Effects and Mechanism of Blue Light on Monascus in Liquid Fermentation
Abstract
:1. Introduction
2. Results and Discussion
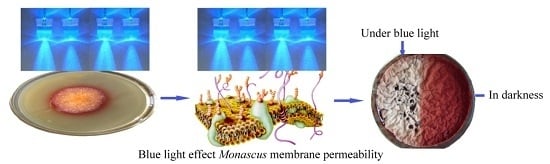
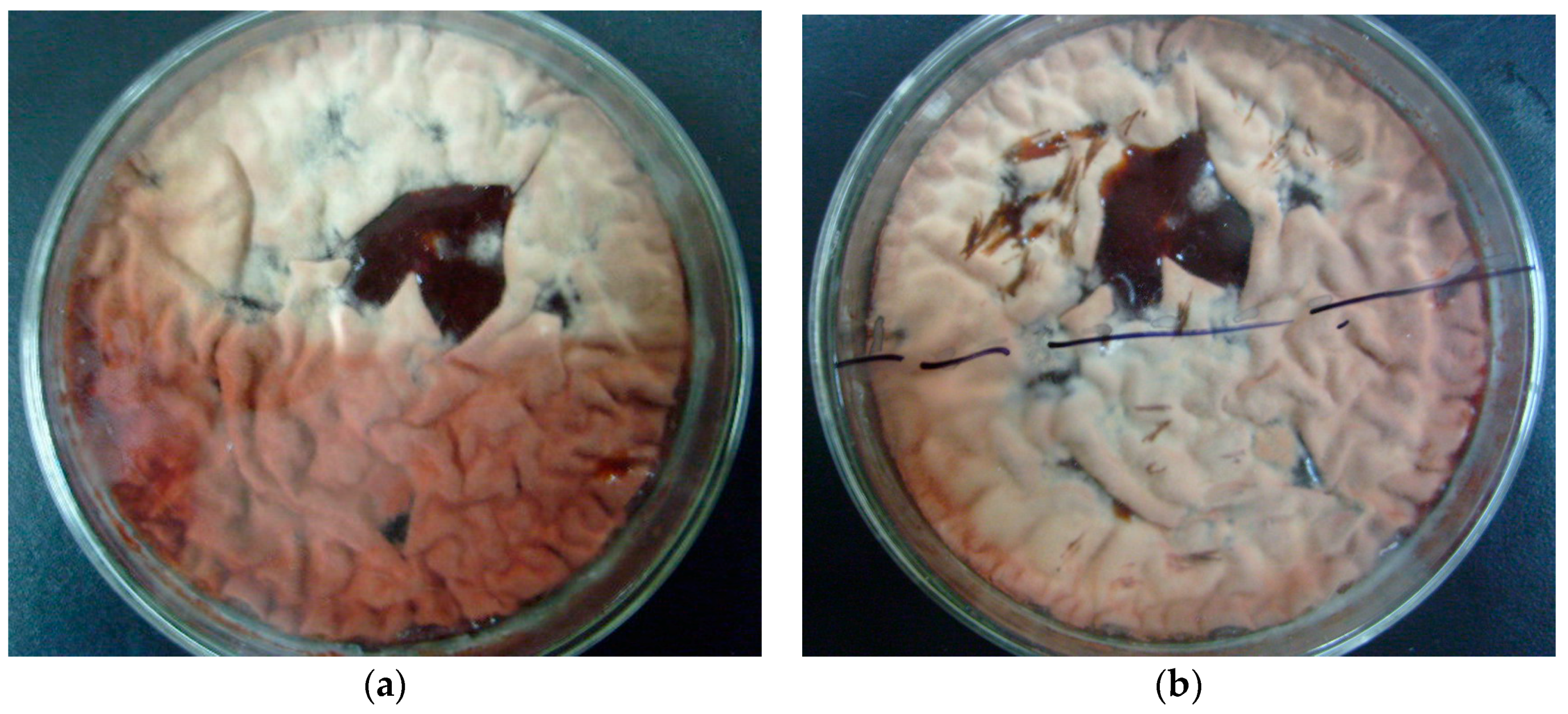
2.1. Effect of Blue Light on Monascus Colony Phenotype in Static Liquid Culture
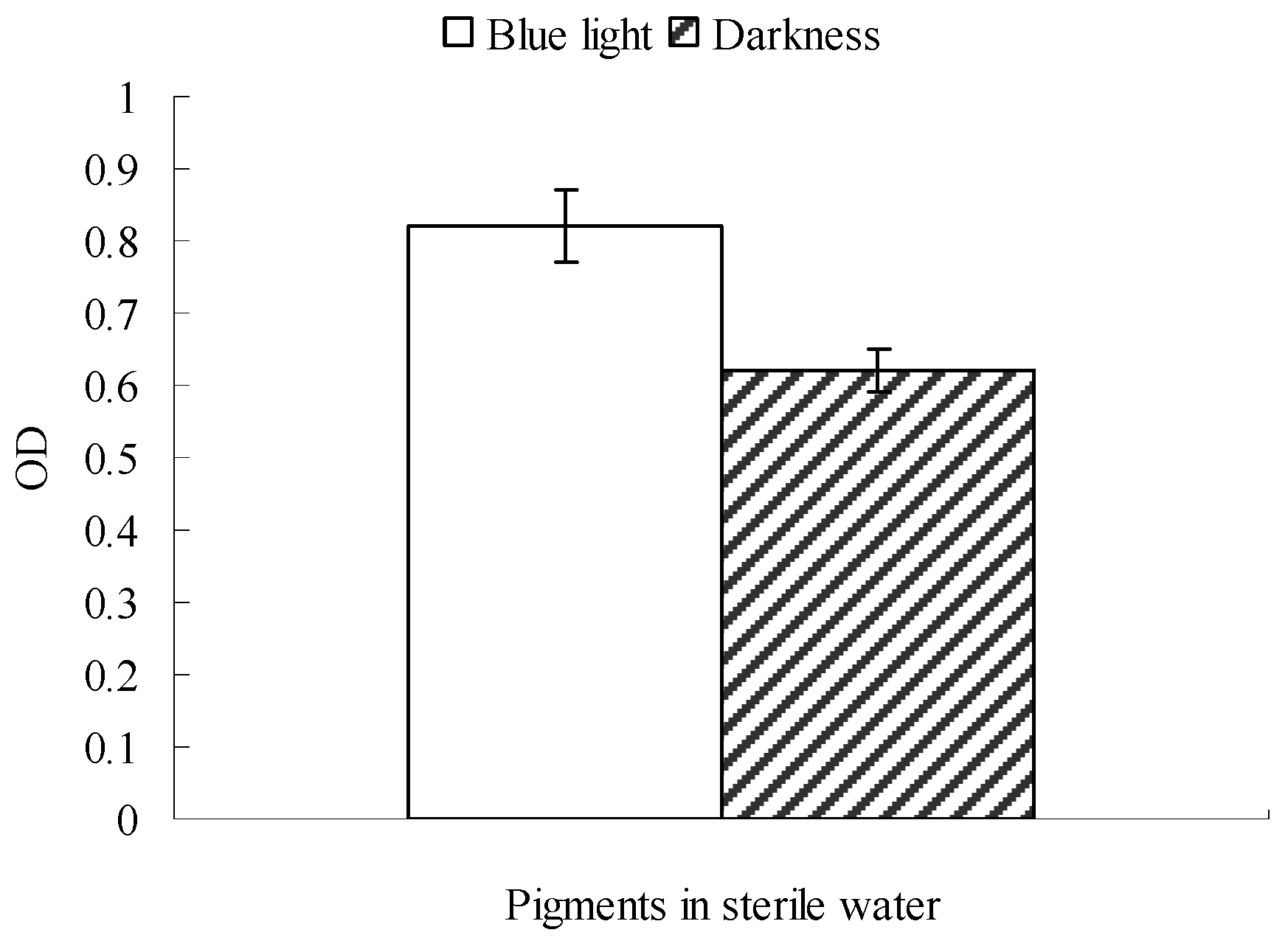
2.2. Possible Explanation for the Reduction in Monascus Pellicle Pigment Content after Blue Light Exposure
2.3. Effect of Blue Light on Monascus Mycelium Permeability
2.4. Effect of Blue Light on the Composition of Monascus Mycelium Wall
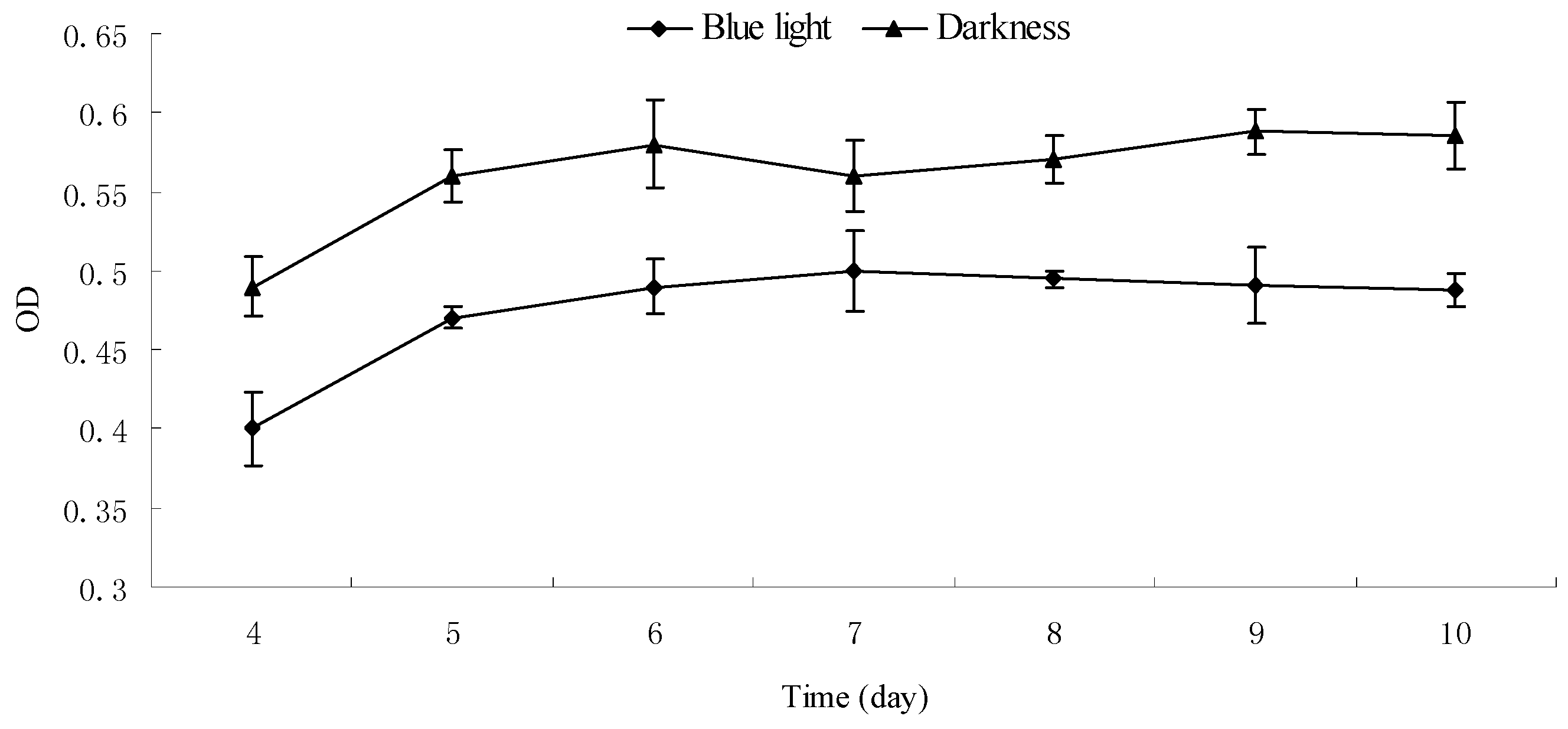
2.5. Effect of Blue Light on the Growth and Pigment Yield of Monascus Cultured by Liquid Oscillation
2.6. Influence and Mechanism of Blue Light on Monascus Citrinin Yield in Liquid-State Fermentation
2.7. Effect of Blue Light on Citrinin Degradation
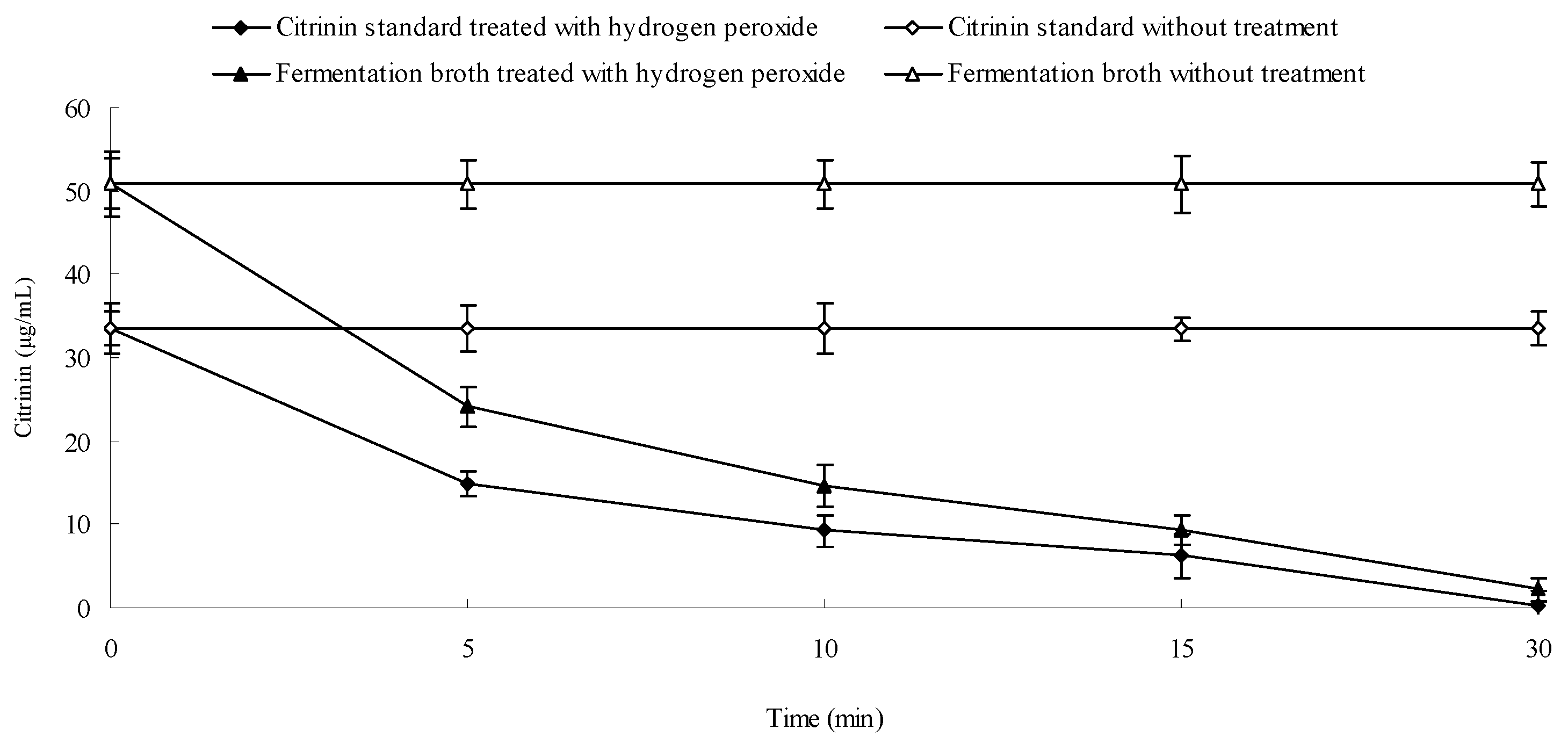
2.8. Effect of Hydrogen Peroxide on the Stability of Citrinin
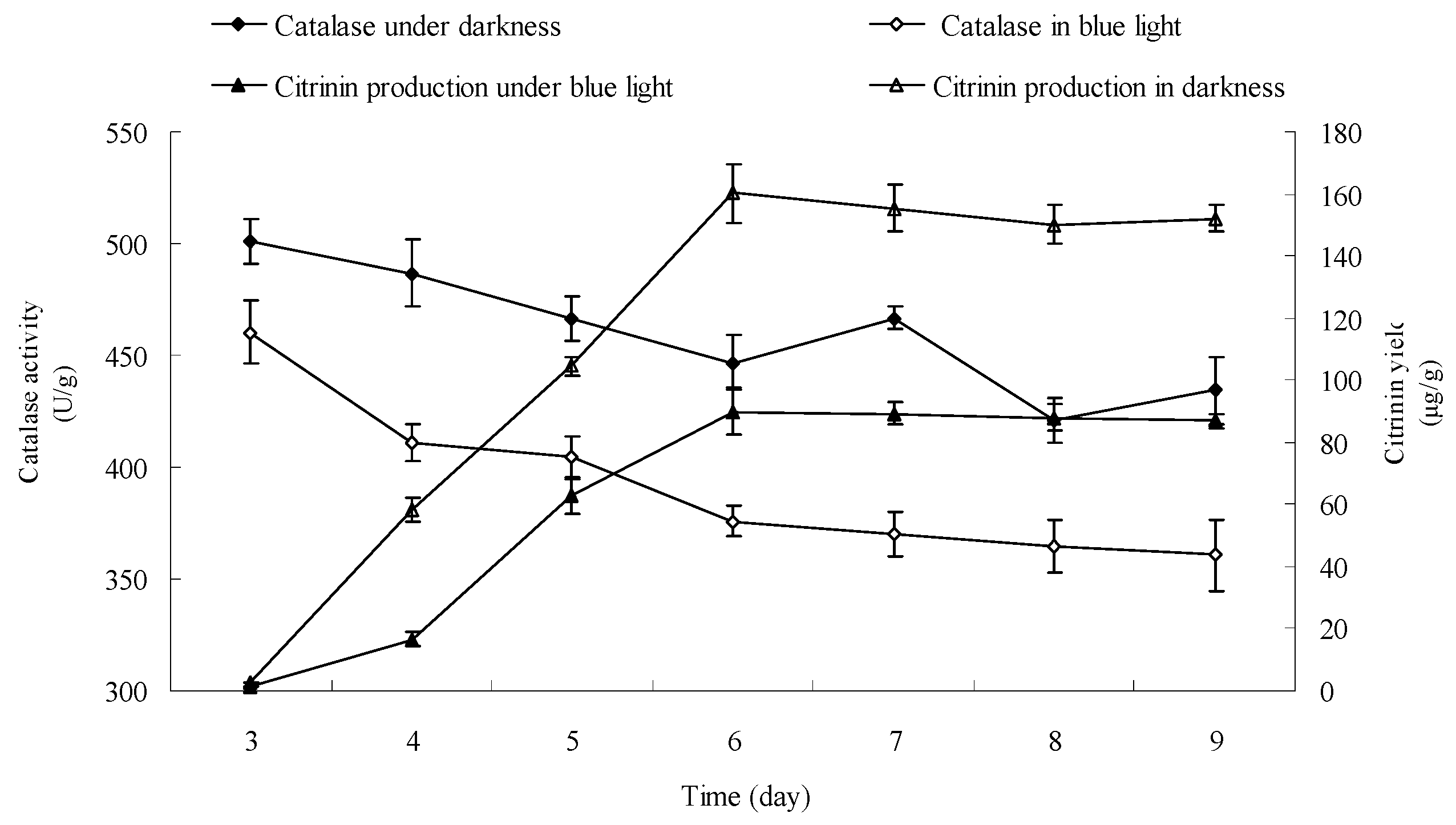
2.9. Effect of Blue Light on Catalase Activity in Monascus Mycelium in Liquid Fermentation
3. Materials and Methods
3.1. Strain and Culture
3.2. Inoculum Preparation
3.3. Stationary Liquid Culture of Monascus
3.4. Effect of Blue Light on Monascus Resting Pellicle Permeability
3.5. Oscillatory Liquid Fermentation of Monascus
3.6. Dynamic Monitoring of Dry Weight of Mycelium during Liquid Fermentation
3.7. Determination of Monascus Pigment Yield in Liquid Fermentation
3.7.1. Determination of Pigment Yield in Fermentation Broth
3.7.2. Determination of Pigment Yield in Monascus Mycelium
3.7.3. Determination of Total Pigment Yield in Unit Mycelium Dry Weight
3.8. Determination of Citrinin Yield
3.8.1. Pretreatment of Monascus Sample
3.8.2. Condition of HPLC
3.9. Determination of Monascus Mycelium Wall Component
3.10. Effects of Blue Light on Monascus Pigments Stability
3.11. Effects of Blue Light on Citrinin Stability
3.12. Effect of Hydrogen Peroxide on Citrinin Stability
3.13. Determination of Catalase Activity in Monascus Mycelium during Fermentation Process
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vendruscolo, F.; Meinicke Bühler, R.M.; Cesar de Carvalho, J.; de Oliveira, D.; Moritz, D.E.; Schmidell, W.; Ninow, J.L. Monascus: A Reality on the Production and Application of Microbial Pigments. Appl. Biochem. Biotechnol. 2016, 178, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Singgih, M.; Julianti, E. Food Colorant from Microorganisms[M]//Beneficial Microorganisms in Food and Nutraceuticals; Springer International Publishing: Cham, Switzerland, 2015; pp. 265–284. [Google Scholar]
- Sung, J.; Shin, J.Y.; Kim, H.; Baek, G.-H.; Yu, K.-W.; Yeon, J.; Lee, J. Anti-obesity and Anti-hyperlipidemic Activities of Fermented Coffee with Monascus ruber Mycelium by Solid-State Culture of Green Coffee Beans. J. Korean Soc. Food Sci. Nutr. 2014, 43, 341–348. [Google Scholar] [CrossRef]
- Kwon, C.S. Effect of Red Yeast (Monascus purpureus) Rice Supplemented Diet on Lipid Profiles and Antioxidant Activity in Hypercholesterolemic Rats. J. Korean Soc. Food Sci. Nutr. 2014, 43, 16–23. [Google Scholar] [CrossRef]
- Hsu, L.C.; Liang, Y.H.; Hsu, Y.W.; Kuo, Y.H.; Pan, T.M. Anti-inflammatory properties of yellow and orange pigments from Monascus purpureus NTU 568. J. Agric. Food Chem. 2013, 61, 2796–2802. [Google Scholar] [CrossRef] [PubMed]
- Rochín-Medina, J.J.; Gutiérrez-Dorado, R.; Sánchez-Magaña, L.M.; Reyes Moreno, C. Enhancement of nutritional properties, and antioxidant and antihypertensive potential of black common bean seeds by optimizing the solid state bioconversion process. Int. J. Food Sci. Nutr. 2015, 66, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.P.; Li, Y.Q.; Yan, J.; Cui, K.Y. Antibacterial Characteristics of Orange Pigment Extracted from Monascus Pigments against Escherichia coli. Czech J. Food Sci. 2016, 34, 197–203. [Google Scholar]
- Lee, C.L.; Lin, P.Y.; Hsu, Y.W.; Pan, T.M. Monascus-fermented monascin and ankaflavin improve the memory and learning ability in amyloid β-protein intracerebroventricular-infused rat via the suppression of Alzheimer’s disease risk factors. J. Funct. Foods 2015, 18, 387–399. [Google Scholar] [CrossRef]
- Chen, C.L.; Chang, K.Y.; Pan, T.M. Monascus purpureus NTU 568 fermented product improves memory and learning ability in rats with aluminium-induced Alzheimer’s disease. J. Funct. Foods 2016, 21, 167–177. [Google Scholar] [CrossRef]
- Muro Urista, C.; Gracida Rodríguez, J.; Abreu Corona, A.; Ainhoa Arana, C.; Alejandro Téllez, G. Pigments from fungi, an opportunity of production for diverse applications. Biologia 2016, 71, 1067–1079. [Google Scholar] [CrossRef]
- Blanc, P.J.; Loret, M.O.; Goma, G. Production of citrinin by various species of Monascus. Biotechnol. Lett. 1995, 17, 291–294. [Google Scholar] [CrossRef]
- Ali, N.; Blaszkewicz, M.; Mohanto, N.C.; Rahman, M.; Alim, A.; Hossain, K.; Degen, G.H. First results on citrinin biomarkers in urines from rural and urban cohorts in Bangladesh. Mycotoxin Res. 2015, 31, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Q.; Wang, W.; Hu, J.; Hu, C. Effect of oxygen supply on Monascus pigments and citrinin production in submerged fermentation. J. Biosci. Bioeng. 2015, 119, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Hajjaj, H.; François, J.M.; Goma, G.; Blanc, P.I. Effect of amino acids on red pigments and citrinin production in Monascus ruber. J. Food Sci. 2012, 77, M156–M159. [Google Scholar] [CrossRef] [PubMed]
- Haggblom, P.; Unestam, T. Blue light inhibits mycotoxin production and increases total lipids and pigmentation in Alternaria alternate. Appl. Microbiol. Biotechnol. 1979, 38, 1074–1077. [Google Scholar]
- Fanelli, F.; Geisen, R.; Schmidt-Heydt, M.; Mule, G. Light regulation of mycotoxin biosynthesis: New perspectives for food safety. World Mycotoxin J. 2016, 9, 129–146. [Google Scholar] [CrossRef]
- Fanelli, F.; Reveglia, P.; Masi, M. Influence of light on the biosynthesis of ophiobolin A by Bipolaris maydis. Nat. Prod. Res. 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Röhrig, J.; Kastner, C.; Fischer, R. Light inhibits spore germination through phytochrome in Aspergillus nidulans. Curr. Genet. 2013, 59, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Patakova, P. Monascus secondary metabolites: production and biological activity. J. Ind. Microbiol. Biotechnol. 2013, 40, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Bühler, R.M.M.; Müller, B.L.; Moritz, D.E.; Vendruscolo, F.; de Oliveira, D.; Ninow, J.L. Influence of light intensity on growth and pigment production by Monascus ruber in submerged fermentation. Appl. Biochem. Biotechnol. 2015, 176, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xue, C.; Chen, M.; Wu, S.; Li, Z.; Wang, C. Effects of blue light on pigment biosynthesis of Monascus. J. Microbiol. 2016, 54, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Mori, A.; Kii, T.; Okuno, T.; Usui, Y.; Sato, F.; Sammoto, H.; Watanabe, A.; Kariyama, M. Light effects on cell development and secondary metabolism in Monascus. J. Ind. Microbiol. Biotechnol. 2005, 32, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Babitha, S.; Soccol, C.R.; Pandey, A. Effect of stress on growth, pigment production and morphology of Monascus sp. in solid cultures. J. Basic Microbiol. 2007, 47, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Heydt, M.; Cramer, B.; Graf, I.; Lerch, S.; Humpf, H.U.; Geisen, R. Wavelength-dependent degradation of ochratoxin and citrinin by light in vitro and in vivo and its implications on Penicillium. Toxins 2012, 4, 1535–1551. [Google Scholar] [CrossRef] [PubMed]
- Hajjaj, H.; Klaebe, A.; Goma, G.; Blanc, P.J.; Barbier, E. Medium-chain fatty acids affect citrinin production in the filamentous fungus Monascus ruber. Appl. Environ. Microbiol. 2000, 66, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Zigman, S.; Reddan, J.; Schultz, J.B.; McDaniel, T. Structural and Functional Changes in Catalase Induced by Near-UV Radiation. Photochem. Photobiol. 1996, 63, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, W.; Chen, X.; Cai, J.; Wang, C.; He, W. Effects and Mechanism of Blue Light on Monascus in Liquid Fermentation. Molecules 2017, 22, 385. https://doi.org/10.3390/molecules22030385
Zhang X, Liu W, Chen X, Cai J, Wang C, He W. Effects and Mechanism of Blue Light on Monascus in Liquid Fermentation. Molecules. 2017; 22(3):385. https://doi.org/10.3390/molecules22030385
Chicago/Turabian StyleZhang, Xiaowei, Wenqing Liu, Xiying Chen, Junhui Cai, Changlu Wang, and Weiwei He. 2017. "Effects and Mechanism of Blue Light on Monascus in Liquid Fermentation" Molecules 22, no. 3: 385. https://doi.org/10.3390/molecules22030385