Synthesis and Biological Activities of Ethyl 2-(2-pyridylacetate) Derivatives Containing Thiourea, 1,2,4-triazole, Thiadiazole and Oxadiazole Moieties
Abstract
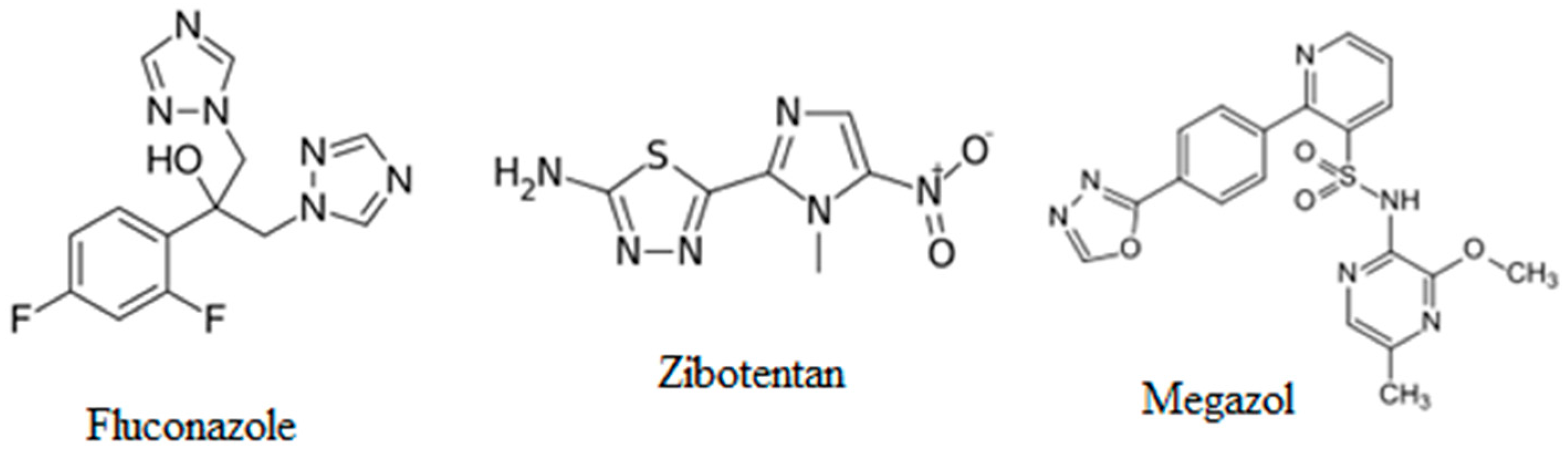
:1. Introduction
2. Results and Discussion
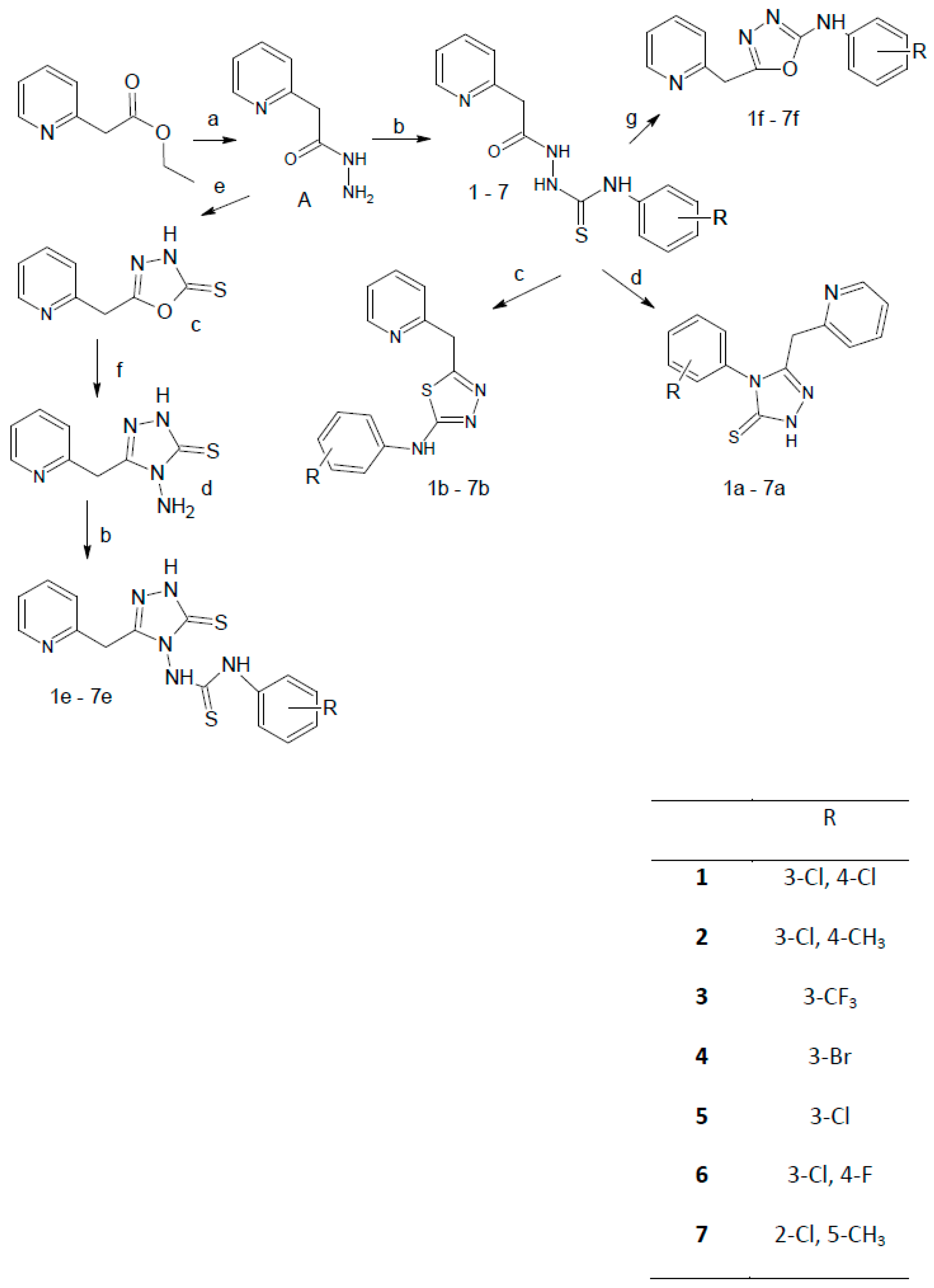
2.1. Chemistry
2.2. Biological Studies
3. Materials and Methods
3.1. Apparatus, Materials and Analysis
3.2. General Procedure of the Synthesis of N-(Phenylsubstituted)-2-(pyridin-2-ylacetyl)hydrazinecarbo-thioamide Derivatives
3.3. General Procedure for the Synthesis of 5-(Pyridin-2-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione Derivatives
3.4. General Procedure for the Synthesis of 5-(Pyridin-2-ylmethyl)-1,3,4-thiadiazol-2-amine Derivatives
3.5. 5-(Pyridin-2-ylmethyl)-1,3,4-oxadiazole-2(3H)-thione (c)
3.6. 4-Amino-5-(pyridin-2-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione
3.7. General Procedure for the Synthesis of 1-(Subtituted phenyl)-3-[3-(pyridin-2-ylmethyl)-5-thioxo-1,5-dihydro-4H-1,2,4-triazol-4-yl]thiourea Derivatives
3.8. General Procedure for the Synthesis of N-(Substituted-phenyl)-5-(pyridin-2-ylmethyl)-1,3,4-oxadiazol-2-amine Derivatives
3.9. Biological Assays
3.9.1. In Vitro Evaluation of Antimicrobial Activity
3.9.2. Media, Growth Conditions and Antimicrobial Activity Assays
3.9.3. Cytotoxicity and Antiviral Assays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shelke, S.H.; Mhaske, P.C.; Kasam, S.K.; Babade, V.D. Synthesis and pharmacological evaluation of a novel series of 2-((2-Aryl thiazol-4-yl)methyl)-5-(alkyl/alkylnitrile thio)-1,3,4-oxadiazole derivatives as possible antifungal agents. J. Heterocycl. Chem. 2014, 51, 1893–1897. [Google Scholar] [CrossRef]
- Wang, B.L.; Zhang, Y.; Liu, X.H.; Zhang, L.Y.; Zhan, Y.Z.; Zhang, X.; Wang, L.Z.; Li, Y.H.; Li, Z.M. Synthesis and biological activity of novel dimethylpyrazole and piperazine-containing (bis)1,2,4-triazole derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 34–41. [Google Scholar] [CrossRef]
- Ayati, A.; Emami, S.; Asadipour, A.; Shafiee, A.; Foroumadi, A. Recent applications of 1,3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem. 2015, 15, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, V.K.; Verma, P.K.; Dhanda, A.; Ranjan, S. 1,2,4-triazole derivatives as potential scaffold for anticonvulsant activity. Cent. Nerv. Syst. Agents Med. Chem. 2015, 15, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.P.; Quan, Z.S. 3,4-DHQLO and triazole and its related analogues with anticonvulsant effects. Mini Rev. Med. Chem. 2016, 16, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Plech, T.; Kaproń, B.; Luszczki, J.J.; Paneth, A.; Siwek, A.; Kołaczkowski, M.; Żołnierek, M.; Nowak, G. Studies on the anticonvulsant activity of 4-alkyl-1,2,4-triazole-3-thiones and their effect on GABAergic system. Eur. J. Med. Chem. 2014, 30, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Plech, T.; Luszczki, J.J.; Wujec, M.; Flieger, J.; Pizoń, M. Synthesis, characterization and preliminary anticonvulsant evaluation of some 4-alkyl-1,2,4-triazoles. Eur. J. Med. Chem. 2013, 60, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cao, Y.; Zhan, P.; Pannecouque, C.; Balzarini, J.; De Clercq, E.; Liu, X. Synthesis and anti-HIV evaluation of novel 1,2,4-triazole derivatives as potential non-nucleoside HIV-1 reverse transcriptase inhibitors. Lett. Drug Des. Discov. 2016, 10, 27–34. [Google Scholar]
- Aneja, R.; Rashad, A.A.; Li, H.; Kalyana Sundaram, R.V.; Duffy, C.; Bailey, L.D.; Chaiken, I. Peptide triazole inactivators of HIV-1 utilize a conserved two-cavity binding site at the junction of the inner and outer domains of Env gp120. J. Med. Chem. 2015, 14, 3843–3858. [Google Scholar] [CrossRef] [PubMed]
- Chapleo, C.B.; Myers, M.; Myers, P.L.; Saville, J.F.; Smith, A.C.B.; Stillings, M.R.; Tulloch, I.F.; Walter, D.S.; Welbourn, A.P. Substituted 1,3,4-thiadiazoles with anticonvulsant activity. 1. Hydrazines. J. Med. Chem. 1986, 29, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Chapleo, C.B.; Myers, P.L.; Smith, A.C.; Stillings, M.R.; Tulloch, I.F.; Walter, D.S. Substituted 1,3,4-thiadiazoles with anticonvulsant activity. 4. Amidines. J. Med. Chem. 1988, 31, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Myers, M.; Gadie, B.; Nelson, A.J.; Pape, R.; Saville, J.F.; Doxey, J.C.; Berridge, T.L. Antihypertensive thiadiazoles. 1. Synthesis of some 2-aryl-5-hydrazino-1,3,4-thiadiazoles with vasodilator activity. J. Med. Chem. 1988, 31, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Myers, M.; Gadie, B.; Hale, S.A.; Horsley, A.; Nelson, A.J.; Pape, R.; Saville, J.F.; Doxey, J.C.; Berridge, T.L. Antihypertensive thiadiazoles. 2. Vasodilator activity of some 2-aryl-5-guanidino-1,3,4-thiadiazoles. J. Med. Chem. 1988, 31, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, G.; Pignatello, R.; Mazzone, S.; Panico, A.; Penisi, G.; Castana, R.; Mazzone, P. Synthesis and local anesthetic activity of alkylaminoacyl derivatives of 2-amino-1,3,4-thiadiazole. Farmaco 1993, 48, 1207–1224. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.Y.; Lai, S.Y.; Pan, S.L.; Jow, G.M.; Chern, J.W.; Guh, J.H. Investigation of anticancer mechanism of thiadiazole-based compound in human non-small cell lung cancer A549 cells. Biochem. Pharmacol. 2003, 66, 115–124. [Google Scholar] [CrossRef]
- Xu, F.; Jia, Y.; Wen, Q.; Wang, X.; Zhang, L.; Zhang, Y.; Yang, K.; Xu, W. Synthesis and biological evaluation of N-(4-hydroxy-3-mercaptonaphthalen-1-yl)amides as inhibitors of angiogenesis and tumor growth. Eur. J. Med. Chem. 2013, 64, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Hajimahdi, Z.; Zarghi, A.; Zabihollahi, R.; Aghasadeghi, M.R. Synthesis, biological evaluation, and molecular modeling studies of new 1,3,4-oxadiazole- and 1,3,4-thiadiazole-substituted 4-oxo-4H-pyrido[1,2-a]pyrimidines as anti-HIV-1 agents. Med. Chem. Res. 2013, 22, 2467–2475. [Google Scholar] [CrossRef]
- Song, Y.; Connor, D.T.; Sercel, A.D.; Sorenson, R.J.; Doubleday, R.; Unangst, P.C.; Roth, B.D.; Beylin, V.G.; Gilbertsen, R.B.; Chan, K.; et al. Synthesis, structure-activity relationships, and in vivo evaluations of substituted di-tert-butylphenols as a novel class of potent, selective, and orally active cyclooxygenase-2 inhibitors. 2. 1,3,4- and 1,2,4-thiadiazole series. J. Med. Chem. 1999, 42, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Labanauskas, L.; Kalcas, V.; Udrenaite, E.; Gaidelis, P.; Brukstus, A.; Dauksas, V. Synthesis of 3-(3,4-dimethoxyphenyl)-1H-1,2,4-triazole-5-thiol and 2-amino-5-(3,4-dimethoxyphenyl)-1,3,4-thiadiazole derivatives exhibiting anti-inflammatory activity. Pharmazie 2001, 56, 617–619. [Google Scholar] [PubMed]
- Hanna, M.A.; Girges, M.M.; Rasala, D.; Gawinecki, R. Synthesis and pharmacological evaluation of some novel 5-(pyrazol-3-yl)thiadiazole and oxadiazole derivatives as potential hypoglycemic agents. Arzneimittelforschung 1995, 45, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.; Akhtar, T.; Al-Masoudi, N.A.; Stoeckli-Evans, H.; Hameed, S. Synthesis, crystal structure and anti-HIV activity of 2-adamantyl/adamantylmethyl-5-aryl-1,3,4-oxadiazoles. Med. Chem. 2012, 8, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, W.A.; El-Essawy, F.A.; Ali, O.M.; Nasr, B.S.; Abdalla, M.M.; Abdel-Rahman, A.A. Anti-HIV activity of new substituted 1,3,4-oxadiazole derivatives and their acyclic nucleoside analogues. Z. Naturforsch. C 2009, 64, 773–778. [Google Scholar] [CrossRef] [PubMed]
- El-Emam, A.A.; Al-Deeb, O.A.; Al-Omar, M.; Lehmann, J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorg. Med. Chem. 2004, 12, 5107–5113. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, V.; Shalini, S.; Sundram, K.; Sokkalingam, A.D. QSAR study of substituted 1,3,4-oxadiazole naphthyridines as HIV-1 integrase inhibitors. Eur. J. Med. Chem. 2010, 45, 2791–2797. [Google Scholar] [CrossRef] [PubMed]
- Dimova, V.; Perisic-Janjic, N. Solvatochromism studies on UV spectra of 4,5-disubstituted-1,2,4-triazoline-3-thiones. Maced. J. Chem. Chem. Eng. 2009, 28, 79–89. [Google Scholar]
- Lindsay, D.S.; Rippey, N.S.; Cole, R.A.; Parsons, L.C.; Dubey, J.P.; Tidwell, R.R.; Blagburn, B.L. Examination of the activities of 43 chemotherapeutic agents against Neospora caninum tachyzoites in cultured cells. Am. J. Vet. Res. 1994, 55, 976–981. [Google Scholar] [PubMed]
- Salgin-Gökşen, U.; Gökhan-Kelekçi, N.; Göktaş, O.; Köysal, Y.; Kiliç, E.; Işik, S.; Aktay, G.; Ozalp, M. 1-Acylthiosemicarbazides, 1,2,4-triazole-5(4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: Synthesis, analgesic-anti-inflammatory and antimicrobial activities. Bioorg. Med. Chem. 2007, 15, 5738–5751. [Google Scholar] [CrossRef] [PubMed]
- Tehranchian, S.; Akbarzadeh, T.; Fazeli, M.R.; Jamalifar, H.; Shafiee, A. Synthesis and antibacterial activity of 1-[1,2,4-triazol-3-yl] and 1-[1,3,4-thiadiazol-2-yl]-3-methylthio-6,7-dihydrobenzo[c]thiophen-4(5H)ones. Bioorg. Med. Chem. Lett. 2005, 15, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Turan-Zitouni, G.; Kaplancikli, Z.A.; Yildiz, M.T.; Chevallet, P.; Kaya, D. Synthesis and antimicrobial activity of 4-phenyl/cyclohexyl-5-(1-phenoxyethyl)-3-[N-(2-thiazolyl)acetamido]thio-4H-1,2,4-triazole derivatives. Eur. J. Med. Chem. 2005, 40, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Kitani, H.; Kuroda, T.; Moriguchi, A.; Ao, H.; Hirayama, F.; Ikeda, Y.; Kawakita, T. Synthesis and structural optimization of 7-(3,3-disubstituted-1-pyrrolidinyl)-1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acids as antibacterial agents. Bioorg. Med. Chem. Lett. 1997, 7, 515–520. [Google Scholar] [CrossRef]
- Tang, T.; Chen, K.X.; Jiang, H.L.; Ji, R.Y. QSAR/QSTR of fluoroquinolones: An example of simultaneous analysis of multiple biological activities using neural network method. Eur. J. Med. Chem. 1988, 33, 647–658. [Google Scholar] [CrossRef]
- Bielenica, A.; Stefanska, J.; Stępień, K.; Napiórkowska, A.; Augustynowicz-Kopeć, E.; Sanna, G.; Madeddu, S.; Boi, S.; Giliberti, G.; Wrzosek, M.; et al. Synthesis, cytotoxicity and antimicrobial activity of thiourea derivatives incorporating 3-(trifluoromethyl)phenyl moiety. Eur. J. Med. Chem. 2015, 101, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Bielenica, A.; Kedzierska, E.; Fidecka, S.; Maluszynska, H.; Miroslaw, B.; Koziol, A.E.; Stefanska, J.; Madeddu, S.; Giliberti, G.; Sanna, G. Synthesis, Antimicrobial and Pharmacological Evaluation of Thiourea-derivatives of 4H-1,2,4-triazole. Lett. Drug Des. Discov. 2015, 12, 263–276. [Google Scholar] [CrossRef]
- Stefanska, J.; Nowicka, G.; Struga, M.; Szulczyk, D.; Koziol, A.E.; Augustynowicz-Kopec, E.; Napiorkowska, A.; Bielenica, A.; Filipowski, W.; Filipowska, A.; et al. Antimicrobial and anti-biofilm activity of thiourea derivatives incorporating a 2-aminothiazole scaffold. Chem. Pharm. Bull. 2015, 63, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Uto, Y.; Ueno, Y.; Kiyotsuka, Y.; Miyazawa, Y.; Kurata, H.; Ogata, T.; Yamada, M.; Deguchi, T.; Konishi, M.; Takagi, T.; et al. Synthesis and evaluation of novel stearoyl-CoA desaturase 1 inhibitors: 1′-{6-[5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl]pyridazin-3-yl}-3,4-dihydrospiro[chromene-2,4′-piperidine] analogs. Eur. J. Med. Chem. 2010, 45, 4788–4796. [Google Scholar] [CrossRef] [PubMed]
- Yale, H.L.; Losee, K.; Martins, J.; Holsing, M.; Perry, F.M.; Bernstein, J. Chemotherapy of experimental tuberculosis. VIII. The synthesis of acid hydrazides, Their derivatives and related compounds. J. Am. Chem. Soc. 1953, 75, 1933–1934. [Google Scholar] [CrossRef]
- Dobosz, M.; Struga, M.; Chodkowska, A.; Jagiełło-Wójtowicz, E.; Stepniak, J.; Koziol, A.E. Synthesis and some pharmacological properties of 3-(4-phenyl-5-oxo-1,2,4-triazolin-1-ylmethyl)-1,2,4-triazolin-5-thione derivatives. Acta Pol. Pharm. 2002, 59, 281–290. [Google Scholar] [PubMed]
- Plech, T.; Kaproń, B.; Paneth, A.; Wujec, M.; Czarnomysy, R.; Bielawska, A.; Bielawski, K.; Trotsko, N.; Kuśmierz, E.; Paneth, P. Search for human DNA topoisomerase II poisons in the group of 2,5-disubstituted-1,3,4-thiadiazoles. J. Enzym. Inhib. Med. Chem. 2015, 30, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Isobe, T.; Ishikawa, T. 2-Chloro-1,3-dimethylimidazolium chloride. 2. Its application to the construction of heterocycles through dehydration reactions. J. Org. Chem. 1999, 64, 6989–6992. [Google Scholar] [CrossRef]
- Gavrilyuk, J.I.; Lough, A.J.; Batey, R.A. Parallel Solution Phase Synthesis of a Library of Amino acid derived 2-Arylamino-[1,3,4]-oxadiazoles. Tetrahedron Lett. 2008, 49, 4746–4749. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts, 2004; Approved Guideline. NCCLS document M44-A; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2009. [Google Scholar]
- CrysAlis PRO; Agilent Technologies Ltd.: Yarnton, UK, 2014.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sobi, M.; El-sayed, R.; Abdullah, M. The Effect of Non Ionic Surfactants Containing Triazole, Thiadiazole and Oxadiazole as Inhibitors of the Corrosion of Carbon Steel in 1M Hydrochloric Acid. J. Surfactants Deterg. 2013, 16, 937–946. [Google Scholar] [CrossRef]
- Drzewiecka, A.; Koziol, A.E.; Borowski, P.; Sanna, G.; Giliberti, G.; La Colla, P.; Zawadowski, T.; Struga, M. Structural and antivirial studies of dipetalactone and its methyl derivative. J. Mol. Struct. 2013, 150, 1054–1055. [Google Scholar] [CrossRef]
- Pauwels, R.; Balzarini, J.; Baba, M.; Snoeck, R.; Schols, D.; Herdewijn, P.; Desmyter, J.; de Clercq, E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 1988, 20, 309–321. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available.
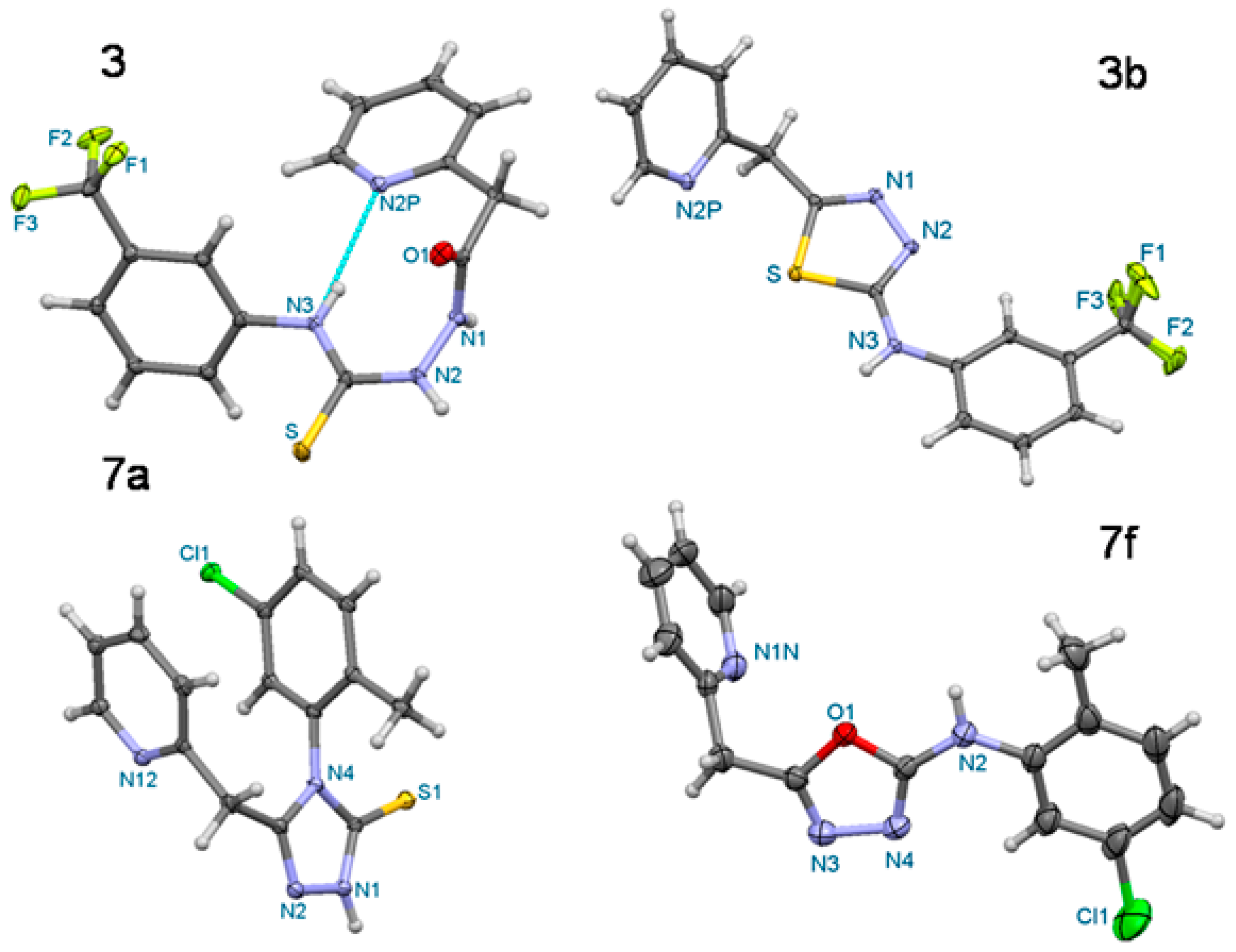
| Compound | 3 | 3b | 7a | 7f |
|---|---|---|---|---|
| Empirical formula | C15H13F3N4OS | C15H11F3N4S | C16H14ClN3S | C15H13ClN4O |
| Formula weight | 354.35 | 336.34 | 315.81 | 300.74 |
| Temperature | 80(2) K | 100(2) K | 120(2) K | 295(2) K |
| Wavelength (Å) | 0.71073 | 0.71073 | 1.54184 | 1.54184 |
| Crystal system | monoclinic | monoclinic | triclinic | monoclinic |
| Space group | P21/n | P21/c | P-1 | I2/a |
| Unit cell dimensions | ||||
| a (Å) | 4.6532(9) | 16.152(5) | 7.417(2) | 15.738(2) |
| b (Å) | 34.942(18) | 7.668(2) | 8.601(2) | 13.945(2) |
| c (Å) | 9.800(3) | 11.594(4) | 12.111(3) | 13.254(2) |
| α (°) | 90 | 90 | 106.72(2) | 90 |
| β (°) | 91.17(5) | 90.99(3) | 92.49(2) | 90.94(1) |
| γ (°) | 90 | 90 | 95.86(2) | 90 |
| Volume (Å−3) | 1593.1(10) | 1435.7(8) | 733.9(3) | 2908.4(7) |
| Z | 4 | 4 | 2 | 8 |
| F(000) | 728 | 688 | 328 | 1248 |
| Density (calcd) (g/cm3) | 1.477 | 1.556 | 1.429 | 1.374 |
| Absorpt. coeff. (mm-1) | 0.246 | 0.263 | 3.591 | 2.364 |
| Max. and min. transmission | 0.985 and 0.8996 | 0.9844 and 0.8886 | 0.990 and 0.9833 | 0.798 and 0.6493 |
| Crystal size (mm) | 0.44 × 0.07 × 0.06 | 0.46 × 0.13 × 0.06 | 0.22 × 0.20 × 0.18 | 0.20 × 0.20 × 0.10 |
| θ range for data coll. (°) | 2.72 to 30.39°. | 2.94 to 28.75°. | 3.82 to 73.65°. | 4.24 to 73.95°. |
| Index ranges | −5 ≤ h ≤ 5, −49 ≤ k ≤ 49, −12 ≤ l ≤ 13 | −21 ≤ h ≤ 21, −9 ≤ k ≤ 10, −11 ≤ l ≤ 15 | −9 ≤ h ≤ 9, −10 ≤ k ≤ 10, −11 ≤ l ≤ 15 | −13 ≤ h ≤ 19, −16 ≤ k ≤ 11, −16 ≤ l ≤ 12 |
| Reflections collected | 8587 | 8611 | 4813 | 3609 |
| Independent reflections | 4085 [R(int) = 0.0466] | 3364 [R(int) = 0.0353] | 2849 [R(int) = 0.0251] | 2419 [R(int) = 0.0189] |
| Data/parameters | 4085/229 | 3364/239 | 2849/195 | 2419/196 |
| Goodness-of-fit on F2 | 1.109 | 1.061 | 1.086 | 1.049 |
| Final R indices [I > 2σ (I)] | R1 = 0.0594 wR2 = 0.1229 | R1 = 0.0398 wR2 = 0.0956 | R1 = 0.0432 wR2 = 0.1012 | R1 = 0.0448 wR2 = 0.1240 |
| R indices (all data) | R1 = 0.0871 wR2 = 0.1354 | R1 = 0.0600 wR2 = 0.1001 | R1 = 0.0490 wR2 = 0.1050 | R1 = 0.0580 wR2 = 0.1382 |
| Extinction coefficient | -- | -- | -- | 0.0017(2) |
| Δρmax/min (e Å−3) | 0.38/−0.38 | 0.32/−0.24 | 0.58/−0.33 | 0.18/−0.29 |
| CCDC No. | 1539080 | 1539081 | 1539083 | 1539082 |
| Compounds | MT-4 | HIV-1IIIB |
|---|---|---|
| a CC50 | b EC50 | |
| (μM) | ||
| 1 | >100 | >100 |
| 2 | >100 | >100 |
| 3 | >100 | >100 |
| 4 | >100 | >100 |
| 5 | >100 | >100 |
| 6 | >100 | >100 |
| 7 | >100 | >100 |
| 1a | >100 | >100 |
| 2a | >100 | >100 |
| 3a | >100 | >100 |
| 4a | >100 | >100 |
| 5a | >100 | >100 |
| 6a | >100 | >100 |
| 7a | >100 | >100 |
| 1b | >100 | >100 |
| 2b | 47 | >47 |
| 3b | 70.6 | >70.6 |
| 4b | 74.0 | >74.0 |
| 5b | >100 | >100 |
| 6b | >100 | >100 |
| 7b | >100 | >100 |
| EFV | 45.0 | 0.003 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szulczyk, D.; Tomaszewski, P.; Jóźwiak, M.; Kozioł, A.E.; Lis, T.; Collu, D.; Iuliano, F.; Struga, M. Synthesis and Biological Activities of Ethyl 2-(2-pyridylacetate) Derivatives Containing Thiourea, 1,2,4-triazole, Thiadiazole and Oxadiazole Moieties. Molecules 2017, 22, 409. https://doi.org/10.3390/molecules22030409
Szulczyk D, Tomaszewski P, Jóźwiak M, Kozioł AE, Lis T, Collu D, Iuliano F, Struga M. Synthesis and Biological Activities of Ethyl 2-(2-pyridylacetate) Derivatives Containing Thiourea, 1,2,4-triazole, Thiadiazole and Oxadiazole Moieties. Molecules. 2017; 22(3):409. https://doi.org/10.3390/molecules22030409
Chicago/Turabian StyleSzulczyk, Daniel, Piotr Tomaszewski, Michał Jóźwiak, Anna E. Kozioł, Tadeusz Lis, David Collu, Filippo Iuliano, and Marta Struga. 2017. "Synthesis and Biological Activities of Ethyl 2-(2-pyridylacetate) Derivatives Containing Thiourea, 1,2,4-triazole, Thiadiazole and Oxadiazole Moieties" Molecules 22, no. 3: 409. https://doi.org/10.3390/molecules22030409






