3.1. Definition of Bioavailability and Challenges to Determine Pentacyclic Triterpene Bioavailability in a Complex Matrix
“The term bioavailability is used to indicate the fraction of an orally administered dose that reaches the systemic circulation as intact drug, taking into account both absorption and local metabolic degradation” [
45]. In order to measure the absolute oral bioavailability
F, the plasma drug concentration versus time curves are determined in a group of subjects following oral and intravenous administration. Then, areas under the plasma concentration time curve (AUC) are used to estimate the fraction AUC
oral/AUC
intravenous corrected by the oral and intravenous dose following this formula:
In the case of pentacyclic triterpenes consumed in medicinal plants and foods, the first step to determine their bioavailability is to evaluate their bioaccessibility, which can be defined as the fraction of ingested nutrients that is released from the food matrix in the gastrointestinal lumen and thereby becomes available for intestinal uptake [
46,
47].
Foods and medicinal plant matrices have a critical role on the bioavailability of triterpenes because before reaching the small intestine, they undergo digestion within the mouth, stomach and duodenum. They are thus submitted to mechanical actions, enzymatic activities and different pH conditions. In addition, food items are often eaten in conjunction with other foods containing proteins, carbohydrates, fat, fibers and minerals. Proteins, carbohydrates and fibers are able to interact with phytochemical compounds [
48,
49]; thereby, they might reduce the absorption of lipophilic compounds, such as triterpenes. The presence of fat appears also of major importance, since the solubilization and micellarization of lipophilic compounds are necessary steps prior to absorption [
47].
The water insolubility and lipophilicity of pentacyclic triterpenes strongly influence their interactions with components of the absorptive surface within the gastrointestinal tract. The lipophilicity of a compound can be characterized by its partition coefficient between octanol and water (P
ow), octanol being assumed to have a similar lipophilicity as cell membranes. This coefficient may be used as one of the predictors of drug absorption by passive diffusion [
50]. Indeed, intestinal permeability increases with log P
ow until values of two, where it reaches a plateau [
51]. In contrast, for log P
ow of four onwards, the permeability decreases with log P
ow because compounds with low aqueous solubility will partition at a slower rate from the cell membrane to the extracellular fluids (transcellular route) [
52]. Partition coefficients of pentacyclic triterpenes are reported in
Table 1.
Other physicochemical properties of the compounds to be absorbed should also be considered, such as molecular weight, H-bonding with the solvent, intramolecular H-bonding, intermolecular H-bonding, crystallinity, rate of dissolution, polymorphic forms, salt form and ionic charge status [
55,
56]. In addition, considering that bioactive compounds are consumed in food products and medicinal plants, better knowledge of their physicochemical properties might help to understand their interactions with complex matrices. It is generally accepted that for oral absorption, a molecule should have no more than five hydrogen bond donors and 10 hydrogen bond acceptors, a molecular mass less than 500 and log P not greater than five. As many natural products, triperpenes are an exception to the “rule of five” [
57]. Undoubtedly, the activities of digestive enzymes and of the gut microbiota also affect the absorption of bioactive compounds [
47]. In general, only aglycones can be absorbed in the small intestine. Prior to absorption, glycosides of pentacyclic triterpenes will thus most probably have to be hydrolysed by intestinal enzymes or by bacterial enzymes in the large intestine.
The degree of absorption of a compound is also related to the surface area over which the absorption is occurring and the time the compound spends in contact with that region [
56]. Among the other factors that can influence the degree of absorption of a compound, the pH of the medium from which absorption occurs, the rate of dissolution of the compound and host factors, such as nutrient status, age, genotype, physiological state, infectious disease state or body secretions, are considered as of high importance [
56].
The oral bioavailability of a compound also depends on its metabolism, which consists of its biotransformation into other compounds that are usually more water-soluble and more readily excreted in the urine. These biotransformations occur mainly in the liver, but they can also occur in the gastrointestinal tissue, lung, kidney, brain and even blood [
56]. They are catalyzed by enzymes that are commonly referred to as drug-metabolizing enzymes, including phase I and phase II metabolizing enzymes. In addition, phase III transporters are responsible for the elimination of the processed or unprocessed compounds from the cells. All together, these proteins provide a barrier against drug penetration and play crucial roles in drug absorption, distribution and excretion [
58,
59].
Since the determination of pentacyclic triterpene bioavailability in a complex matrix is riddled with obstacles, bioaccessibility is often not taken into account in studies dealing with the bioavailability of pentacyclic triterpenes. Most studies use pure compounds (isolated from medicinal plants, foods or chemically synthesized), precluding the extrapolation of the results obtained to more practical situations where the compounds of interest are consumed in a complex matrix. In addition, in these studies, the terms “absorption” and “bioavailability” are often considered as interchangeable, although absorption represents only one of the steps involved in the passage of a compound from its site of administration into the systemic circulation.
Studies on pentacyclic triterpene bioavailability have been carried out using in vitro assays, animal models and humans. In order to improve the bioavailability of these compounds, different approaches have been performed, including solubility or absorption site affinity increase by technological or chemical modifications of the compounds, the design of micelles, liposomes and nanoparticles [
47]. These studies are outlined below.
3.2. In Vitro Studies Carried out with Pentacyclic Triterpenes to Predict the In Vivo Bioavailability
In order to reach the bloodstream, released compounds have to cross the intestinal barrier. This can happen by different transport means: passive paracellular diffusion, passive transcellular diffusion, facilitated transport by membrane proteins, active (carrier-mediated) transport and exo-, endo- or trans-cytosis [
52].
In vitro models have been developed to study the absorptive processes of compounds administered orally. The reported works indicate good correlation among both in vitro cellular-based and non-cellular-based models and in vivo results [
52]. In vitro methods have also been optimized to evaluate the permeability of poorly soluble compounds in order to ensure a high level of accuracy [
60].
Lupeol, a lupane-type triterpene, occurs in fruits and vegetables, such as mango, green pepper and strawberries [
31]. Although its bioactivities have been well described, only one manuscript [
61] reports on a lupeol in vitro permeability study. In this study, permeability experiments were carried out using a Caco-2 cell monolayer grown in a bicameral system. In that kind of experiment, the cell monolayer is allowed to develop on a permeable membrane delimiting two compartments called “apical” and “basolateral”. As these cells get polarized during the differentiation step that follows confluency and since they form tight junctions, the two compartments get physically separated. The apical side represents the intestinal lumen, and the basolateral side represents the systemic circulation. The substance of interest can then be added to the apical compartment, and the system is left to manage the transport of the substance during a determined period of time, often ranging from one to three hours. After the incubation, the medium on each side, as well the cells themselves, is collected and submitted to extraction of the compound of interest for further quantification.
Caco-2 cell monolayers can be used to predict drug transport by different pathways across the intestinal epithelium. The apparent permeability coefficient (
Papp), which is a measure of the compound’s ability to cross the intestinal barrier, is calculated using Equation (2), where
Q is the amount of compounds (µg) transported over time
t (s),
A is the surface area of the porous membrane (cm
2) and
CD is the initial concentration added in the apical side (µg/cm
3). Substances with a
Papp value below 1 × 10
−6 cm/s, between 1 and 10 × 10
−6 cm/s and above 10 × 10
−6 cm/s, are respectively considered as poorly (0%–20%), moderately (20%–70%) or well orally absorbed (70%–100%) in humans [
62].
Examining the permeability of lupeol in nanoparticles containing 16% (
w/
v) of lupeol, Chairez-Ramírez et al. [
61] reported that from the results of transport at 8 h of incubation, only a small fraction of lupeol (traces) was transported from the apical to the basolateral side. However, the authors concluded that although transport across the Caco-2 cell model had not been observed, the best anti-inflammatory effects were observed at the highest dose of pure lupeol (20 µM) and the lowest dose of the nanonutraceutical compound (5 µM), suggesting that this difference could be related to an increased bioavailability of encapsulated lupeol.
The pentacyclic triterpene betulinic acid is another lupane-type triterpene that possesses anticancer and anti-HIV activity [
8], but besides these effects, its low aqueous solubility results in a low effective concentration and limited absorption in the gastrointestinal tract, seriously limiting its therapeutic applications. Betulinic acid derivatives have thus been synthesized in order to modulate the water solubility of the betulinic acid moiety. These derivatives have been tested with a non-cellular model called the parallel artificial membrane permeability assay (PAMPA™, Millipore, Watertown, MA, USA), in order to rapidly predict the passive transport of betulinic acid derivatives (
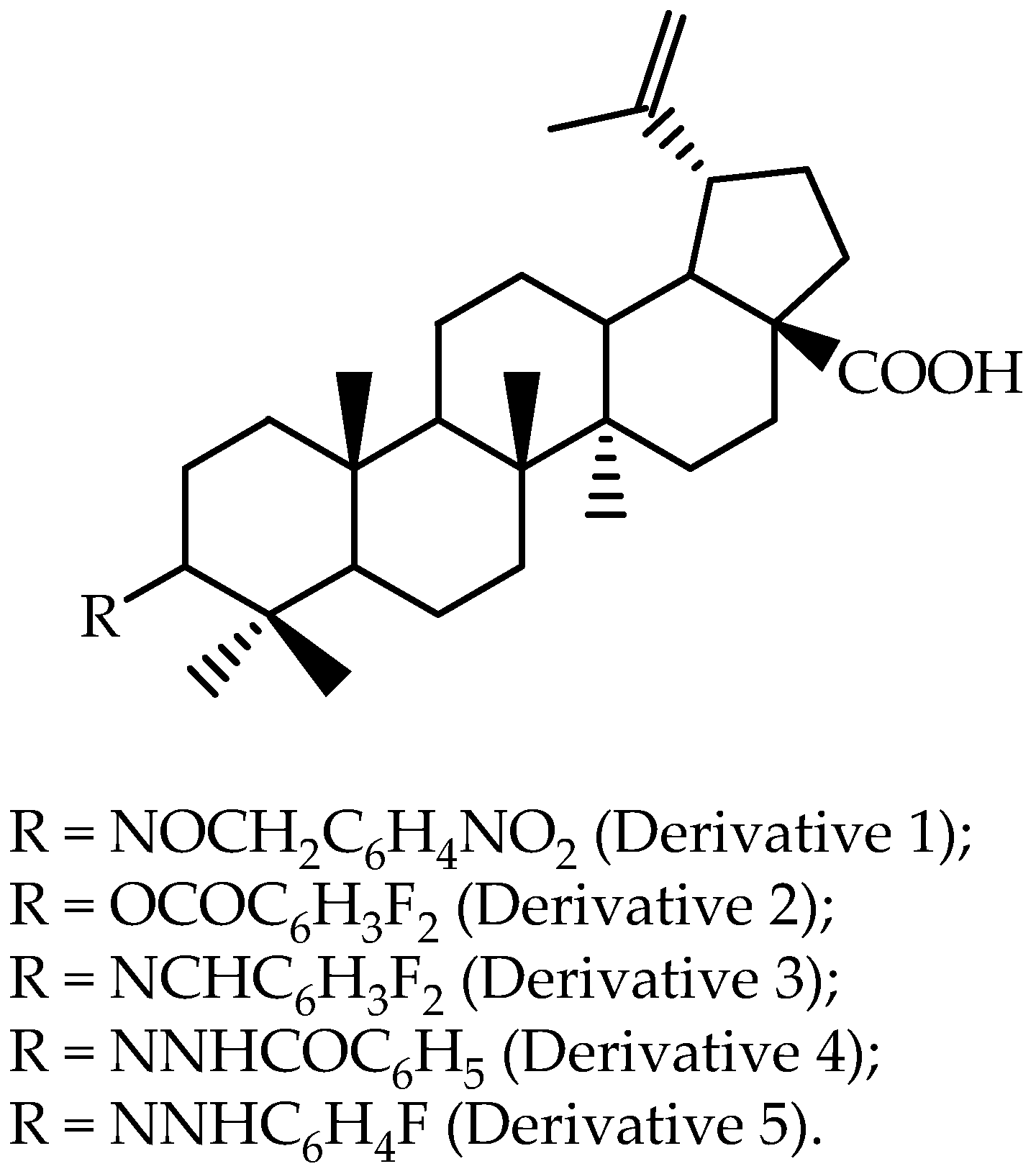
Figure 2) across a lipid layer that mimics the intestinal lipid bi-layer [
63]. The permeability of the betulinic acid derivatives was between 4.9% to 32.7%. Betulinic acid was not detected using this assay considering the limit of quantitation (3 µg/mL). The authors concluded that the betulinic acid derivatives fall in the group of “moderate” to “poor” permeable compounds when compared to known drugs.
Permeability studies were also carried out with oleanolic acid using in vitro Caco-2 cells [
64]. Jeong et al. [
64] reported that the
Papp of oleanolic acid in the apical to basolateral direction at 10 and 20 µM (1.1–1.3 × 10
−6 cm/s) was similar to that of a low-permeability standard atenolol (0.25 × 10
−6 cm/s), suggesting that oleanolic acid may be poorly absorbed. In addition, Jeong et al. [
64] found that there was no significant difference between the
Papp for the apical to basolateral direction and the
Papp for the basolateral to apical direction, which also suggests that the transport of oleanolic acid across the intestinal barrier occurs by passive diffusion and is not effluxed by the transporter.
An oral solid dispersion of oleanolic acid prepared by using spray freeze drying technology was evaluated in different formulations in in vitro transport studies using the Caco-2 cell monolayer [
65]. The authors reported that the presence of sodium caprate as the wetting agent and permeation enhancer in the formulation increased the permeation of oleanolic acid through the Caco-2 cell monolayer in 2 h by 2.76-times (
p < 0.05). The increased permeability occurred with a concomitant reduction in the transepithelial electrical resistance (TEER), which is a widely-accepted quantitative technique to measure the integrity of the cell monolayer grown on membrane inserts in cell culture models [
66]. Therefore, the authors suggested that this is indicative of an increased transport through the paracellular route via opening of the cellular tight junctions.
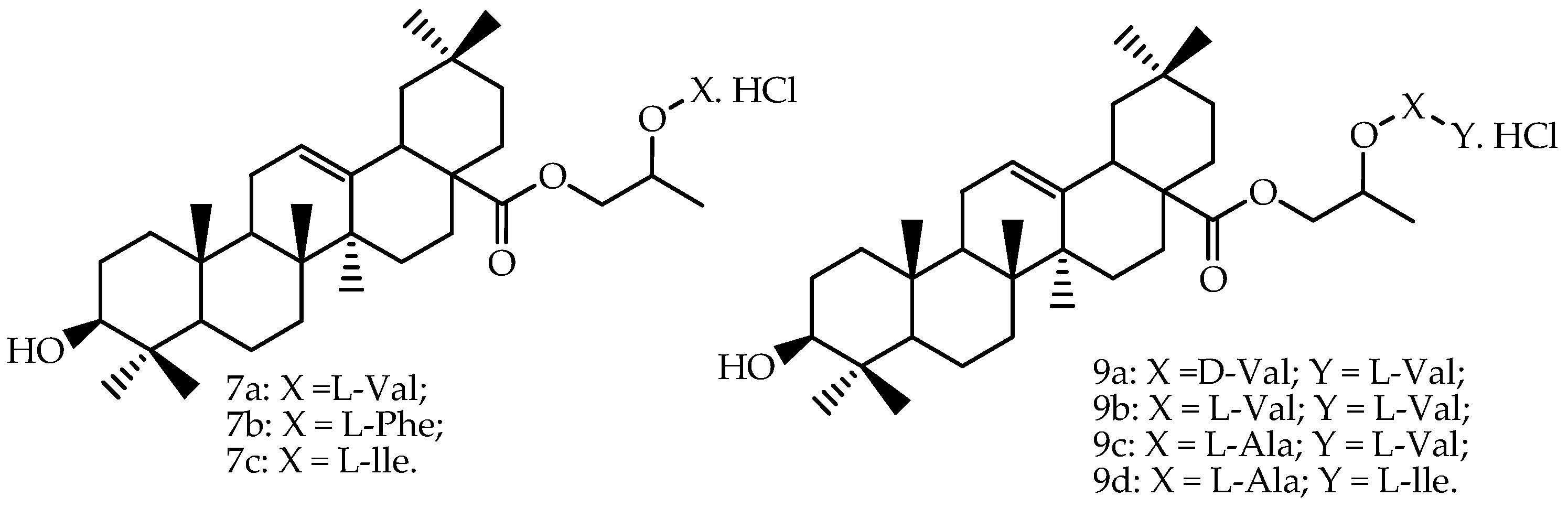
Prodrugs of oleanolic acid (
Figure 3) were also evaluated in Caco-2 permeability experiments, and all prodrugs showed the following increases of permeability through the Caco-2 cell monolayer compared to oleanolic acid:
7a (5.27-fold),
9b (3.31-fold),
9a (2.26-fold),
7b (2.10-fold),
7c (2.03-fold),
9c (1.87-fold) and
9d (1.39-fold) [
66]. The authors mentioned that the transepithelial electrical resistance (TEER) was little changed, indicating that the tight junctions were not opened.
One permeability study was performed in vitro with ursolic acid, either as free compound and in an ethanol extract from
Salvia officinalis L. That study used human intestinal epithelial Caco-2 cell monolayers [
68]. The content in ursolic acid of the ethanol extract from
S. officinalis was determined by HPLC analysis as 2.6 ± 0.4 g/L. Ursolic acid and the
S. officinalis extract at 2, 5, 10 and 20 µM (non-cytotoxic concentrations) were added to the apical chamber of the bicameral system, and then, basolateral solutions were collected after 0.5, 1, 2 and 4 h. After 4 h, the authors also analyzed the apical and cellular compartments. The uptake of ursolic acid as free compound and ursolic acid in
S. officinalis extract increased linearly and significantly from 0.03 ± 0.01–0.2 ± 0.04 µM/h/cm
2 and was not saturable across the evaluated concentrations (5–20 µM) and the tested time points (0.5–4 h), suggesting an uptake by passive diffusion. No significant differences were found between ursolic acid as free compound or ursolic acid in the plant extract since the permeability coefficients were of 2.8 ± 0.1 × 10
−6 cm/s for ursolic acid free compound and 2.5 ± 0.4 × 10
−6 cm/s for ursolic acid in plant extract.
Yuan et al. [
69] reported an investigation of asiatic acid absorption using Caco-2 cell line in vitro model
. After transportation of 2 µM of asiatic acid across the Caco-2 cell monolayer from the apical to basolateral and from basolateral to apical side, the permeabilities of asiatic acid were determined to be more than 1 × 10
−5 cm/s, which indicates a good absorption. The results obtained with a rat intestinal perfusion model also showed that the permeabilities were more than 1 × 10
−5 cm·s
−1, confirming the absorption results in the Caco-2 cell absorption model.
A significant number of studies reported investigations of the absorption of boswellic acids in human Caco-2 cell lines. Krüger et al. [
70] examined the permeability of 11-keto-β-boswellic acid and 3-acetyl-11-keto-β-boswellic acid, as well as their interaction with three transporters: the organic anion transporter polypeptides family member 1B3 (OATP1B3), the multidrug resistance-associated protein 2 (MRP2) and P-glycoprotein. They also evaluated a
B. serrata extract. The experiments were carried out using 10 µM of 11-keto-β-boswellic acid and 9.5 µM of 3-acetyl-11-keto-β-boswellic acid as free compounds and in the extract. Considering the apparent permeability coefficient obtained for the isolated 11-keto-β-boswellic acid (
Papp = 1.69 × 10
−6 cm/s), this compound can be classified as moderately absorbed. The
Papp value determined for this compound in the crude extract was 2.14 × 10
−6 cm/s. The isolated 3-acetyl-11-keto-β-boswellic acid was not detected when permeability studies were carried out using the isolated compound and the complex extract. The authors also reported that at the end of absorptive transport experiments, a significant loss of mass balance was detected for both compounds, but as they carried out control experiments in the absence of the Caco-2 monolayer, they concluded that these compounds were mainly accumulated in and/or adsorbed on the cell monolayer. Both compounds modulated the activity of OATP1B3 and MRP2 transporters, indicating that therapeutic relevant interactions with other anionic drugs may be expected. The authors also concluded that both compounds are not substrates of P-glycoprotein.
Permeability studies on Caco-2 cell lines were also carried out with 11-keto-β-boswellic acid and 3-acetyl-11-keto-β-boswellic acid by Hüsh et al. [
71]. Interestingly, these authors developed formulations that increased the solubility of boswellic acids up to 54-times. One of the formulations (extract-phospholipid complex) increased the mass flux of 11-keto-β-boswellic and 3-acetyl-11-keto-β-boswellic acids by eight- and 15-times, respectively, in comparison with a non-formulated extract.
Other boswellic acids were evaluated in Caco-2 permeability studies by Gerbeth et al. [
72], and the obtained apparent permeability coefficients are presented in
Table 2. These authors reported that the permeabilities of 11-keto-β-boswellic and 3-acetyl-11-keto-β-boswellic acids were underestimated in previous experiments and that their adapted Caco-2 model, which was closer to physiological conditions thanks to the addition of bovine serum albumin to the basolateral side and the use of modified fasted state simulated intestinal fluid on the apical side, provided better prediction of the absorption in vivo. Considering the classification of compounds in poorly, moderately and well absorbable based on the apparent permeability coefficient values reported by Yee et al. [
62], 11-keto-β-boswellic and 3-acetyl-11-keto-β-boswellic acids can be considered as well absorbable (70%–100%) according to the results obtained by Gerbeth et al. [
72].
3.3. Bioavailability of Bioactive Pentacyclic Triterpenes In Vivo
Betulinic acid bioavailability has been reported in in vivo studies by Udeani et al. [
73] and Godugu et al. [
74]. Udeani et al. [
73] performed a pharmacokinetic study on betulinic acid and showed that it is widely distributed in several tissues after a 500-mg/kg intraperitoneal administration to mice. In this study, serum samples were obtained after a 250 or 500 mg/kg intraperitoneal dose of betulinic acid. A two-compartment, first order model was selected for pharmacokinetic modeling. Godugu et al. [
74] reported an improvement of pharmacokinetic parameters of betulinic acid when this compound was evaluated in an optimized formulation in spray-dried mucoadhesive microparticles. These authors reported a significant increase in the oral bioavailability of betulinic acid (
Table 3) that resulted in a superior anticancer effect when evaluated in mice A549 orthotopic lung cancer models and in metastatic tumor models. The lung tumor weights and volumes were significantly reduced upon oral administration of this formulation at the dose of 100 mg/kg, daily for three weeks.
Other studies have reported on different formulation approaches of betulinic acid, such as complexation with gamma-cyclodextrin [
75], beta-cyclodextrin [
76] and nanoemulsion [
77], but in these manuscripts, the authors reported only an improvement of the anticancer effect evaluated in in vitro tests on tumor cell lines and in vivo tests on experimental animal models.
The pharmacokinetics of different betulinic acid derivatives have also been studied [
78,
79,
80] and the chemical structures of these derivatives are presented in
Figure 4.
Rajendran et al. [
63] reported that, based on in vitro results, a dihydro-betulinic acid derivative modified at the C-3 position (4-nitrobenzyl-oximino), as presented as Derivative 1 in
Figure 2, was selected to perform an in vivo assay using male Wistar rats. Non-compartmental analysis was selected for pharmacokinetic modeling, and the reported data are presented in
Table 3. The authors found that this Derivative 1 showed favorable pharmacokinetic characteristics and better in vivo antitumor efficacy as compared to betulinic acid in a human colon cancer xenograft model.
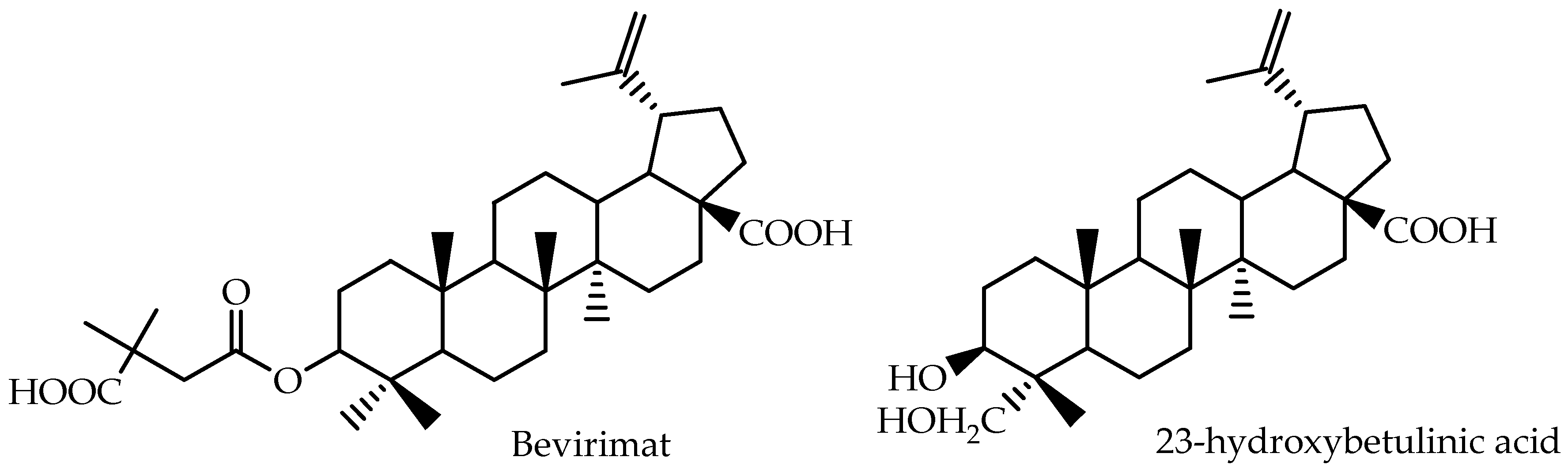
Yang et al. [
78] developed a new assay based on liquid chromatography/mass spectrometry for the quantitative analysis of 23-hydroxybetulinic acid in mouse plasma after intragastric administration. A two-compartment, first order model was selected for pharmacokinetic modeling, and the reported data are also presented in
Table 3. 23-hydroxybetulinic acid was found to have a long elimination half-live of 25.6 h and low bioavailability of 2.3%.
Bevirimat [3-O-(3′,3′-dimethylsuccinyl)-betulinic acid], a betulinic acid derivative, represents a promising class of anti-HIV agents with a novel mechanism. It inhibits HIV-1 maturation by blocking the cleavage of p25 to functional p24, resulting in the production of noninfectious HIV-1 particles [
80]. Martin et al. [
79] calculated AUC
p.o for different bevirimat oral doses and showed that the compound presents a dose-proportional pharmacokinetics during repeated dosing for 10 days. The pharmacokinetic parameters of bevirimat were estimated using non-compartmental methods, and the reported data for Day 10 are presented in
Table 3.
According to the literature, in mouse, rat or dog plasma, betulinic acid was reported to be 99.99% bound to serum proteins [
81].
Regarding oleanolic acid, pharmacokinetic parameters of this triterpene and of two amino acid ester prodrugs of oleanolic acid (
5a and
6f,
Figure 5) were reported by Cao et al. [
82] after oral administration to rats that received by oral gavage 300 mg/kg of oleanolic acid and its prodrugs (
Table 4). Standard non-compartmental analysis was performed for the estimation of the absorption profile using Kinetica
®, Version 4.4 (Thermo Electron Corporation, New York, NY, USA). The authors concluded that the water solubility of the prodrugs of oleanolic acid was greater than that of oleanolic acid. The permeability studies with rats in a single-pass intestinal perfusion model showed that all of the prodrugs had higher membrane effective permeability, and
5a and
6f exhibited enhanced oral bioavailability of oleanolic acid in rats.
In another work [
67], the same group of researchers also determined the pharmacokinetic parameters of two amino acid/dipeptide diester prodrugs with a propylene glycol link to oleanolic acid (
7a and
9b), and the reported data are presented in
Table 4. Compared to the ethylene glycol-linked amino acid/dipeptide diester prodrugs of oleanolic acid synthesized by Cao et al. [
82], the results from this study revealed that part of the propylene glycol-linked amino acid/dipeptide diester prodrugs showed better stability, permeability, affinity and bioavailability. The chemical structures of these derivatives are presented in
Figure 3. Standard non-compartmental analysis was performed for the estimation of the absorption profile using Kinetica
®, Version 4.4.
Jeong et al. [
64] reported on the oleanolic acid pharmacokinetic parameters in rats after intravenous injection at doses of 0.5, 1 and 2 mg/kg and oral administration at doses of 10, 25 and 50 mg/kg (
Table 4). According to these authors, oleanolic acid was also metabolically unstable following incubation with rat liver microsomes in the presence of NADPH. These authors concluded that the low bioavailability of 0.7% of oleanolic acid determined after oral administration to rats may be due to a poor gastrointestinal absorption and subsequent hepatic microsomal metabolism.
Pharmacokinetic parameters of some formulations of oleanolic acid were determined by Tong et al. [
65] after 50 mg/kg oral administration to rats. The authors evaluated three different formulations: Formula B (solid dispersion of oleanolic acid in polyvinylpyrrolidone-40 matrix using spray freeze drying), Formula F (solid dispersion of oleanolic acid in polyvinylpyrrolidone-40 matrix using spray freeze drying and the addition of sodium caprate) and Formula G (solid dispersion of oleanolic acid sodium salt in polyvinylpyrrolidone-40 matrix using spray freeze drying and the addition of sodium caprate). The reported data are presented in
Table 4. Comparison of Formulas B and G revealed that the addition of sodium caprate resulted in an initial boost of oleanolic acid concentration in the plasma, reaching a peak within the first 13–18 min and dropping progressively. The influence of the sodium salt form on the oral bioavailability of oleanolic acid was assessed by comparing the results that were achieved with Formulas F and G, and the authors concluded that the replacement of oleanolic acid with its sodium salt did not exert significant impact on either the plasma concentration-time profile or the associated kinetic parameter estimates.
Xi et al. [
83] developed a self-nanoemulsified drug delivery system of oleanolic acid and evaluated in vivo oral bioavailability in rats and compared this formulation to the commercially-available oleanolic acid tablet. The pharmacokinetic parameters are presented in
Table 4. According to the authors, the self-nanoemulsified drug delivery system of oleanolic acid showed a 2.4-fold increase in the oral bioavailability of oleanolic acid and an increased mean retention time of oleanolic acid in rat plasma.
Jiang et al. [
84] reported dual strategies to improve oral bioavailability of oleanolic acid. They carried out a pharmacokinetic study with a solidified phospholipid complex (oleanolic acid phospholipid complex (OPCH)) composed of oleanolic acid phospholipid complex and hydroxyapatite and the same complex added with ketoconazole (KCZ), since this compound is a noncompetitive inhibitor of CYP3A enzymes. The study was performed in rats after oral administration of 50 mg/kg of oleanolic acid, oleanolic acid phospholipid complex and oleanolic acid phospholipid complex added with ketoconazole. The reported pharmacokinetic parameters are presented in
Table 4. The formulation of solidified phospholipid complex and co-administration of ketoconazole improved the bioavailability of oleanolic acid by increasing the solubility and permeability in combination with inhibiting the metabolism of oleanolic acid.
Yang et al. [
85] developed a self-microemulsifying drug delivery system to enhance the solubility and bioavailability of oleanolic acid, and a pharmacokinetic study was carried out in rats to compare the developed formulation with the conventional tablet. The reported pharmacokinetic parameters are presented in
Table 4. The self-microemulsifying drug delivery system increased the bioavailability of oleanolic acid to 507% by keeping the drug in a dissolved form that contributed to enhance the absorption. In addition, the developed drug delivery system forms a fine oil/water microemulsion with a droplet size of less than 100 nm that provides a large interfacial surface area for the drug. The authors also reported that the high surfactant content in the developed formulation may increase the permeability by disturbing the cell membrane.
A nanosuspension of oleanolic acid stabilized with sucrose ester was developed by Li et al. [
86] who carried out pharmacokinetic studies in rats following intravenous (2 mg/kg) and oral administration (10 and 20 mg/kg). The authors compared the developed formulation with an oleanolic acid coarse suspension. The reported non-compartmental model pharmacokinetic parameters are presented in
Table 4. The oral bioavailability of the oleanolic acid nanosuspension was 6–7-times higher than that of the oleanolic acid coarse suspension.
Oleanolic acid can also be found in grape skins and raisins (
Vitis vinifera L.). According to Kanellos et al. [
89], the level of oleanolic acid in human plasma reached its highest concentration (24.4 ± 14.4 ng/mL) 4 h post-consumption of 144 g of raisins.
Pharmacokinetic studies of oleanolic acid were carried out in beagle dog by Shi et al. [
87] and Li et al. [
88]. Shi et al. [
87] determined pharmacokinetic parameters of oleanolic acid after oral and intravenous administration of calenduloside E, a triterpene saponin of oleanolic acid conjugated with glucuronic acid, while Li et al. [
88] determined pharmacokinetic parameters of a solid dispersion of oleanolic acid prepared with fumed silica by a supercritical fluid technology (
Table 4). According to Shi et al. [
87], oleanolic acid was found in the plasma after administration of oral doses of calenduloside E, indicating its formation from its glucuronic acid conjugate. In fact, after oleanolic acid formation in the gut, this compound is absorbed and then transported to the liver where it is converted back to its glucuronic acid conjugate. This conjugate undergoes biliary excretion and is transported through the bile to the gut where it is again hydrolyzed to oleanolic acid, and the cycle is repeated. On the basis of the AUC values (
Table 4), Li et al. [
88] concluded that the solid dispersion of oleanolic acid prepared with fumed silica bioavailability was 1.9-fold higher, as compared with commercial tablets.
Concerning maslinic acid, the main pentacyclic triterpene found in the leaves and fruits of
Olea europaea L. [
43], pharmacokinetic parameters of this triterpene were determined after intravenous (1 mg/kg) and oral (50 mg/kg) administration to rats [
90]. Plasma concentrations of maslinic acid versus time were analyzed following a non-compartmental approach by population-based compartmental modeling with the nonlinear mixed-effects approach. Estimates were confirmed by non-compartmental calculations. Some of the pharmacokinetic parameters estimated through the noncompartmental and compartmental approach were: AUC
0→∞ µmol·h/L = 5.06 and AUC
0→∞ µmol·h/L = 5.17 after intravenous injection; AUC
0→∞ µmol·h/L = 14.87 and AUC
0→∞ µmol·h/L = 12.43 after oral administration; C
0 = 32.79 µM and C
0 = 17.61 µM after intravenous injection; C
max = 5.36 µM and C
max = 4.03 µM after oral administration. The oral bioavailability of maslinic acid was determined to be 5.13%. This low bioavailability could be due to either a first-pass effect of the compound at the gut wall or the liver or a poor gastrointestinal absorption.
Regarding ursolic acid, concentrations in mice tissues and plasma were determined after intravenous administration of 15 mg/kg of ursolic acid dissolved in a 10-mL mixture of ethanol and polyethylene glycol 400 (1:1) and 15 mg/kg of ursolic acid phospholipid nanoparticles [
91]. The plasma concentration of ursolic acid reached after 12 h of intravenous administration in phospholipid nanoparticles (2.07 ng/mL) was higher than the plasma concentration reached with the ursolic acid solution (0.82 ng/mL).
Other formulation approaches have been developed for improving the dissolution properties and bioavailability of ursolic acid. Nanoparticles were prepared using different procedures by Zhi-Qiang et al. [
92] and Yang et al. [
93], and liposomes were prepared by Yang et al. [
94]. Pharmacokinetic studies were carried out by these authors in rats or mice, and the reported results are presented in
Table 5. It should be highlighted from the results achieved by Zhi-Qiang et al. [
92] that the oral bioavailability of ursolic acid nanoparticles prepared using
d-α-tocopheryl polyethylene glycol 1000 succinate was 27.5-fold higher than that of the ursolic acid free compound.
Asiatic acid, an ursane type triterpene, is the bioactive constituent of
Centella asiatica (L.) Urb. extract that is marketed by Syntex in a number of European Union countries and Canada under the trade name Madecassol
® to treat various dermatological conditions, including burns [
95]. Asiatic acid is found in
C. asiatica as free triterpene and as asiaticoside (triterpenoid saponin in which the aglycone is the triterpene asiatic acid). The bioavailability of asiatic acid was studied in 12 healthy male and female volunteers after oral administration of equimolar doses of asiatic acid (12 mg) and asiaticoside (24 mg). Pharmacokinetic parameters were determined, and the difference in the mean AUC between treatments was less than 2% (AUC
0→12 h ng·h/mL = 614 ± 250 after asiatic acid administration on Day 10 of a twice daily regime and AUC
0→12 h ng·h/mL = 606 ± 316 after asiaticoside administration on Day 10 of a twice daily regime). However, the C
max reached after asiatic acid administration was higher (C
max = 97.8 ± 43.5 ng/mL) than the C
max reached after asiaticoside administration (C
max = 65.1 ± 30.4 ng/mL), and T
max was slightly shorter on asiatic acid (T
max = 4.0 ± 2.5 h) than asiaticoside (T
max = 5.4 ± 4.3 h). Asiaticoside thus contributes to the plasma levels of asiatic acid after Madecassol
® administration though in vivo hydrolysis of asiaticoside into asiatic acid. According to the authors, the combination of asiatic acid and asiaticoside in Madecassol
® provides both a rapid and a prolonged availability of asiatic acid for maintained therapeutic effectiveness during the dose interval.
Yuan et al. [
69] reported the determination of plasma concentrations of asiatic acid after oral (20 mg/kg) and intravenous (2 mg/kg) administration, and the pharmacokinetic parameters were calculated by using DAS Version 3.0 according to the non-compartmental model. According to the authors, asiatic acid can be absorbed into blood rapidly since the maximum plasma concentration was reached at 30 min (C
max = 0.394 ng/mL). However, the small
t1/2 = 0.348 h suggested that asiatic acid may be metabolized quickly by hepatic enzymes, and the authors confirmed this hypothesis through investigations on the metabolic rate of asiatic acid in rat liver microsomes. The absolute oral bioavailability of asiatic acid was determined to be 16.25%.
Lingling et al. [
96] developed solid lipid nanoparticles of asiatic acid tromethamine salt to enhance the oral bioavailability and performed a pharmacokinetic study in rats. The main pharmacokinetic parameters were determined for solid lipid nanoparticles of asiatic acid tromethamine salt: AUC
0→∞ µg·h/L = 2347.1 ± 238.4; C
max = 680.0 ± 233.5 µg/L and T
max = 0.25 ± 0.0 h. The pharmacokinetic parameters were also determined for asiatic acid tromethamine salt: AUC
0→∞ µg·h/L = 929.9 ± 238.4; C
max = 184.0 ± 70.8 µg/L and T
max = 0.25 ± 0.0 h.
Corosolic acid, another ursane-type triterpene, is one of the bioactive triterpenes found in
Potentilla discolor Bunge, which has been used for diabetes in China for a long time [
97]. Li et al. [
97] studied the pharmacokinetics of corosolic acid after oral administration of the
P. discolor extract (1.33 g/kg) to normal and diabetic rats. Pharmacokinetic parameters of corosolic acid revealed significant differences between normal and diabetic rats. The AUC
0→∞ and C
max were elevated in diabetic rats (AUC
0→∞ mg·h/L = 3.26 ± 0.28 and C
max = 0.49 ± 0.03 mg/L) and compared with the values obtained for normal rats (AUC
0→∞ mg·h/L = 1.42 ± 0.04 and C
max = 0.31 ± 0.07 mg/L). The results showed a double-peak profile in the corosolic acid plasma concentration after around 0.5 and 2 h, with peak concentrations around 0.25 and 0.27 µg/mL, respectively. These data are different from those obtained in a study reported by Liu et al. [
98], in which a maximum plasma concentration of 0.30 µg/mL within 3 h was reached at 9.2 min and presented a single-peak profile after oral administration of corosolic acid (20 mg/kg). According to Li et al. [
97], these differences might be due to the fact that in the study carried out by Liu et al. [
98], corosolic acid was provided as pure compound (20 mg/kg), while in the study of Li et al. [
97], corosolic acid was administered in the form of herbal extract (45.3 mg/kg of corosolic acid in 1.33 g/kg of herbal extract). The AUC
0→∞ of corosolic acid in normal rats was only 1.42 mg·h/L, suggesting that only traces of corosolic acid can be absorbed into the blood when the compound is orally administered in an herbal extract.
Boswellic acids, which are constituents of
B. serrata gum resin extract, are some of the most studied pentacyclic triterpenes, and several clinical studies have confirmed their anti-inflammatory and antitumor activities [
99]. Pharmacokinetic studies have been carried out in humans, and the reported data are presented in
Table 6.
Sharma et al. [
19] carried out pharmacokinetic study of 11-keto-β-boswellic acid in twelve healthy male volunteers between 18 and 50 years of age after oral single dose of Wok Vel™ capsule containing the standardized
B. serrata gum extract with a minimum of 65% organic acids or minimum 40% total boswellic acids. Blood samples were withdrawn prior to drug administration and at 30, 60, 120, 150, 180, 210, 240, 300, 360, 480, 600, 720 and 840 min after drug administration. The authors performed a noncompartmental pharmacokinetic analysis of concentration time data. A single dose administration of 333 mg of the standardized
B. serrata did not cause side effects. Considering the elimination half-life, the standardized
B. serrata extract needs to be given orally at the interval of six hours, and the steady state is reached after approximately 30 h.
Sterk et al. [
18] reported that the pharmacokinetic profile of boswellic acids after oral administration of an extract is influenced by food intake. They determined the pharmacokinetic parameters after oral administration of 786 mg of
B. serrata dry extract (55.08% of boswellic acids) in twelve healthy male volunteers. This study was also a single dose study, but was under normal and high-fat meal. Blood samples were collected at 0.5, 1, 2, 3, 4, 8, 12, 18, 24, 36, 48 and 60 h. The pharmacokinetic profile of boswellic acid varied with food intake, and a better absorption of boswellic acids occurred in the high fat-meal due to the presence of bile acids.
Skarke et al. [
17] also determined the pharmacokinetic parameters of 11-keto-β-boswellic and 3-acetyl-11-keto-β-boswellic acids in twelve male volunteers after oral administration of a single oral dose of two Boswelan capsules containing 800 mg of
Boswellia serrata extract. Blood samples were collected at 1, 2, 3, 4, 6, 8, 12 and 24 h after oral dosing. In this study, food intake also affected the bioavailability of boswellic acids, but caused less effect than those caused in the study reported by Sterk et al. [
18].
Novel approaches have been developed to enhance the bioavailability and consequently the bioactivity of boswellic acids in humans, including the synthesis of new derivatives [
100,
101] and the preparation of different formulations composed of
B. serrata extracts [
102,
103] or isolated boswellic acids [
104,
105]. Therefore, all of these approaches may contribute to providing new therapeutic candidates for inflammation, cancer and other diseases.