Scand. J. Infect. Dis. 1999, 31, 179–184. [Google Scholar] [PubMed]
- Oo, T.Z.; Cole, N.; Garthwaite, L.; Willcox, M.D.; Zhu, H. Evaluation of synergistic activity of bovine lactoferricin with antibiotics in corneal infection. J. Antimicrob. Chemother. 2010, 65, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- MilkAMP database. A database dedicated to milk antimicrobial peptides. Available online: http://milkampdb.org/BLF0084 (accessed on 11 December 2015).
- Yamauchi, K.; Tomita, M.; Gieh, T.; Ellison, R. Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect. Immun. 1993, 61, 719–728. [Google Scholar] [PubMed]
- Ulvatne, H.; Haukland, H.H.; Samuelsen, O.; Krämer, M.; Vorland, L.H. Proteases in Escherichia coli and Staphylococcus aureus confer reduced susceptibility to lactoferricin B. J. Antimicrob. Chemother. 2002, 50, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Romo, T.D.; Bradney, L.A.; Greathouse, D.V.; Grossfield, A. Membrane binding of an acyl-lactoferricin B antimicrobial peptide from solid-state NMR experiments and molecular dynamics simulations. Biochim. Biophys. Acta 2011, 1808, 2019–2030. [Google Scholar] [CrossRef] [PubMed]
- Branen, J.; Davidson, P.M. Activity of hydrolysed lactoferrin against foodborne pathogenic bacteria in growth media: The effect of EDTA. Lett. Appl. Microbiol. 2000, 30, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Qamruddin, A.; Alkawash, M.; Soothill, J. Antibiotic susceptibility of Stenotrophomonas maltophilia in the presence of Lactoferrin. Antimicrob. Agents Chemother. 2005, 49, 4425–4426. [Google Scholar] [CrossRef] [PubMed]
- Vergel, C.; Rivera, Z.J.; Rosas, J.E.; García, J.E. Efficient Synthesis of Peptides with 4-Methylpiperidine as Fmoc Removal Reagent by Solid Phase Synthesis. J. Mex. Chem. Soc. 2014, 58, 386–392. [Google Scholar]
- García, J.E.; Fierro, R.; Puentes, A.; Cortés, J.; Bermúdez, A.; Cifuentes, G.; Vanegas, M.; Patarroyo, M.E. Monosacharides modulate HCV E2 Protein-derived peptide biological properties. Biochem. Biophys. Res. Commun. 2007, 355, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Solarte, V.; Rosas, J.E.; Rivera, Z.J.; Arango, M.L.; García, J.E.; Vernot, J.A. A Tetrameric Peptide Derived from Bovine Lactoferricin Exhibits Specific Cytotoxic Effects against Oral Squamous-Cell Carcinoma Cell Lines. Biomed. Res. Int. 2015, 2015, 630179. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds used in this paper are available from the authors.
| Antibacterial Activity | ||||||
|---|---|---|---|---|---|---|
| Peptide | E. coli ATCC 11775 | S. maltophilia ATCC 13636 | S. enteritidis ATCC 13076 | |||
| Code | MIC | MBC | MIC | MBC | MIC | MBC |
| LfcinB * | 100 (32.2) | 100 (32.2) | >200 (>64.4) | >200 (>64.4) | 100 (32.2) | 50 (16.1) |
| LfcinB 17–31 | 25 (12.5) | 25 (12.5) | >200 (>100) | >200 (>100) | >200 (>100) | 200 (100) |
| LfcinB 20–25 | 12.5 (12.5) | 12.5 (12.5) | >200 (>203) | >200 (>203) | 100 (102) | 100 (102) |
| LfcinB (20–25)2 | 6.2 (2.8) | 6.2 (2.8) | 50 (22.4) | 50 (22.4) | 12.5 (5.6) | 12.5 (5.6) |
| LfcinB (20–25)4 | 25 (5.5) | 25 (5.5) | >200 (>44) | 200 (44) | 200 (44) | 100 (22) |
| LfcinB (20–25)Cyc | 25 (21) | 25 (21) | 200 (84) | 200 (84) | 50 (42) | 50 (42) |
| LfcinB 21–25Pal | 12.5 (8.5) | 12.5 (8.5) | >200 (>136) | 200 (136) | 25 (17) | 25 (17) |
| BLF | >200 (>2.5) | >200 (>2.5) | >200 (>2.5) | >200 (>2.5) | >200 (>2.5) | >200 (>2.5) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huertas Méndez, N.D.J.; Vargas Casanova, Y.; Gómez Chimbi, A.K.; Hernández, E.; Leal Castro, A.L.; Melo Diaz, J.M.; Rivera Monroy, Z.J.; García Castañeda, J.E. Synthetic Peptides Derived from Bovine Lactoferricin Exhibit Antimicrobial Activity against E. coli ATCC 11775, S. maltophilia ATCC 13636 and S. enteritidis ATCC 13076. Molecules 2017, 22, 452. https://doi.org/10.3390/molecules22030452
Huertas Méndez NDJ, Vargas Casanova Y, Gómez Chimbi AK, Hernández E, Leal Castro AL, Melo Diaz JM, Rivera Monroy ZJ, García Castañeda JE. Synthetic Peptides Derived from Bovine Lactoferricin Exhibit Antimicrobial Activity against E. coli ATCC 11775, S. maltophilia ATCC 13636 and S. enteritidis ATCC 13076. Molecules. 2017; 22(3):452. https://doi.org/10.3390/molecules22030452
Chicago/Turabian StyleHuertas Méndez, Nataly De Jesús, Yerly Vargas Casanova, Anyelith Katherine Gómez Chimbi, Edith Hernández, Aura Lucia Leal Castro, Javier Mauricio Melo Diaz, Zuly Jenny Rivera Monroy, and Javier Eduardo García Castañeda. 2017. "Synthetic Peptides Derived from Bovine Lactoferricin Exhibit Antimicrobial Activity against E. coli ATCC 11775, S. maltophilia ATCC 13636 and S. enteritidis ATCC 13076" Molecules 22, no. 3: 452. https://doi.org/10.3390/molecules22030452






