The Novel Property of Heptapeptide of Microcin C7 in Affecting the Cell Growth of Escherichia coli
Abstract
:1. Introduction
2. Results
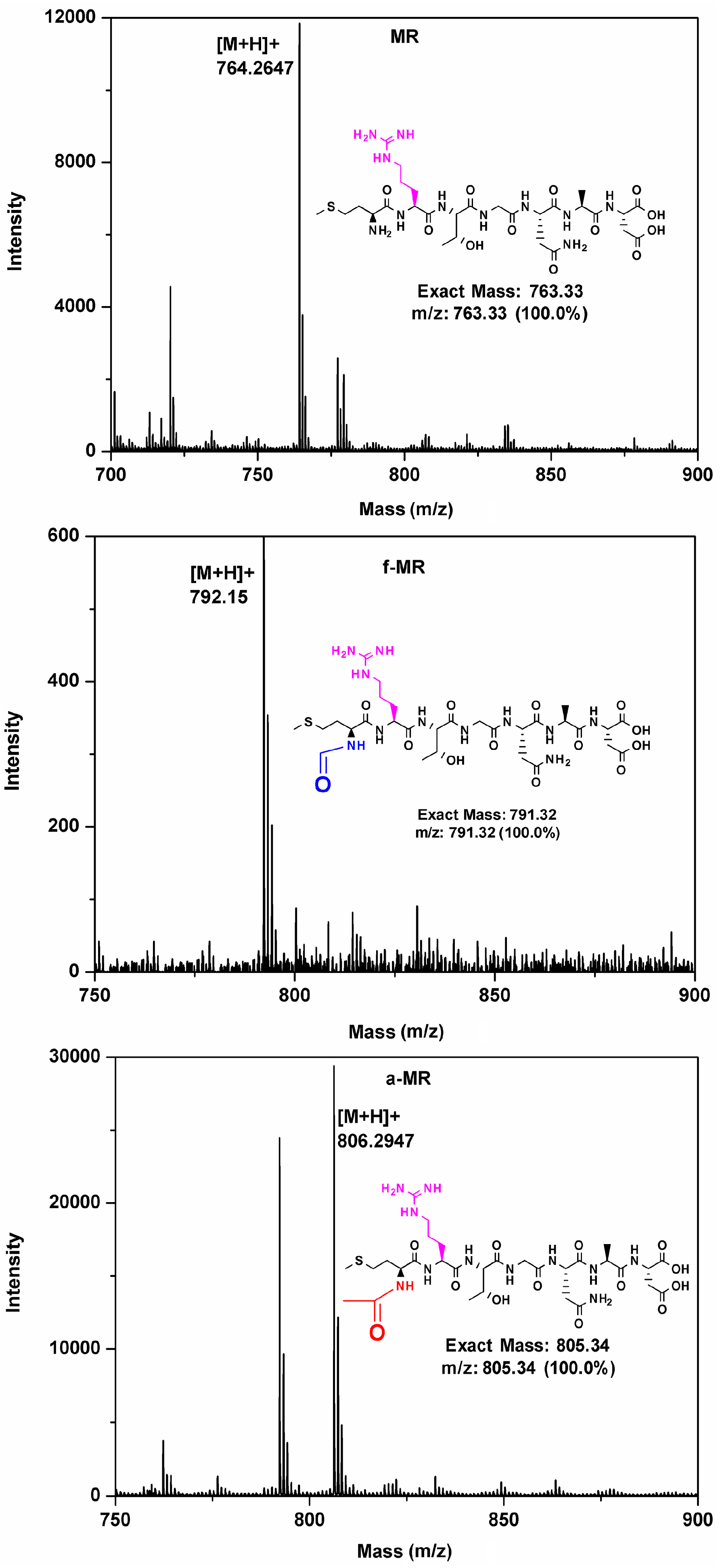
2.1. Peptide Synthesis
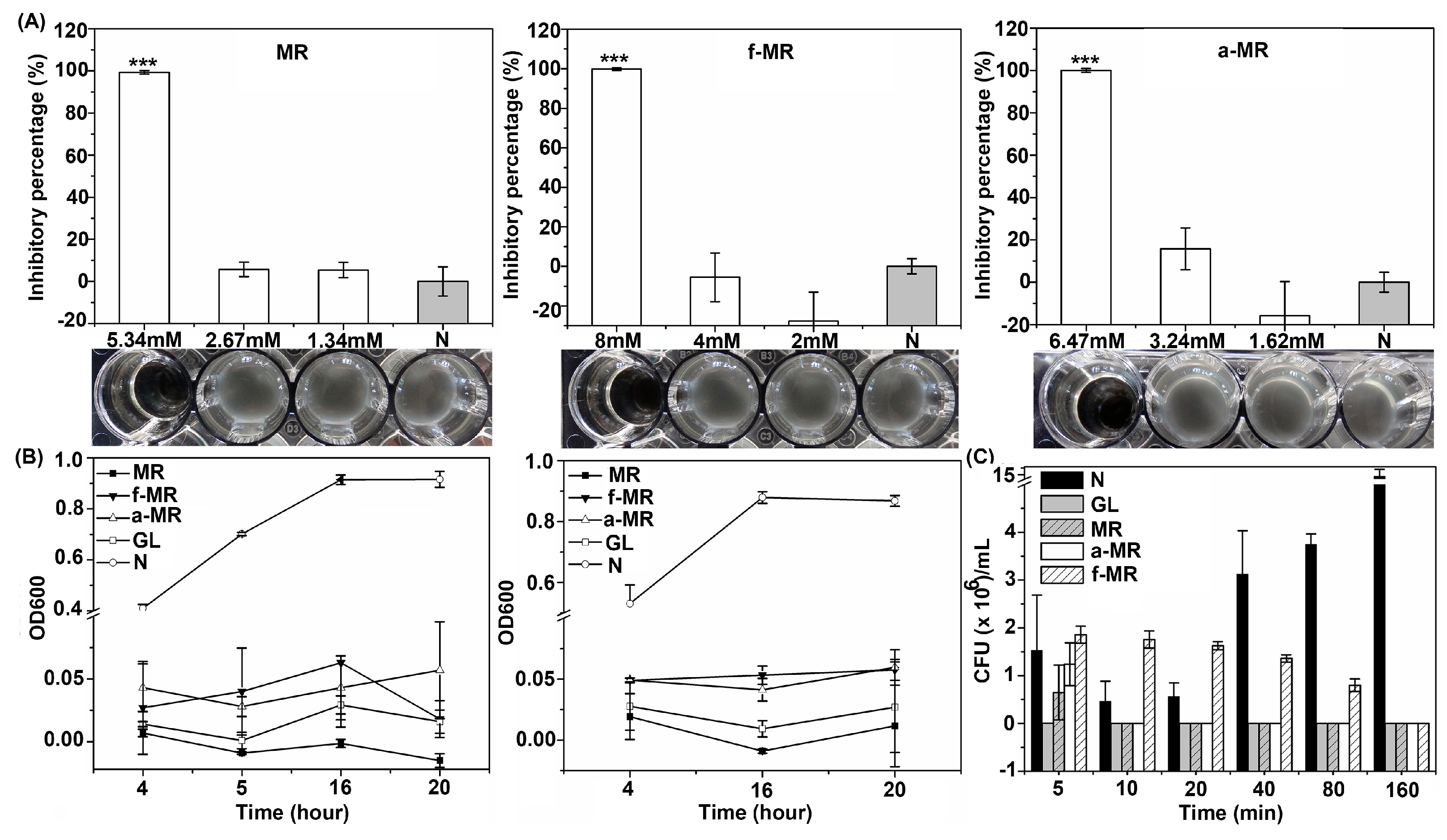
2.2. Cell Growth
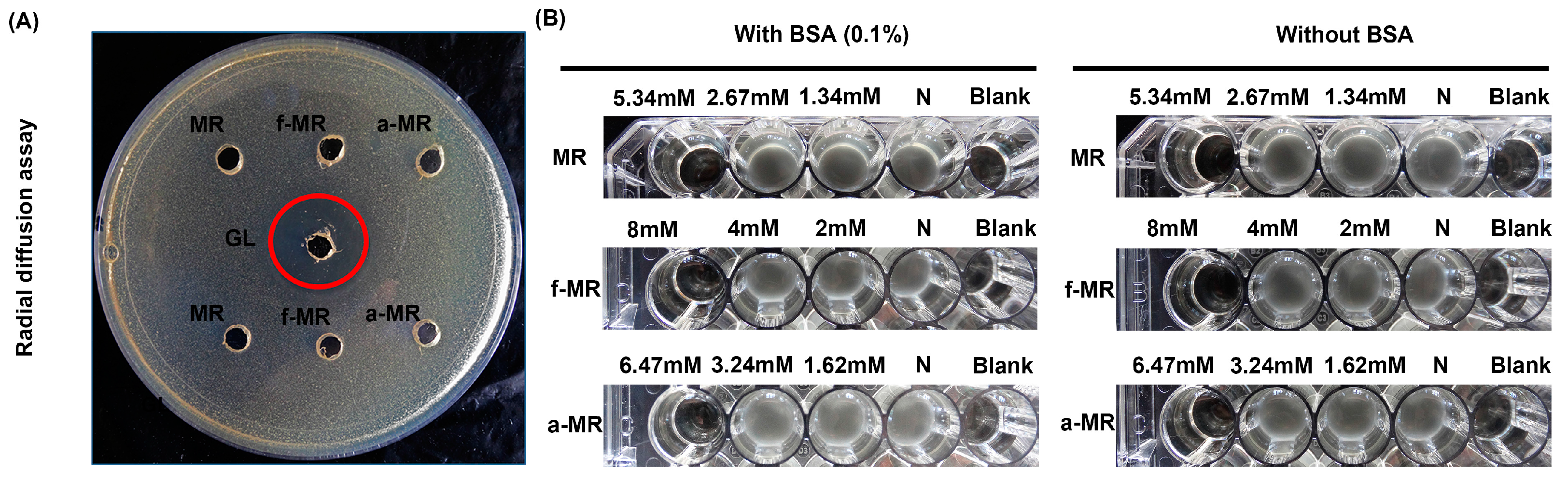
2.3. Media Effect
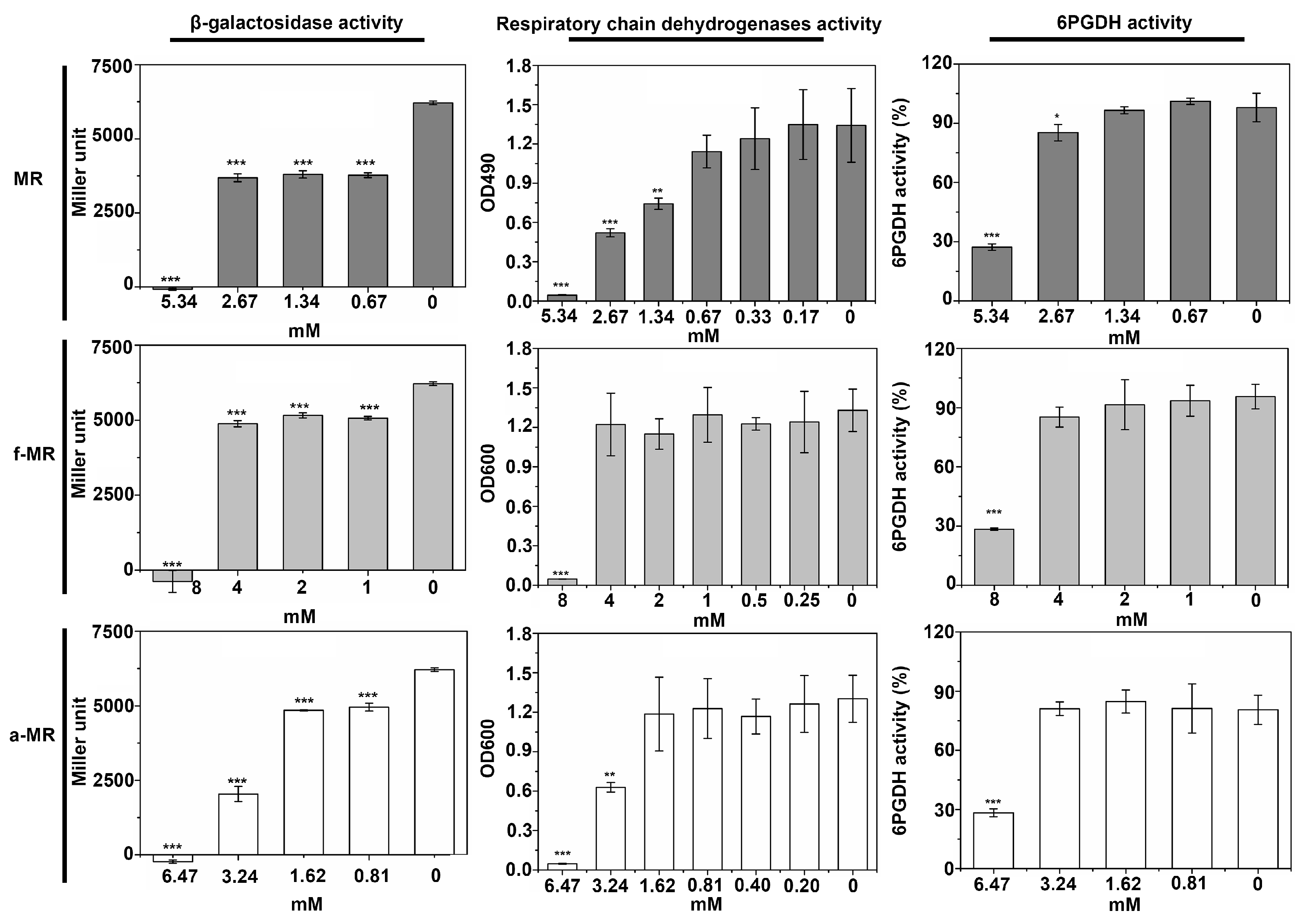
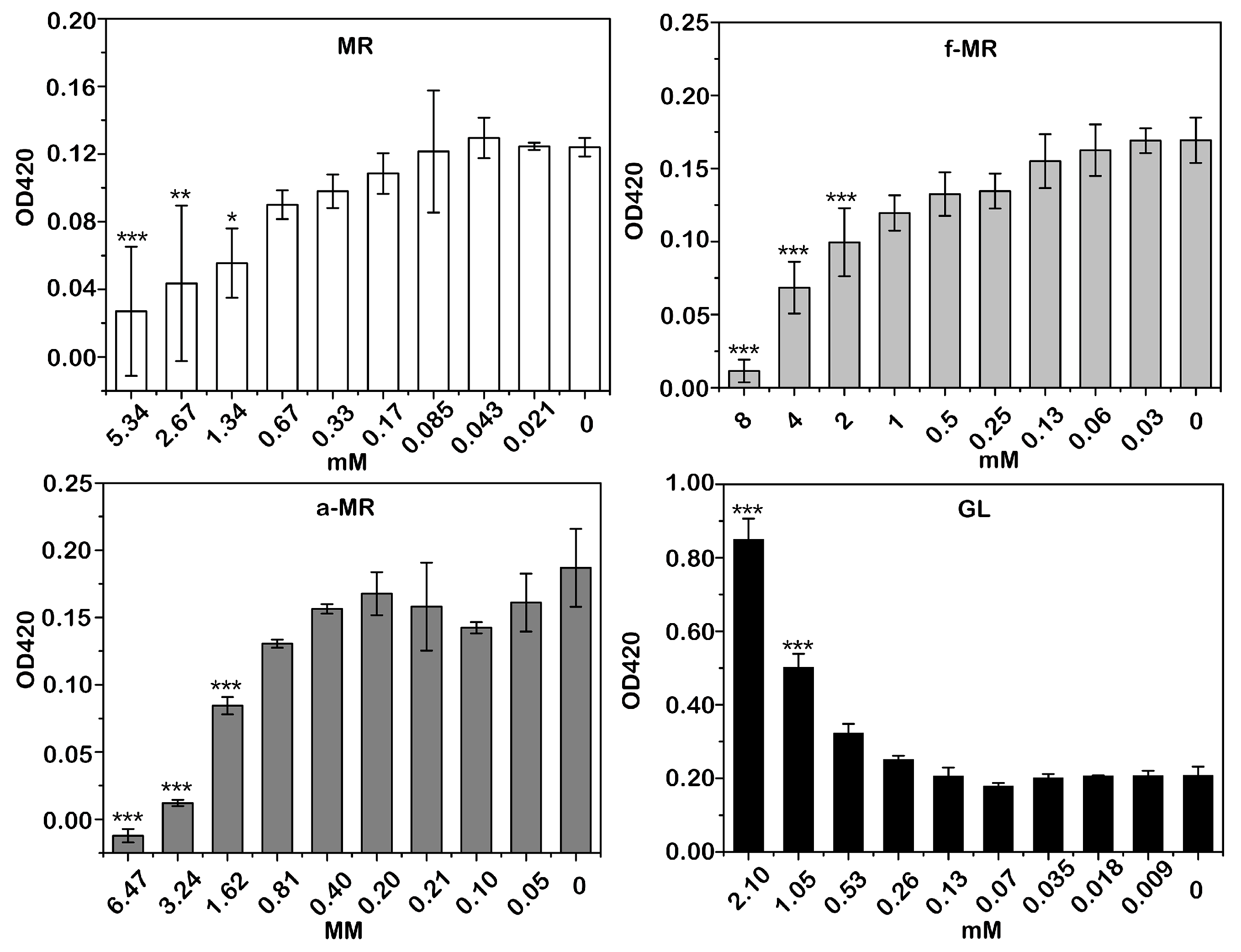
2.4. Enzyme Activity
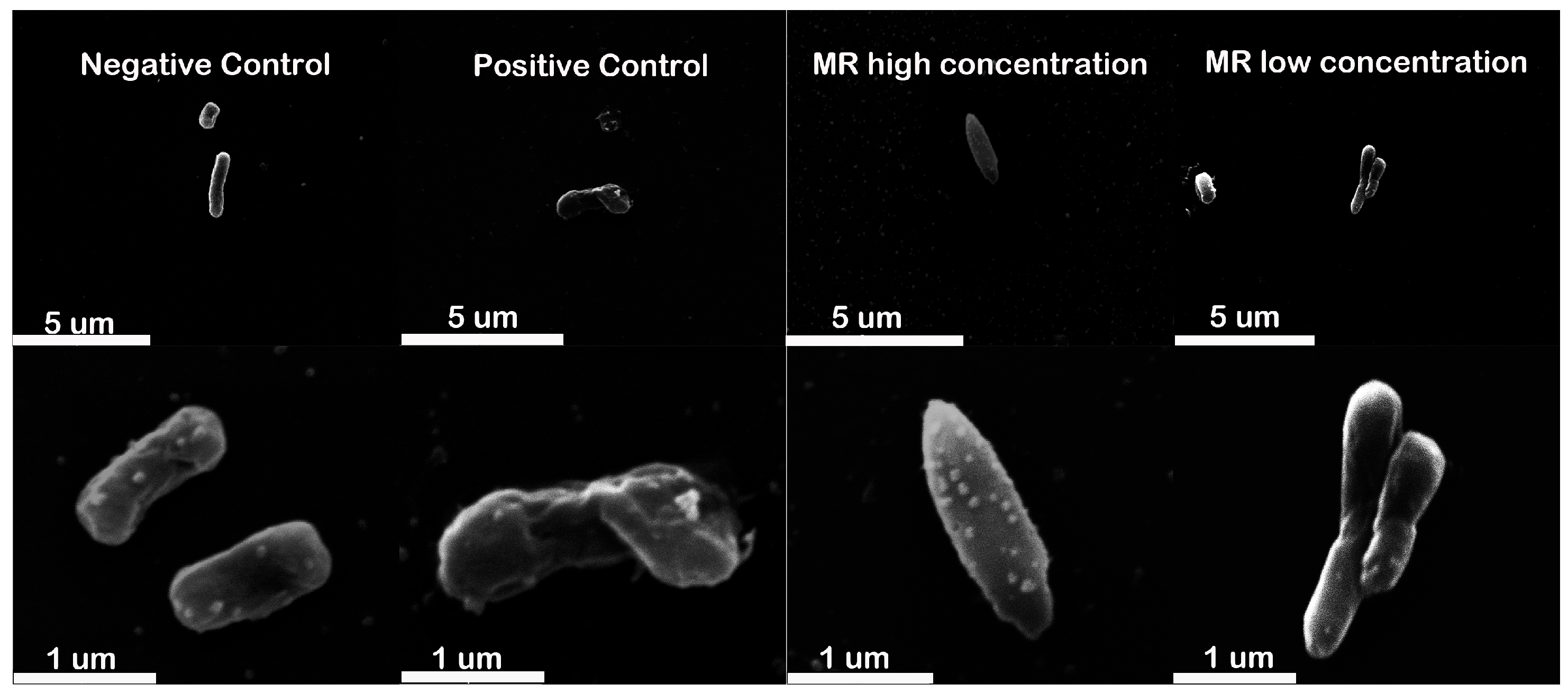
2.5. Cell Morphology
2.6. Membrane Integrity
2.7. Effects of Outer Membrane Permeabilization Agents
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Peptide Synthesis and Purification
4.3. Cell Growth
4.4. Kill Time Assay
4.5. Enzyme Activity Assays
4.6. Scanning Electron Microscopy
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arias, C.A.; Murray, B.E. A New Antibiotic and the evolution of resistance. N. Engl. J. Med. 2015, 372, 1168–1170. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.P.; Bu, D.P.; Carrique-Mas, J.; Fèvre, E.M.; Gilbert, M.; Grace, D.; Hay, S.I.; Jiwakanon, J.; Kakkar, M.; Kariuki, S.; et al. Antibiotic resistance is the quintessential one health issue. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Vondenhoff, G.H.M.; van Aerschot, A. Microcin C: Biosynthesis, mode of action, and potential as a lead in antibiotics development. Nucleosides Nucleotides Nucleic Acids 2011, 30, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Duquesne, S.; Destoumieux-Garzon, D.; Peduzzi, J.; Rebuffat, S. Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat. Prod. Rep. 2007, 24, 708–734. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Tikhonov, A.; Metlitskaya, A.; Severinov, K.; Nair, S.K. Structure and function of a serine carboxypeptidase adapted for degradation of the protein synthesis antibiotic microcin C7. Proc. Natl. Acad. Sci. USA 2012, 109, 4425–4430. [Google Scholar] [CrossRef] [PubMed]
- Kulikovsky, A.; Serebryakova, M.; Bantysh, O.; Metlitskaya, A.; Borukhov, S.; Severinov, K.; Dubiley, S. The molecular mechanism of aminopropylation of peptide-nucleotide antibiotic Microcin C. J. Am. Chem. Soc. 2014, 136, 11168–11175. [Google Scholar] [CrossRef] [PubMed]
- Van de Vijver, P.; Vondenhoff, G.H.M.; Kazakov, T.S.; Semenova, E.; Kuznedelov, K.; Metlitskaya, A.; van Aerschot, A.; Severinov, K. Synthetic Microcin C analogs targeting different aminoacyl-tRNA synthetases. J. Bacteriol. 2009, 191, 6273–6280. [Google Scholar] [CrossRef] [PubMed]
- Severinov, K.; Nair, S.K. Microcin C: Biosynthesis and mechanisms of bacterial resistance. Future Microbiol. 2012, 7, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Novikova, M.; Metlitskaya, A.; Datsenko, K.; Kazakov, T.; Kazakov, A.; Wanner, B.; Severinov, K. The Escherichia coli Yej transporter is required for the uptake of translation inhibitor Microcin C. J. Bacteriol. 2007, 189, 8361–8365. [Google Scholar] [CrossRef] [PubMed]
- Vondenhoff, G.H.M.; Blanchaert, B.; Geboers, S.; Kazakov, T.; Datsenko, K.A.; Wanner, B.L.; Rozenski, J.; Severinov, K.; van Aerschot, A. Characterization of peptide chain length and constituency requirements for YejABEF-Mediated uptake of Microcin C Analogues. J. Bacteriol. 2011, 193, 3618–3623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazakov, T.; Vondenhoff, G.H.M.; Datsenko, K.A.; Novikova, M.; Metlitskaya, A.; Wanner, B.L.; Severinov, K. Escherichia coli peptidase A, B, or N can process translation inhibitor Microcin C. J. Bacteriol. 2008, 190, 2607–2610. [Google Scholar] [CrossRef] [PubMed]
- Metlitskaya, A.; Kazakov, T.; Vondenhoff, G.H.M.; Novikova, M.; Shashkov, A.; Zatsepin, T.; Semenova, E.; Zaitseva, N.; Ramensky, V.; van Aerschot, A.; Severinov, K. Maturation of the translation inhibitor Microcin C. J. Bacteriol. 2009, 191, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Rebuffat, S. Microcins in action: amazing defence strategies of enterobacteria. Biochem. Soc. Trans. 2012, 40, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Guijarro, J.I.; González-Pastor, J.E.; Baleux, F.; Millán, J.L.S.; Castilla, M.A.; Rico, M.; Moreno, F.; Delepierre, M. Chemical structure and translation inhibition studies of the antibiotic Microcin C7. J. Biol. Chem. 1995, 270, 23520–23532. [Google Scholar] [CrossRef] [PubMed]
- Metlitskaya, A.; Kazakov, T.; Kommer, A.; Pavlova, O.; Praetorius-Ibba, M.; Ibba, M.; Krasheninnikov, I.; Kolb, V.; Khmel, I.; Severinov, K. Aspartyl-tRNA synthetase is the target of peptide nucleotide antibiotic Microcin C. J. Biol. Chem. 2006, 281, 18033–18042. [Google Scholar] [CrossRef] [PubMed]
- Konno, K.; Hisada, M.; Fontana, R.; Lorenzi, C.C.; Naoki, H.; Itagaki, Y.; Miwa, A.; Kawai, N.; Nakata, Y.; Yasuhara, T.; et al. Anoplin, a novel antimicrobial peptide from the venom of the solitary wasp Anoplius samariensis. Biochim. Biophys. Acta 2001, 1550, 70–80. [Google Scholar] [CrossRef]
- Jindřichová, B.; Burketová, L.; Novotná, Z. Novel properties of antimicrobial peptide anoplin. Biochem. Biophys. Res. Commun. 2014, 444, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Won, A.; Khan, M.; Gustin, S.; Akpawu, A.; Seebun, D.; Avis, T.; Leung, B.O.; Hitchcock, A.P.; Ianoul, A. Investigating the effects of l- to d-amino acid substitution and deamidation on the activity and membrane interactions of antimicrobial peptide anoplin. Biochim. Biophys. Acta. 2011, 1808, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Hurdle, J.G.; O’Neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Chen, H.; Zhong, C.; Kim, J.E.; Zhang, Y.H.P. High-Throughput screening of coenzyme preference change of thermophilic 6-Phosphogluconate dehydrogenase from NADP+ to NAD+. Sci. Rep. 2016, 6, 32644. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.; Paop, N.; Barra, D.; Simmaco, M.; Bozzi, A.; di Giulio, A.; Rinaldi, A.C. Effects of the antimicrobial peptide temporin L on cell morphology, membrane permeability and viability of Escherichia coli. Biochem. J. 2004, 380, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.; Richard, B.; Martha, C.; Mike, D.; Heather, F.; Vincent, A.F.; Simon, F.; Brendan, F.G.; Hancock, R.E.; David, H.; et al. Alternatives to antibiotics—A pipeline portfolio review. Lancet Infect Dis. 2016, 16, 239–251. [Google Scholar]
- Hancock, R.E.; Wong, P.G. Compounds which increase the permeability of the Pseudomonas aeruginosa outer membrane. Antimicrob. Agents Chemother. 1984, 26, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Vaara, M. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 1992, 56, 395–411. [Google Scholar] [PubMed]
- Olga, B.; Marina, S.; Inna, Z.; Kulikovskya, A.; Tsibulskayaa, D.; Svetlana, D.; Severinov, K. Enzymatic synthesis and functional characterization of bioactive Microcin C-Like compounds with altered peptide sequence and length. J. Bacteriol. 2015, 197, 3133–3141. [Google Scholar]
- Bantysh, O.; Serebryakova, M.; Makarova, K.S.; Dubiley, S.; Datsenko, K.A.; Severinov, K. Enzymatic synthesis of bioinformatically predicted Microcin C-Like compounds encoded by diverse bacteria. mBio 2014, 5, e01059-14. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.A.; Lehrer, R.I. Designer assays for antimicrobial peptides. In Antibacterial Peptide Protocols; Shafer, W.M., Ed.; Humana Press: Totowa, NJ, USA, 1997; pp. 169–186. [Google Scholar]
- Gläser, R.; Harder, J.; Lange, H.; Bartels, J.; Christophers, E.; Schröder, J.M. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat. Immunol. 20005, 6, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Yi, K.S.; Matsuzaki, K.; Kim, M.S.; Kim, S.C. Structure-activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: The proline hinge is responsible for the cell-penetrating ability of buforin II. Proc. Natl. Acad. Sci. USA 2000, 97, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Bartesaghi, A.; Merk, A.; Banerjee, S.; Matthies, D.; Wu, X.; Milne, J.L.; Subramaniam, S. 2.2 Å resolution cryo-EM structure of β-galactosidase in complex with a cell-permeant inhibitor. Science 2015, 348, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.P.D.S.; Arcisio-Miranda, M.; Costa, S.T.B.; Konno, K.; Ruggiero, J.R.; Procopio, J.; Neto, J.R. Study of the mechanism of action of anoplin, a helical antimicrobial decapeptide with ion channel-like activity, and the role of the amidated C-terminus. J. Pept. Sci. 2008, 14, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Zgurskaya, H.I.; López, C.A.; Gnanakaran, S. Permeability barrier of Gram-Negative cell envelopes and approaches to bypass It. ACS Infect. Dis. 2015, 1, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Breukink, E.; de Kruijff, B. Lipid II as a target for antibiotics. Nat. Rev. Drug Discov. 2006, 5, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Aoki, N.; Ishii, Y.; Tateda, K.; Saga, T.; Kimura, S.; Kikuchi, Y.; Kobayashi, T.; Tanabe, Y.; Tsukada, H.; Gejyo, F.; Yamaguchi, K. Efficacy of Calcium-EDTA as an inhibitor for Metallo-β-Lactamase in a mouse model of Pseudomonas aeruginosa pneumonia. Agents Chemother. 2010, 54, 4582–4588. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Budge, L.P.; Driscoll, C.D.; Willardson, B.M.; Allman, G.W.; Savage, P.B. Incremental conversion of Outer-Membrane permeabilizers into potent antibiotics for Gram-Negative bacteria. J. Am. Chem. Soc. 1999, 121, 931–940. [Google Scholar] [CrossRef]
- Brogden, K.A.; de Lucca, A.J.; Bland, J.; Elliott, S. Isolation of an ovine pulmonary surfactant-associated anionic peptide bactericidal for Pasteurella haemolytica. Proc. Natl. Acad. Sci. USA 1996, 93, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Burkoth, T.S.; Fafarman, A.T.; Charych, D.H.; Connolly, M.D.; Zuckermann, R.N. Incorporation of unprotected heterocyclic side chains into peptoid oligomers via Solid-Phase submonomer synthesis. J. Am. Chem. Soc. 2003, 125, 8841–8845. [Google Scholar] [CrossRef] [PubMed]
- Arhin, F.F.; Belley, A.; McKay, G.A.; Moeck, G. Characterization of the in vitro activity of novel lipoglycopeptide antibiotics. In Current Protocols in Microbiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Ocampo, P.S.; Lázár, V.; Papp, B.; Arnoldini, M.; Abel zur Wiesch, P.; Busa-Fekete, R.; Fekete, R.; Csaba, P.; Martin, A.; Sebastian, B. Antagonism between bacteriostatic and bactericidal antibiotics is prevalent. Antimicrob. Agents Chemother. 2014, 58, 4573–4582. [Google Scholar] [CrossRef] [PubMed]
- Segev-Zarko, L.; Saar-Dover, R.; Brumfeld, V.; Mangoni Maria, L.; Shai, Y. Mechanisms of biofilm inhibition and degradation by antimicrobial peptides. Biochem. J. 2015, 468, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, M.; Lee, M.W.; Mansbach, R.A.; Song, Z.; Bao, Y.; Peek, R.M., Jr.; Yao, C.; Chen, L.F.; Ferguson, A.L.; Wong, G.C.; Cheng, J. Helical antimicrobial polypeptides with radial amphiphilicity. Proc. Natl. Acad. Sci. USA 2015, 13155–13160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bremer, H. Control of the Escherichia coli rrnB P1 promoter strength by ppGpp. J. Biol. Chem. 1995, 270, 11181–11189. [Google Scholar] [CrossRef] [PubMed]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y.; Yang, O.U.; Sheng, Y.; Chen, Y.B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Papapetridis, I.; van Dijk, M.; Dobbe, A.P.; Metz, B.; Pronk, J.T.; van Maris, A.J.A. Improving ethanol yield in acetate-reducing Saccharomyces cerevisiae by cofactor engineering of 6-phosphogluconate dehydrogenase and deletion of ALD6. Microb. Cell Fact. 2016, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Kin Tam, T.; Sun, F.; You, C.; Percival Zhang, Y.H. A high-energy-density sugar biobattery based on a synthetic enzymatic pathway. Nat. Commun. 2014, 5, 3026. [Google Scholar] [CrossRef] [PubMed]
- Marcellini, L.; Giammatteo, M.; Aimola, P.; Mangoni, M.L. Fluorescence and electron microscopy methods for exploring antimicrobial peptides mode(s) of action. In Antimicrobial Peptides: Methods and Protocols; Giuliani, A., Rinaldi, C.A., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 249–266. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, R.; Zeng, H.; Zhao, D.; Liu, R.; Xu, X. The Novel Property of Heptapeptide of Microcin C7 in Affecting the Cell Growth of Escherichia coli. Molecules 2017, 22, 432. https://doi.org/10.3390/molecules22030432
Ran R, Zeng H, Zhao D, Liu R, Xu X. The Novel Property of Heptapeptide of Microcin C7 in Affecting the Cell Growth of Escherichia coli. Molecules. 2017; 22(3):432. https://doi.org/10.3390/molecules22030432
Chicago/Turabian StyleRan, Rensen, Huan Zeng, Dong Zhao, Ruiyuan Liu, and Xia Xu. 2017. "The Novel Property of Heptapeptide of Microcin C7 in Affecting the Cell Growth of Escherichia coli" Molecules 22, no. 3: 432. https://doi.org/10.3390/molecules22030432





